Transcatheter aortic valve replacement (TAVR) swiftly evolved from a disrupting technology towards mainstream therapy in the field of severe symptomatic aortic stenosis. A series of randomized evaluations established its role in treating severe aortic stenosis patients across all surgical risk categories, paving the way for an extension of its indications to younger low-risk patients with a longer life expectancy.
Over the past two decades, transcatheter aortic valve replacement (TAVR) swiftly evolved from a disrupting technology towards mainstream therapy in the field of severe symptomatic aortic stenosis. A series of randomized evaluations established its role in treating severe aortic stenosis patients across all surgical risk categories, paving the way for an extension of its indications to younger low-risk patients with a longer life expectancy.
- transcatheter aortic valve replacement
- transcatheter aortic valve implantation
- coronary artery disease
- myocardial infarction
1. Introduction
2. Conduction Disturbances
Conduction disturbances, i.e., high-degree atrioventricular block (HAVB) requiring permanent pacemaker implantation (PPI) and new-onset left bundle branch block (LBBB), represent the most common complication of transcatheter aortic valve replacement (TAVR). Thus, reswearchers will briefly review the current knowledge regarding the incidence and clinical impact of these conduction disturbances and reflect upon the challenges posed by these complications going forward in the TAVR setting. An extensive discussion of the mechanisms underlying these conduction disturbances, their natural history, predictors, and management is beyond the scope of the present researchview and can be found elsewhere [6][7][8][9][10][6,7,8,9,10].3. New-Onset Persistent Left Bundle Branch Block
3.1. Incidence
With newer-generation devices, rates of 6% to 77% have been reported [6][7][6,7]. The incidence of new-onset LBBB with the SAPIEN 3 prosthesis ranges from 6% to 29% [11][12][13][14][15][16][17][18][11,12,13,14,15,16,17,18]. The prospective MARE study reported the lowest rate with this iteration of the balloon-expandable device at 6.0% while the randomized PARTNER 3 trial demonstrated a 22% rate of 30 days new-onset LBBB, which was 3-fold higher than the rate of the surgical group [11][19][11,19]. Regarding the self-expandable EVOLUT R/PRO system, the MARE study also found a low 8.0% rate of persistent LBBB. Nonetheless, other studies reported an incidence ranging from 18.0% to 44.2% [20][21][22][23][20,21,22,23]. Regarding other self-expandable systems, the PORTICO valve (Abbott Medical) showed rates of approximately 12% [24][25][24,25] while rates of 10.3% to 13.1% have been reported with the ACURATE Neo prosthesis (Boston Scientific) [22][23][26][27][22,23,26,27].3.2. Clinical Impact
3.2.1. High-Degree Atrioventricular Block and Permanent Pacemaker Implantation
Three meta-analyses reported an approximately 2-fold higher rate of PPI associated with new-onset LBBB at mid-term (≈1 year) follow-up [28][29][30][28,29,30]. A significant impact of new-onset LBBB upon the risk of progression towards HAVB and PPI has consistently been reported either in-hospital [13][31][32][33][34][13,31,32,33,34] or at follow-up [32][33][35][36][37][38][32,33,35,36,37,38]. Furthermore, with the exception of the PARTNER I trial analysis [32], the vast majority of studies reported HAVB to be the leading indication (>70%) for PPI at follow-up. Some studies suggested that a QRS duration > 150–160 ms in the setting of new-onset LBBB was associated with a higher risk of late onset HAVB and sudden death [39][40][39,40], particularly when associated with a PR interval prolongation (>240 ms) [40][41][42][40,41,42].3.2.2. Left Ventricular Ejection Fraction (LVEF) and Hospitalization for Heart Failure (HHF)
LBBB may be associated with deleterious ventricular remodeling and deterioration of left ventricular function [43]. Several studies have reported an impaired LVEF recovery after TAVR among new-onset LBBB patients [32][33][35][36][37][38][44][45][32,33,35,36,37,38,44,45]. This observation did not translate into a consistently increased risk of hospitalization for heart failure (HHF) in individual studies. Nevertheless, the largest meta-analysis to date reported an increased 1-year HHF risk associated with new-onset LBBB (RR = 1.35; 95% CI: 1.05–1.72) [30].3.2.3. Mortality
Although it may act through the risk of progression to HAVB (and sudden death) and progressive heart failure as a result of LBBB-induced dyssynchrony, the effect of LBBB on all-cause and cardiovascular mortality has been inconsistent across studies. Regueiro et al. found an increased cardiovascular mortality risk in a meta-analysis of 5 studies, while only a trend was apparent for all-cause mortality combining data from 8 studies [28]. In their updated meta-analysis, Faroux et al. confirmed the deleterious impact upon cardiovascular mortality (RR = 1.46; 95% CI: 1.20–1.72), and unraveled a detrimental impact on all-cause mortality (RR = 1.32; 95% CI: 1.17–1.49) pooling data from 8 studies (5906 patients) and 12 studies (7792 patients), respectively [30].4. Permanent Pacemaker Implantation
4.1. Incidence
According to a recent systematic review, post-TAVR rates of PPI with newer-generation devices range from 2.3% to 36.1% [8][46][8,46]. Rates were 4% to 24% with the Edwards SAPIEN 3 valve, lower than those reported with the Medtronic EVOLUT R/PRO ranging from 14.7% to 31.3% [21][46][21,46]. Interestingly, the risk of PPI at 30 days post-procedure was not significantly different between the TAVR and surgical group in the PARTNER 3 trial, whereas it remained higher after implantation of a self-expandable valve in the EVOLUT Low-Risk trial [19][47][19,47]. With the PORTICO valve, rates ranging from 9.8% to 28.1% have been reported [24][48][24,48]. Overall, the ACURATE Neo prosthesis demonstrated the lowest rates ranging from 2.3% to 11.5% [26][49][26,49]. In the SCOPE I and SCOPE II randomized comparisons, the post-procedural rate of PPI with the ACURATE Neo was similar to the incidence observed with the SAPIEN 3 and significantly lower than the rate reported with the EVOLUT R/PRO, respectively [23][49][23,49].4.2. Clinical Impact
Left Ventricular Ejection Fraction and Hospitalization for Heart Failure The impact of PPI on the evolution of LVEF after TAVR has been inconsistent from one study to another. Some studies suggested a significant decrease in LVEF at follow-up among patients undergoing PPI [36][50][51][52][36,50,51,52], while others reported no meaningful association [53][54][55][56][57][53,54,55,56,57]. These discrepancies may stem from differing pacing indications, pacing dependency, and populations across studies as deleterious effects of right ventricular pacing are more likely to occur in younger patients subjected to a high ventricular pacing percentage over a longer period [45]. PPI post-TAVR has been linked to a higher 1-year risk of HHF in a recent meta-analysis of crude study-level data (RR = 1.18 95% CI: 1.03–1.36) [30]. However, individual studies with a longer follow-up reached conflicting adjusted results [45][54][58][45,54,58].4.3. Mortality
Faroux et al., reported an increased risk of 1-year all-cause mortality among pacemaker recipients post-TAVR (RR = 1.17 95% CI: 1.11–1.25) [30]. As previously discussed for HHF, long-term studies with a multivariable analysis reached inconsistent results regarding the independent impact of PPI in this finding [45][54][58][45,54,58]. This observation, along with the fact that PPI was not associated with an increased 1-year cardiovascular mortality in the meta-analysis by Faroux et al. [30], raises the issue of potential residual confounding in the association between PPI and post-TAVR mortality. Figure 1 summarizes the effects of new-onset LBBB and PPI on TAVR outcomes.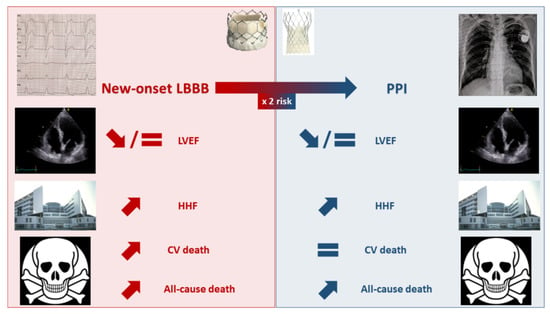
5. Unmet Needs
5.1. Pre-Procedural Management
Several studies have demonstrated that a significant proportion of TAVR candidates displayed HAVB or severe bradyarrhythmia during pre-procedural ECG monitoring either with 24-h in-hospital telemetry [59] or with ambulatory systems (patch ECG recorder, mobile telemetry), allowing a prolonged (≥7 days) recording [60][61][60,61]. Overall, approximately 3% of patients had HAVB episodes pre-TAVR, among whom 2/3 underwent pre-procedural PPI [62]. Furthermore, almost half of the patients with pre-TAVR bradyarrhythmic events benefited from a treatment change [59][60][59,60]. Bradyarrhythmic events were especially frequent among patients with 1st-degree AVB and right bundle branch block (RBBB) occurring in 30% and 47% of them, respectively [60]. Finally, among patients who received a pacemaker post-TAVR, 30% had HAVB episodes pre-TAVR [59][60][59,60]. These data suggest that pre-procedural ECG monitoring may be an appealing strategy to streamline patients care, especially those with the highest conduction disturbances risk (e.g., pre-existent RBBB and/or 1st-degree AVB). Nonetheless, further randomized studies are necessary to delineate the optimal indications, duration, clinical impact, and cost-effectiveness of pre-TAVR ambulatory ECG monitoring.5.2. Procedural Management
Several approaches have been proposed in recent years to reduce the occurrence of conduction disturbances during TAVR. Jilaihawi et al. reported the MInimizing Depth According to the membranous Septum (MIDAS) approach, which suggests that a systematic pre-procedural evaluation of the length of the membranous septum below the aortic annulus plane may help tailor the implantation strategy to each patient anatomy [63]. Indeed, the performance of TAVR among increasingly younger and “healthier” patients may imply a need for future coronary re-access. Therefore, the optimal patient-specific implantation depth may result from a compromise between the need to prevent conduction disturbances and to access coronary artery during long-term follow-up. Consequently, in patients with a membranous septum length > 5 mm, considered at low risk of conduction disturbances by Jilaihawi et al., a deeper prosthesis position may be tolerated as long as it does not result in significant paravalvular leak [63]. Moreover, several studies have advocated for a higher implantation of transcatheter heart valves, especially using the cusp-overlap projection, which overlap coronary cusps and isolates the non-coronary cusp, thus providing a better appreciation of the implantation depth [64][65][66][64,65,66]. These reports usually demonstrated an approximately 2-fold lower rate of PPI associated with the use of the cusp-overlap technique.5.3. Post-Procedural Management
One of the main issues regarding post-TAVR conduction disturbances has been the differing management strategies across centers and operators resulting from the lack of consensus, which explain the major differences observed in PPI rates and impact post-TAVR. Several experts’ consensus and guidelines have been published in recent years [9][10][67][9,10,69], which should facilitate a uniform post-procedural management, and allow the performance of large-scale, prospective studies to better describe the long-term impact of these conduction disturbances. Another persistent challenge is the management of conduction disturbances not representing firm PPI indications, i.e., new-onset LBBB and significant PR or QRS prolongation (≥40 ms, especially if PR is >240 ms or QRS > 150 ms). Several studies have demonstrated the safety of using ambulatory ECG monitoring post-TAVR to expedite patients’ discharge and guide PPI in such cases [11][62][68][69][70][71][11,62,70,71,72,73]. Overall, in these studies, delayed HAVB rates have ranged from 5% to 10% and from 10% to 15% approximately, at 30 days and 1-year post-TAVR, respectively. Interestingly, in the largest study to date, encompassing 459 TAVR recipients, the rate of delayed HAVB was higher among patients with new-onset first degree AVB than in patients with new-onset LBBB [70][72]. Another study demonstrated that the delta between baseline and day 2 post-procedure in PR interval but not in QRS duration was significantly associated with episodes of delayed HAVB [71][73]. These data suggest that the prolongation of the atrioventricular conduction on the surface ECG may not be a benign occurrence resulting from a supra Hisian injury and that rwesearchers may need to pay greater attention to this modification. On the other hand, some groups have proposed the use of in-hospital electrophysiological studies (EPS) to guide PPI post-TAVR. Studies focusing on this strategy are usually of limited sample size and used various EPS protocols as well as different HV interval cut-offs to retain an indication for PPI [2][72][2,74]. Therefore, the level of evidence seems weaker than for ambulatory ECG monitoring. Nonetheless, these studies have overall demonstrated an excellent negative predictive value of EPS in the post-TAVR setting with a somewhat lower positive predictive value [2][72][2,74]. The recent European pacing guidelines granted ambulatory ECG monitoring and EPS-guided strategies (EPS being performed at day 3 post-procedure and an HV interval > 70 ms being used to retain an indication for PPI) the same grade of recommendations in TAVR recipients with new-onset or worsened conduction disturbances [10]. Defining whether ambulatory ECG monitoring or EPS-guided strategies represent the best and more cost-effective option in the post-procedural management of TAVR-related conduction disturbances remains a major unmet need, which is currently addressed by the Clinical Monitoring Strategy Versus Electrophysiology-guided Algorithmic Approach With a New LBBB After TAVI (COME-TAVI) study (NCT03303612). Finally, among TAVR recipients with pre-existent depressed LVEF (<50%) and requiring PPI or with large new-onset LBBB (>150 ms), the role of cardiac resynchronization has not been properly studied yet. Table 1 summarizes ongoing studies regarding conduction disturbances in the setting of TAVR.| NCT Number | Study Name | Planned Number of Patients | Target Population | Design and Timing | Intervention | Main Outcomes |
|---|---|---|---|---|---|---|
| NCT03810820 | Remote ECG Monitoring of TAVI Patients | 240 | Consecutive candidates to outpatient TAVR | Observational, prospective, pre and post-procedure | Mobile cardiac telemetry (m-CARDS) before and after TAVR | Feasibility/patients’ adherence. Timeliness of medical assessment. Any new conduction disturbances up to 30 days. |
| NCT04139616 | PROMOTE | 2000 | All TAVR recipients without prior pacemaker | Observational, prospective, post-procedure | Application of a pre-specified algorithm for the management of conduction disturbances post-TAVR | Implementation of the algorithm. Incidence of PPI and sudden cardiac death up to 1 year |
| NCT02659137 | HESITATE | 100 | All TAVR recipients without pre-existent conduction disturbances | Observational, prospective, per and post-procedure | EPS during the procedure | Measurement of the HV interval upon occurrence of a LBBB. Location of the LBBB |
| NCT04454177 | SMART TAVR | 100 | All TAVR patients | Observational, prospective, post-procedure |
Huawei smart watch | Composite of death and rehospitalization, rates of conduction disturbances and PPI at 30 days |
| NCT04489095 | Conduction Disease After Transcatheter Aortic Valve Replacement | 200 | All TAVR recipients without prior pacemaker | Prospective, observational, per and post-procedure | EPS immediately before and after TAVR and the next day | Correlation between delta values of EPS findings and high-grade conduction disturbances at 1 year |
| NCT02482844 | LBBB-TAVI | 200 | TAVR recipients with new-onset LBBB | Observational, prospective, post-procedure |
EPS with PPI if HV interval >70 ms and implantable cardiac monitoring if <70 ms. |
Incidence of HAVB at 1 year |
| NCT04128384 | HOM TAVI | 200 | All TAVR recipients without prior pacemaker | Observational, prospective, per and post-procedure | Limited EPS including HV- and AH-intervals measurements pre- and post-TAVR | Incidence of HAVB and persistence of new-onset LBBB at 2 years |
| NCT03303612 | COME TAVI | 200 | TAVR recipients with new-onset LBBB | Randomized, prospective, post-procedure | Group 1: EPS-based strategy Group 2: Clinical follow-up with implantable cardiac monitoring. |
Incidence of the composite of cardiovascular hospitalization, syncope or death at 1 year. Incidence of HAVB at 1 year. Cost-effectiveness. |
| NCT02768064 | PAMIT | 120 | All TAVR recipients without prior pacemaker | Randomized, prospective, per and post-procedure | Experimental: Flexible screwed temporary pacemaker Active Comparator: Stiff standard temporary pacemaker |
Incidence of pericardial effusion, electrode dislocation, and other temporary pacing complications at 1 week |
| NCT04482816 | PHYS-TAVI | 24 | TAVR recipients with HAVB pacing indication after TAVR and LVEF > 50% | Randomized, prospective, post-procedure | Experimental: Physiological (His system) pacing Active Comparator: Right ventricular pacing |
Composite of survival, NYHA improvement and >25% increase in the 6MWT at 1 year. LVEF at 1 year. |
