Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Amina Yu and Version 1 by Allison Jean Stewart.
Pituitary pars intermedia dysfunction (PPID) is the most common endocrine disorder of geriatric horses, affecting 20–25% of horses over the age of 15 years. Since the disease was first described in 1932, considerable research has been conducted investigating PPID pathophysiology, prevalence of clinical signs, appropriate diagnostic techniques and treatment. In recent years, awareness of PPID among horse owners has grown, and veterinarians are increasingly testing for underlying endocrinopathies. An increase in awareness has led to a substantial increase in research conducted in the field of equine endocrinology.
- endocrine
- geriatric
- hypertrichosis
- insulin dysregulation
- α-melanocyte stimulating hormone (α-MSH)
- proopiomelanocortin (POMC) derived peptides
1. Anatomy and Physiology
The equine pituitary gland is suspended by the infundibular stalk, ventral to the hypothalamus in the sella turcica [10][1]. The pituitary gland is composed of the adenohypophysis (pars distalis, pars intermedia and pars tuberalis), and the neurohypophysis (pars nervosa) [11][2]. In response to physiologic, pathologic or environmentally induced stressors, there is activation of the hypothalamic-pituitary-adrenal axis. This involves the paraventricular dopaminergic neurons of the hypothalamus synthesizing corticotropin-releasing hormone (CRH) which is released in the hypothalamic-hypophyseal portal vessels. There is local action of CRH on the corticotroph cells of the pars distalis, which leads to adrenocorticotropic hormone (ACTH) secretion. Adrenocorticotrophin acts on the zona fasiculata cells of the adrenal glands to synthesise and secrete glucocorticoids. The pars intermedia in horses is composed of melanotrope cells which are directly innervated by hypothalamic periventricular dopaminergic neurons which release dopamine. Dopamine acts on the D2 receptors of the melanotropes to inhibit cell proliferation and transcription of POMCproopiomelanocortin (POMC) [12,13,14][3][4][5]. POMC undergoes processing to produce ACTH, α-MSH, β-END and corticotropin like intermediate lobe peptide (CLIP) [4,15,16,17,18,19][6][7][8][9][10][11]. In the normal equine pituitary gland most ACTH is produced via enzymatic conversion of POMC by prohormone convertase I (PC-1) in the pars distalis [15][7]. ACTH is further cleaved by prohormone convertase 2 (PC-2) to produce α-MSH and CLIP [4,15,16,17,18,19][6][7][8][9][10][11]. β-END is produced via PC-2 conversion of β-lipotropin [20][12] (Figure 1). Melanotrophs are also stimulated by thyrotropin-releasing hormone (TRH) [21][13].
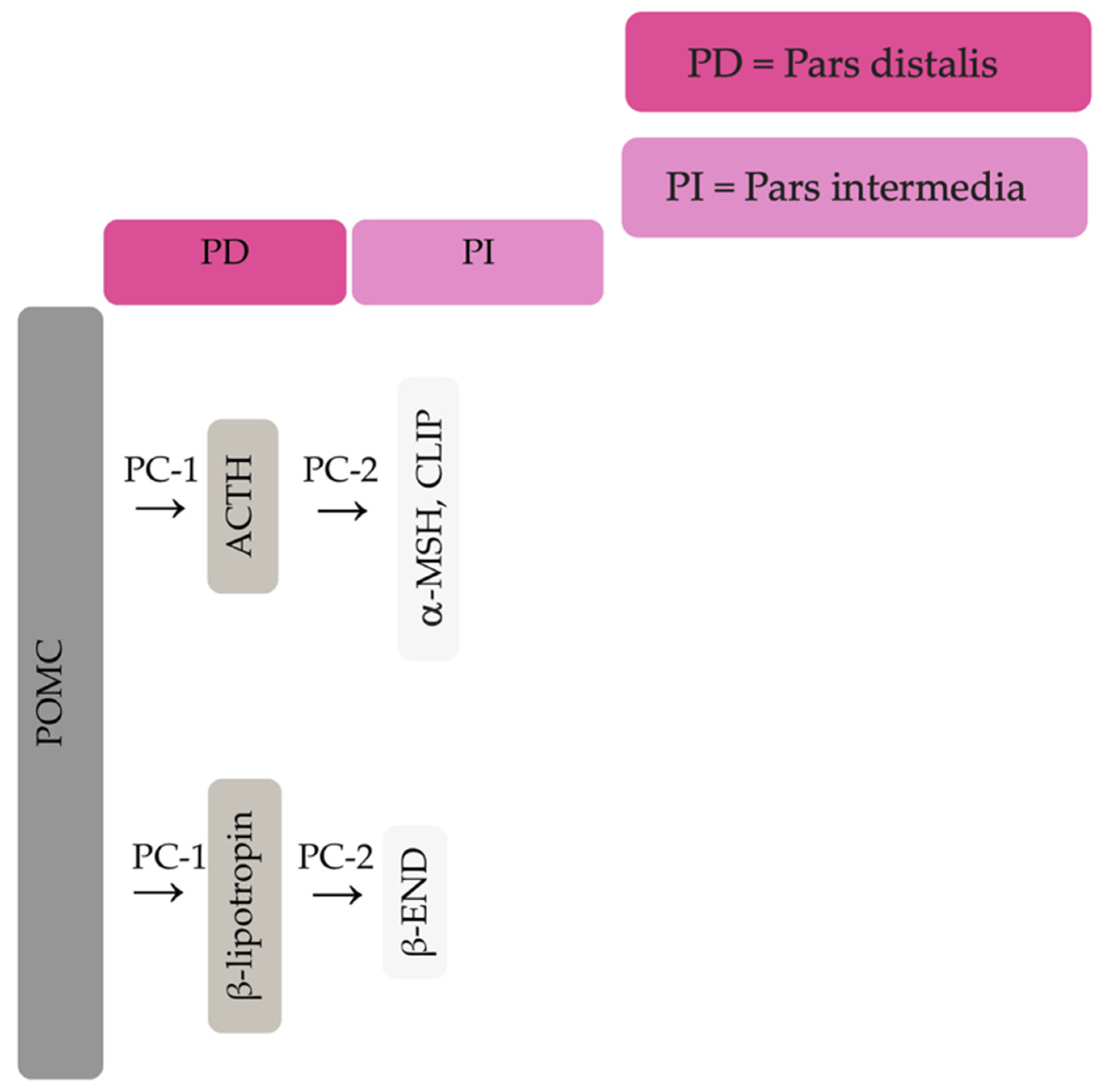
Figure 1. Simplified processing of POMC in the pars distalis by PC-1 to produce ACTH and β-lipotropin. Further PC-2 activity on ACTH and β-lipotropin to produce α-MSH, CLIP and β-END in the pars intermedia.
Closely linked to the hypothalamic-pituitary-adrenal axis is the hypothalamic-pituitary-thyroid axis [22][14]. Endogenous TRH produced by the hypothalamus causes an increase in thyroid stimulating hormone (TSH) produced by the pituitary gland, and an increase in circulating concentrations of thyroxine and triiodothyronine [23][15]. Glucocorticoids exert an inhibitory action on the hypothalamic-pituitary-thyroid axis by reducing TRH secretion by the hypothalamus [24,25][16][17]. Exogenous TRH administration will lead to binding of TRH receptors on the melanotrophs of the pars intermedia and secretion of POMC derived peptides [26][18].
Hypothalamic and pituitary secretions are physiologically influenced by several factors including stress, exercise, illness, photoperiod length, and climate [27,28,29,30][19][20][21][22]. In late summer and autumn, concentrations of α-MSH and ACTH in peripheral blood increase in normal horses, with more pronounced increases in animals with PPID [31][23]. These seasonal changes in ACTH and α-MSH concentrations require seasonally adjusted diagnostic cut-off values to diagnose Pituitary pars intermedia dysfunction (PIDPID) [2,32][24][25].
2. Pathophysiology of PPID
Pituitary pars intermedia dysfunction is one of the most common endocrinopathies in horses; however, the pathophysiology of PPID is incompletely understood [33,34,35,36,37,38][26][27][28][29][30][31]. The disease is caused by loss of dopaminergic inhibition of the pituitary PI due to oxidative-stress and subsequent neurodegeneration of dopaminergic neurons within the hypothalamus [17,39][9][32]. This theory was confirmed recently in a study that aimed to determine whether it was decreased dopamine or dopamine D2 receptor dysfunction that led to PPID. Tyrosine hydroxylase is a biomarker of dopaminergic neurons, and this retrospective study used 28 horses with PPID to measure tyrosine hydroxylase expression within the pars intermedia. A negative correlation between pituitary histomorphological grades and tyrosine hydroxylase was observed, confirming the involvement of reduced dopamine in the pathogenesis of PPID [38][31]. The loss of dopaminergic inhibition on the melanotropes of the pars intermedia results in hyperplasia, microadenoma or macroadenoma formation and overproduction of POMC derived peptides including ACTH and α-MSH (Figure 2) [16][8]. The ACTH secreted by the PI of horses with PPID is largely biologically inactive [17,40,41][9][33][34]. Subsequently, increased plasma ACTH concentrations do not result in adrenal stimulation and hypercortisolaemia [16,17,42,43,44][8][9][35][36][37]. Horses with PPID have resting serum concentrations of free thyroxine and TSH that are lower than age-matched control horses, with normal TSH responses to exogenous TRH administration [24][16]. Because glucocorticoids exert an inhibitory action on the hypothalamic-pituitary-thyroid axis [24[16][17],25], it has been hypothesized that increased glucocorticoid activity does exist, with prolonged tonic suppression of TRH [24][16].
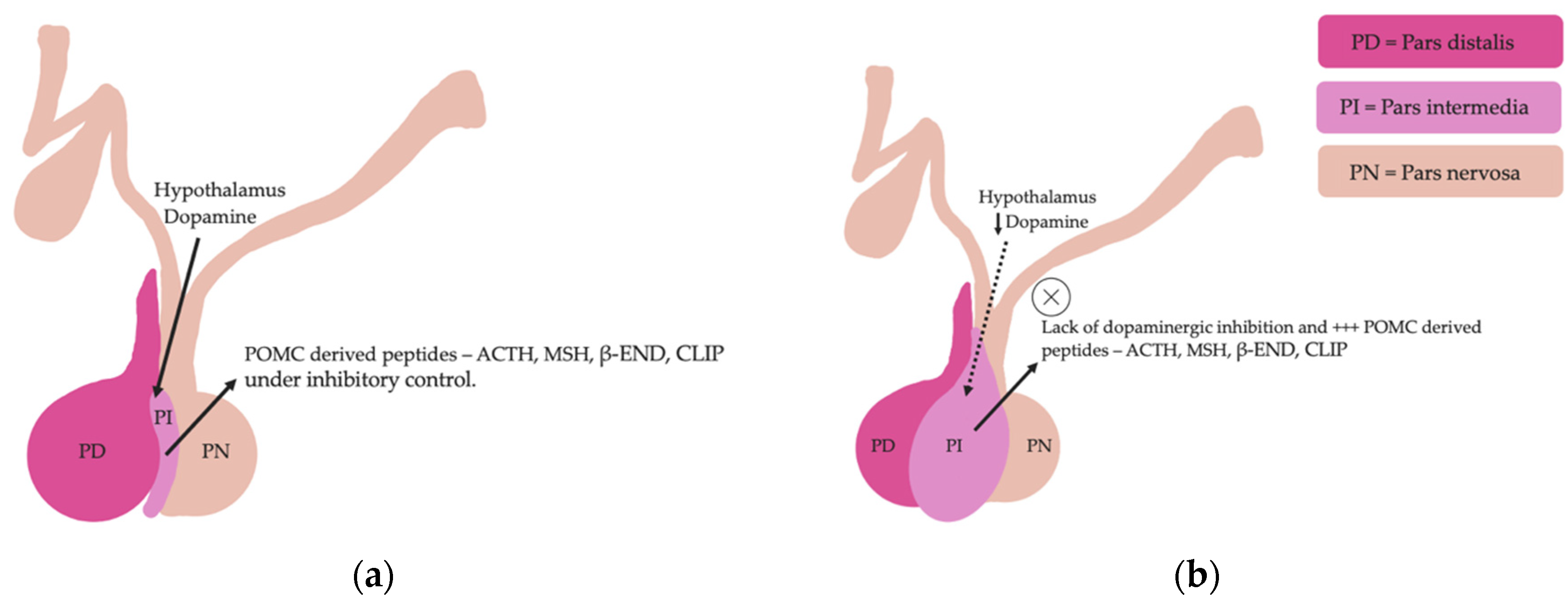
Figure 2. Normal equine pituitary gland (a). Equine pituitary gland affected by PPID demonstrating a loss of dopaminergic inhibition, and subsequent macroadenoma formation of the pituitary pars intermedia and overproduction of POMC derived peptides (b).
Horses with PPID have increased concentrations of α-synuclein in dopaminergic nerve terminals within the pars intermedia compared to healthy geriatric horses [14,45][5][38]. α-synuclein extracted from the pituitary glands of PPID affected horses is misfolded and overexpressed compared to normal and age-matched horses [45][38]. In one study, the ultrastructure of α-synuclein from five each of young, aged and PPID affected horses was assessed using transmission electron microscopy and revealed no α-synuclein fibrils in young and aged horses. However, α-synuclein fibrils were detected in horses with PPID [45][38]. Accumulation of α-synuclein and oxidative stress can lead to cytotoxicity and subsequent degeneration of dopaminergic neurons in humans with Parkinson’s disease [46][39]. Further research is required to determine if misfolded α-synuclein is the cause or result of degeneration of dopaminergic neurons in horses with PPID [45][38].
3. Signalment
Twenty to twenty-five percent of horses over 15 years of age are affected by PPID [2,3,4][6][24][40]. There does not appear to be any sex predilection [3,4][6][40] or any breed predisposition to PPID [3][40]. Previously, despite the high prevalence of PPID in the geriatric equine population, owners were infrequently aware of the disease [4][6]. In 2012, free basal ACTH testing for PPID was well marketed, improving awareness of PPID in both owners and veterinarians [8][41]. However, despite recognising clinical signs, owners may be unlikely to seek veterinary advice [4][6]. Early clinical signs are also easily missed as they can be confused with normal age-related changes [4,47][6][42]. It is not recommended to test horses less than 10 years-of-age unless hypertrichosis is present [16][8] as younger horses are rarely affected by PPID [17][9]. The youngest horses reported with PPID were 7 years of age. This is considered unusual and early onset familial PPID was suspected [17][9].
4. Diagnosis
A presumptive diagnosis of PPID can be made based on the presence of hypertrichosis in aged horses [108,109,110,111,112][43][44][45][46][47]. Laboratory testing is recommended in cases where treatment is financially feasible, where early or severe clinical disease is suspected [54][48], or to determine the response to treatment [54][48]. Currently, testing horses for subclinical PPID is not recommended [54][48]. However, the disease can have severe, life threatening consequences and ability to diagnose subclinical or mild PPID and initiate treatment before end-stage disease may be beneficial [40,47,113][33][42][49]. A recent prospective case series measured basal and TRH-stimulated ACTH concentrations in seven horses with subclinical PPID, and found that TRH-stimulation testing in late summer or early autumn (February and March in Australia) identified most horses that transitioned to clinical PPID over a 3.5 year period [113][49]. This was a small case series, so further research is required to determine if early diagnosis is warranted. Currently, there is no ante-mortem diagnostic test that can be completely relied upon for an accurate diagnosis of PPID [31,32][23][25]. Post-mortem evaluation of pituitary glands via histopathology has been used to confirm laboratory test results [111][46]. A grading system was created to determine the severity of pituitary pars intermedia histological changes [114][50], and the grade has been shown to correlate with laboratory results and clinical signs [114,115][50][51]. However, histopathology remains a subjective test, and concordance between pathologists is only moderate [116][52]. Pituitary size and histomorphology are also affected by season [115][51] and age [117][53] making the use of histopathology in diagnosis of PPID challenging [40][33]. Results of tests in horses that are affected by early or subclinical disease, are often equivocal [113][49]. Untreated PPID cases due to false-negative test results can lead to morbidity through muscle wastage, opportunistic infections, and difficulty thermoregulating. False positive test results may lead to unnecessary treatment. This is not only a financial burden, but the risks associated with long-term treatment with pergolide have not been established [2,118][24][54]. It is imperative that diagnostic tests be interpreted with care to reduce the incidence of false positive and false negative results [32][25]. Current recommendations for diagnosis of PPID are to measure the basal ACTH concentration in horses with obvious clinical signs, utilising seasonally adjusted diagnostic thresholds and an equivocal zone [54][48]. For the diagnosis of early disease, measurement of TRH-stimulated ACTH concentrations is recommended [2,54][24][48]. When finances or availability of laboratory testing of ACTH concentrations are not available, response to therapy with pergolide mesylate may be used as a more practical approach to diagnosing PPID in aged horses with generalised hypertrichosis [54][48]. Other diagnostic techniques include measurement of endogenous α-MSH and the now superseded overnight dexamethasone suppression test. Measurement of endogenous α-MSH, it is not commercially available and did not perform as well as basal ACTH [28,31,119][20][23][55]. Seasonally adjusted DCOV are yet to be established for the overnight dexamethasone suppression test. Although not discussed in this reviewerein, additional diagnostic testing for hyperinsulinemia is recommended for horses with regional adiposity and/or a history of laminitis, as management practices are altered in these horses [120][56].4.1. Basal ACTH
Basal ACTH is the most popular and common test used for diagnosis of PPID as it is easy to perform and requires only a single blood measurement [121][57]. Interpretation of results must take into consideration stress, illness, pain, season, age, sex, and body condition score, as all these factors can increase concentrations of ACTH [30][22]. Breed differences in ACTH concentration have been observed with a greater and prolonged peak in ACTH concentrations in late summer and autumn in Shetland and Welsh ponies compared to Warmbloods, Thoroughbreds and Cob breeds. These differences were not observed outside of late summer and autumn. Arabians and donkeys had ACTH concentrations greater than other breeds for a more prolonged period from May to November [122,123][58][59]. These differences in ACTH concentration do not appear to have led to overrepresentation of these breeds in diagnosis of PPID [3][40]. The test can be performed at any time of day [124,125][60][61]. Performing paired measurement of ACTH has no benefit to performing a single measurement [125][61]. If these results are equivocal, a TRH stimulation test should be performed due to the greater accuracy of this test [2,40][24][33]. The blood sample should be collected in an EDTA tube, centrifuged, kept at 4 °C and analysed as soon as possible after sampling, ideally within 24 h [126,127][62][63]. If the sample cannot be tested immediately, the centrifuged plasma should be frozen as heat results in degradation of ACTH [128][64]. Multiple freeze-thaw cycles should be avoided as this will alter the ACTH concentration [129][65]. In all horses, basal ACTH follows a circannual rhythm that demonstrates a peak in concentration in the autumn months. Basal ACTH was thought to be the most sensitive and specific diagnostic test in the autumn due to increased magnitude of differences in ACTH concentrations between affected and unaffected horses [31][23]. However, more recent literature has demonstrated the accuracy of basal ACTH is reduced in March (September in the Northern hemisphere). Basal ACTH concentration still had good accuracy for diagnosis of PPID in March (accuracy 0.8–0.9), but accuracy was improved to excellent (accuracy > 0.9) in April and May [2][24]. Meta-analyses and systematic reviews have been performed to establish the sensitivity and specificity of basal ACTH. Although not broken down by season, the median sensitivity and specificity of basal ACTH in one study were 75.5% and 95.2% respectively [36][29], while another study reported the mean sensitivity and specificity at 66% and 87% respectively [130][66]. Based on these results, basal ACTH is excellent for ruling out PPID, and less accurate for detecting the condition unless the horse has severe clinical signs. As such, basal ACTH is not recommended for screening purposes or use in horses with early disease [130][66]. However, both studies identified biases, between-study variations and suboptimal study designs and populations. Less biased studies examining the diagnostic accuracy of basal ACTH are required to accurately establish sensitivity and specificity. Monthly reference intervals (RI) and DCOV for the interpretation of ACTH results were established in a study of 106 mature horses. IHerein this study , DCOV were recommended to improve the detection of early PPID, and RI were recommended to reduce the likelihood of false positives [2][24]. ThisIt studywas proposed that determination of basal ACTH concentrations is accurate for diagnosis of PPID in horses, providing these DCOV and RI are used, and the results are interpreted within the clinical context. The TRH stimulation test may improve the accuracy of diagnosis of PPID irrespective of season [2][24]. Another recent retrospective study using a population of 75,892 horses used an indirect approach to calculate diagnostic thresholds. ThisIt studywas similarly proposed that diagnostic thresholds should be used alongside clinical judgement, and the appropriate threshold should be chosen when there is a reason to avoid false positive or false negative results [32][25]. ThDis study established diaagnostic thresholds was established using intervals as short as one week. Inter-weekly variability was low for most of the year, with a nadir in June to December (northern hemisphere) [32][25]. The Equine Endocrinology Group recommends using seasonal thresholds with equivocal zones for interpretation of results. Prior to recommending treatment for horses without strong clinical signs that have basal ACTH results that fall within equivocal zones, re-testing or use of a TRH stimulation test is recommended [54][48].4.2. Thyrotropin-Releasing Hormone (TRH) Stimulation Test
In early PPID or when clinical signs are mild, the TRH stimulation test is useful for increased accuracy of diagnosis [1,2,40,113,121][24][33][49][57][67]. Plasma ACTH is measured before and either 10 or 30 min after administration of 1 mg TRH IV (0.5 mg for ponies). Thyrotropin-releasing hormone increases ACTH and α-MSH concentrations in plasma through stimulation of TRH R1 receptors on the pituitary pars intermedia and pars distalis [1,21,40,119,131][13][33][55][67][68]. Thyrotropin-releasing hormone stimulation in normal horses results in POMC derived peptide secretion by the pars distalis that is restricted by glucocorticoid negative feedback. In horses with PPID, TRH stimulation results in POMC-derived peptide secretion by the melanotrophs of the pars intermedia, and excessive ACTH secretion by hyperplastic or neoplastic melanotrophs that is unaffected by a negative feedback loop, resulting in a dramatic increase in ACTH concentration [1,2,21,26][13][18][24][67]. As with basal ACTH, the TRH stimulation test should be interpreted in accordance with seasonally adjusted DCOV to improve diagnostic accuracy [2,54][24][48]. Several investigators recommend not using the TRH stimulation test in the autumn [40[33][35][48],42,54], and repeatability of the test in autumn appears to be reduced [132][69]. However, recent studies have found that while variation in ACTH concentrations are greater in autumn, it should not impact diagnostic accuracy providing DCOV and RI are used [2,133][24][70]. The disparity in results could be due to the difference in ages of the populations investigated, as well as sample sizes. The TRH stimulation test has been associated with side effects such as yawning, muscle fasciculation, transient coughing, lip-smacking and the flehmen response, but appears safe. The test is applicable for both hospital and ambulatory practice [40][33]. Measuring ACTH concentration 10-min post TRH administration is favoured by ambulatory practitioners for time efficiency, hence its recommendation by the Equine Endocrinology Group (EEG) [54][48]. However, as there is a much larger variation in the ACTH concentration 1 min pre and post the 10 min time point [40[33][35][68][71],42,131,134], compared to 1 min pre and post the 30 min time point [2,132][24][69] (where the ACTH response curve is flatter), some researchers favour using the 30 min time point due to greater accuracy when repeated samples are required [134][71]. Further research is required to determine the variability of ACTH concentrations 30 min post TRH administration [134][71]. Prior to the availability of a compounded product in the USA, most research papers utilise the chemical grade TRH (Sigma-Aldrich Pty Ltd. (subsidiary of Merck), North Ryde BC, Australia or synthetic thyrotropin releasing hormone, Sigma-Aldrich Inc, St. Louis, MO, USA) [2,121,124,127,129,132][24][57][60][63][65][69]. Although TRH is now available as a compounded product in the USA, in Europe and Australia TRH is not available as a sterile registered product, and use tends to be limited to referral hospitals [132,133,134][69][70][71]. In the UK and most of Europe, the use of unlicenced chemicals is restricted, limiting the use of TRH for the diagnosis of PPID.4.3. Dexamethasone Suppression Test
The dexamethasone suppression test for detection of PPID requires two consecutive visits making it a less popular option. It is also less valuable in the identification of early disease, and administration of dexamethasone has been associated with negative consequences such as laminitis [135,136][72][73]. Serum cortisol concentration is measured before and 18–20 h after administration of 0.04 mg/kg dexamethasone IM [28,137,138][20][74][75]. In normal horses, administration of dexamethasone results in negative feedback on the corticotropes of the pars distalis, suppressed ACTH secretion and a reduction in plasma cortisol concentration. However, in horses with PPID, cortisol secretion is maintained as the melanotropes of the pars intermedia continue to secrete large quantities of ACTH [1][67]. Results of the dexamethasone suppression test are frequently inconsistent with TRH stimulation test results [40][33]. There is also some evidence to suggest that season influences the result of the dexamethasone suppression test, with false positives more likely in the autumn months [27,139][19][76]. The dexamethasone suppression test is no longer recommended if laboratory measurement of ACTH concentration is available.4.4. Plasma α-MSH
Unlike ACTH, α-MSH is a direct product of the pars intermedia [21][13]. In horses with PPID, the hyperplastic pars intermedia secretes α-MSH in quantities in excess of ACTH production [21[13][55][68],119,131], indicating that α-MSH may increase earlier in the disease process [5,51][77][78]. Measuring α-MSH has shown to have improved diagnostic accuracy than basal ACTH and may be more useful in detecting early disease [21,28,51,119,131][13][20][55][68][78]. α-MSH has a higher specificity and sensitivity than ACTH in the autumn months, and specificity is also greater than that of basal ACTH in the non-autumn months, but the sensitivity reduces [31][23]. Measurement of α-MSH has been identified as a test that could be useful in the diagnosis of PPID, as unlike ACTH, α-MSH is not known to be confounded by variables such as stress, transportation, exercise and pain [2,21][13][24]. Similar to ACTH, α-MSH concentration is influenced by season and is highest in the autumn months [21,28,51,119][13][20][55][78]. Seasonally adjusted DCOV for basal α-MSH were established using the Youden index and compared to ACTH DCOV, and found that α-MSH and ACTH were highly correlated [31][23]. α-MSH is not as stable as ACTH in chilled whole blood, remaining stable for 8 h prior to centrifugation, plasma separation and freezing [51][78]. Therefore, its use may be restricted in ambulatory practice. Laboratory measurement of α-MSH is not commercially available. Some research has been established investigating α-MSH response to TRH administration, but it is not any more useful than measuring ACTH after TRH administration [131][68]. Increases in α-MSH in response to TRH were substantially greater than ACTH in the autumn months [21[13][55],119], which may complicate the use of the test unless seasonally adjusted DCOVs are established. However, this may also prove to be a valuable diagnostic test if interpreted correctly [119][55].4.5. Imaging
Computed tomography (CT) [107][79] and more recently, magnetic resonance imaging (MRI) [140][80] have been used to evaluate the equine pituitary gland, and both modalities appear to provide adequate detail [107,140][79][80]. Because no laboratory test for PPID can be completely relied upon, imaging may be useful for earlier detection of the disease [140][80]. Imaging may also be useful to assess pituitary size, surrounding structures, and obtain a more accurate prognosis [141][81]. The use of imaging modalities is limited at this stage due to expense and accessibility, but may become a suitable diagnostic technique in the future [107][79].References
- Berne, R.; Levy, M.; Koeppew, B.; Stanton, B. The Hypothalamus and Pituitary Gland; Mosby: St. Louis, MO, USA, 1998.
- Malven, P. Pituitary gland neuroendocrinology. In Proceedings of the 15th Annual Forum of the American College of Veterinary Internal Medicine, Orlando, FL, USA, 1997; p. 462.
- Kemppainen, R.; Zerbe, C.; Sartin, J. Regulation and secretion of proopiomelanocortin peptides from isolated perifused dog pituitary pars intermedia cells. Endocrinology 1989, 124, 2208–2217.
- Kemppainen, R.; Peterson, M. Regulation of alpha-melanocyte-stimulating hormone secretion from the pars intermedia of domestic cats. Am. J. Vet. Res. 1999, 60, 245–249.
- McFarlane, D.; Dybdal, N.; Donaldson, M.; Miller, L.; Cribb, A.E. Nitration and increased α-synuclein expression associated with dopaminergic neurodegeneration in equine pituitary pars intermedia dysfunction. J. Neuroendocrinol. 2005, 17, 73–80.
- McGowan, T.W.; Pinchbeck, G.P.; McGowan, C.M. Prevalence, risk factors and clinical signs predictive for equine pituitary pars intermedia dysfunction in aged horses. Equine Vet. J. 2013, 45, 74–79.
- Carmalt, J.L.; Mortazavi, S.; McOnie, R.C.; Allen, A.L.; Unniappan, S. Profiles of pro-opiomelanocortin and encoded peptides, and their processing enzymes in equine pituitary pars intermedia dysfunction. PLoS ONE 2018, 13, e0190796.
- Heinrichs, M.; Baumgärtner, W.; Capen, C. Immunocytochemical demonstration of proopiomelanocortin-derived peptides in pituitary adenomas of the pars intermedia in horses. Vet. Pathol. 1990, 27, 419–425.
- Orth, D.N.; Holscher, M.A.; Wilson, M.G.; Nicholson, W.E.; Plue, R.E.; Mount, C.D. Equine Cushing’s disease: Plasma immunoreactive proopiolipomelanocortin peptide and cortisol levels basally and in response to diagnostic tests. Endocrinology 1982, 110, 1430–1441.
- Wilson, M.G.; Nicholson, W.E.; Holscher, M.A.; Sherrell, B.J.; Mount, C.D.; Orth, D.N. Proopiolipomelanocortin peptides in normal pituitary, pituitary tumor, and plasma of normal and Cushing’s horses. Endocrinology 1982, 110, 941–954.
- Yoshikawa, H.; Oishi, H.; Sumi, A.; Ueki, H.; Oyamada, T.; Yoshikawa, T. Spontaneous Pituitary Adenomas of the Pars Intermedia in 5 Aged Horses: Histopathological, Immunohistochemical and Ultrastructural Studies. J. Equine Sci. 2001, 12, 119–126.
- Mousa, S.A.; Shakibaei, M.; Sitte, N.; Schäfer, M.; Stein, C. Subcellular Pathways of β-Endorphin Synthesis, Processing, and Release from Immunocytes in Inflammatory Pain. Endocrinology 2004, 145, 1331–1341.
- McFarlane, D.; Beech, J.; Cribb, A. Alpha-melanocyte stimulating hormone release in response to thyrotropin releasing hormone in healthy horses, horses with pituitary pars intermedia dysfunction and equine pars intermedia explants. Domest. Anim. Endocrinol. 2006, 30, 276–288.
- Ferlazzo, A.; Cravana, C.; Fazio, E.; Medica, P. Is There an Interplay Between the Hypothalamus-Pituitary-Thyroid and the Hypothalamus-Pituitary-Adrenal Axes During Exercise-Stress Coping in Horses? J. Equine Vet. Sci. 2018, 62, 85–97.
- Breuhaus, B.A. Thyroid-Stimulating Hormone in Adult Euthyroid and Hypothyroid Horses. J. Vet. Intern. Med. 2002, 16, 109–115.
- Breuhaus, B.A. Thyroid Hormone and Thyrotropin Concentrations and Responses to Thyrotropin-Stimulating Hormone in Horses with PPID Compared with Age-Matched Normal Horses. J. Equine Vet. Sci. 2019, 75, 35–40.
- Abraham, G.; Allersmeier, M.; Schusser, G.F.; Ungemach, F.R. Serum thyroid hormone, insulin, glucose, triglycerides and protein concentrations in normal horses: Association with topical dexamethasone usage. Vet. J. 2011, 188, 307–312.
- Frank, N. Pituitary Pars Intermedia Dysfunction. In Robinson’s Current Therapy in Equine Medicine, 7th ed.; Robinson, N.E., Sprayberry, K.A., Eds.; Elsevier Inc.: Saint Louis, MO, USA, 2015; pp. 574–577.
- Donaldson, M.T.; McDonnell, S.M.; Schanbacher, B.J.; Lamb, S.V.; McFarlane, D.; Beech, J. Variation in plasma adrenocorticotropic hormone concentration and dexamethasone suppression test results with season, age, and sex in healthy ponies and horses. J. Vet. Intern. Med. 2005, 19, 217–222.
- Schreiber, C.M.; Stewart, A.J.; Kwessi, E.; Behrend, E.N.; Wright, J.C.; Kemppainen, R.J.; Busch, K.A. Seasonal variation in results of diagnostic tests for pituitary pars intermedia dysfunction in older, clinically normal geldings. J. Am. Vet. Med. Assoc. 2012, 241, 241–248.
- Secombe, C.; Bailey, S.; de Laat, M.; Hughes, K.; Stewart, A.; Sonis, J.; Tan, R. Equine pituitary pars intermedia dysfunction: Current understanding and recommendations from the Australian and New Zealand Equine Endocrine Group. Aust. Vet. J. 2018, 96, 233–242.
- Stewart, A.J.; Hackett, E.; Bertin, F.R.; Towns, T.J. Cortisol and adrenocorticotropic hormone concentrations in horses with systemic inflammatory response syndrome. J. Vet. Intern. Med. 2019, 33, 2257–2266.
- Mc Gowan, T.W.; Pinchbeck, G.P.; Mc Gowan, C.M. Evaluation of basal plasma α-melanocyte-stimulating hormone and adrenocorticotrophic hormone concentrations for the diagnosis of pituitary pars intermedia dysfunction from a population of aged horses. Equine Vet. J. 2013, 45, 66–73.
- Horn, R.; Stewart, A.J.; Jackson, K.V.; Dryburgh, E.L.; Medina-Torres, C.E.; Bertin, F.R. Clinical implications of using adrenocorticotropic hormone diagnostic cutoffs or reference intervals to diagnose pituitary pars intermedia dysfunction in mature horses. J. Vet. Intern. Med. 2021, 35, 560–570.
- Durham, A.E.; Clarke, B.R.; Potier, J.F.N.; Hammarstrand, R.; Malone, G.L. Clinically and temporally specific diagnostic thresholds for plasma ACTH in the horse. Equine Vet. J. 2021, 53, 250–260.
- McFarlane, D. Advantages and limitations of the equine disease, pituitary pars intermedia dysfunction as a model of spontaneous dopaminergic neurodegenerative disease. Ageing Res. Rev. 2007, 6, 54–63.
- McFarlane, D.; Cribb, A.E. Systemic and pituitary pars intermedia antioxidant capacity associated with pars intermedia oxidative stress and dysfunction in horses. Am. J. Vet. Res. 2005, 66, 2065–2072.
- McGowan, C. Hyperadrenocorticism (Pituitary Pars Intermedia Dysfunction) in Horses, 1st ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 100–114.
- Tatum, R.C.; McGowan, C.M.; Ireland, J.L. Evaluation of the sensitivity and specificity of basal plasma adrenocorticotrophic hormone concentration for diagnosing pituitary pars intermedia dysfunction in horses: A systematic review. Vet. J. 2021, 275, 105695.
- McFarlane, D. Pathophysiology and clinical features of pituitary pars intermedia dysfunction. Equine Vet. Educ. 2014, 26, 592–598.
- Huang, L.; Palmieri, C.; Bertin, F.-R. Correlation of pituitary histomorphometry with dopamine and dopamine D2 receptor expression in horses with pituitary pars intermedia dysfunction. Res. Vet. Sci. 2022, 152, 427–433.
- Saland, L.C. The mammalian pituitary intermediate lobe: An update on innervation and regulation. Brain Res. Bull. 2001, 54, 587–593.
- Beech, J.; Boston, R.; Lindborg, S.; Russell, G.E. Adrenocorticotropin concentration following administration of thyrotropin-releasing hormone in healthy horses and those with pituitary pars intermedia dysfunction and pituitary gland hyperplasia. J. Am. Vet. Med. Assoc. 2007, 231, 417–426.
- Cordero, M.; Shrauner, B.; McFarlane, D. Bioactivity of plasma ACTH from PPID-affected horses compared to normal horses. J. Vet. Intern. Med. 2011, 25, 1431–1438.
- Beech, J.; Boston, R.; Lindborg, S. Comparison of cortisol and ACTH responses after administration of thyrotropin releasing hormone in normal horses and those with pituitary pars intermedia dysfunction. J. Vet. Intern. Med. 2011, 25, 1431–1438.
- Morgan, R.A.; Keen, J.A.; Homer, N.; Nixon, M.; McKinnon-Garvin, A.M.; Moses-Williams, J.A.; Davis, S.R.; Hadoke, P.W.; Walker, B.R. Dysregulation of cortisol metabolism in equine pituitary pars intermedia dysfunction. Endocrinology 2018, 159, 3791–3800.
- Dybdal, N.; Hargreaves, K.; Madigan, J.E.; Gribble, D.; Kennedy, P.; Stabenfeldt, G. Diagnostic testing for pituitary pars intermedia dysfunction in horses. J. Am. Vet. Med. Assoc. 1994, 204, 627–632.
- Fortin, J.S.; Hetak, A.A.; Duggan, K.E.; Burglass, C.M.; Penticoff, H.B.; Schott, H.C., 2nd. Equine pituitary pars intermedia dysfunction: A spontaneous model of synucleinopathy. Sci. Rep. 2021, 11, 16036.
- Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Oxidative stress induced-neurodegenerative diseases: The need for antioxidants that penetrate the blood brain barrier. Neuropharmacology 2001, 40, 959–975.
- Ireland, J.; McGowan, C. Epidemiology of pituitary pars intermedia dysfunction: A systematic literature review of clinical presentation, disease prevalence and risk factors. Vet. J. 2018, 235, 22–33.
- Ireland, J.L.; McGowan, C.M. Translating research into practice: Adoption of endocrine diagnostic testing in cases of equine laminitis. Vet. J. 2021, 272, 105656.
- Horn, R.; Bamford, N.; Afonso, T.; Sutherland, M.; Buckerfield, J.; Tan, R.; Secombe, C.; Stewart, A.; Bertin, F. Factors associated with survival, laminitis and insulin dysregulation in horses diagnosed with equine pituitary pars intermedia dysfunction. Equine Vet. J. 2019, 51, 440–445.
- Carmalt, J.L.; Waldner, C.L.; Allen, A.L. Equine pituitary pars intermedia dysfunction: An international survey of veterinarians’ approach to diagnosis, management, and estimated prevalence. Can. J. Vet. Res. 2017, 81, 261–269.
- Rohrbach, B.; Stafford, J.; Clermont, R.; Reed, S.; Schott, H.; Andrews, F. Diagnostic frequency, response to therapy, and long-term prognosis among horses and ponies with pituitary par intermedia dysfunction, 1993–2004. J. Vet. Intern. Med. 2012, 26, 1027–1034.
- Toribio, R.E. Diagnosing equine pars intermedia dysfunction: Are we there yet? J. Vet. Intern. Med. 2005, 19, 145–146.
- Frank, N.; Andrews, F.M.; Sommardahl, C.S.; Eiler, H.; Rohrbach, B.W.; Donnell, R.L. Evaluation of the combined dexamethasone suppression/ thyrotropin-releasing hormone stimulation test for detection of pars intermedia pituitary adenomas in horses. J. Vet. Intern. Med. 2006, 20, 987–993.
- Van der Kolk, J.; Wensing, T.; Kalsbeek, H.; Breukink, H. Laboratory diagnosis of equine pituitary pars intermedia adenoma. Domest. Anim. Endocrinol. 1995, 12, 35–39.
- Hart, K.; Durham, A.; Frank, N.; McGowan, C.; Schott, H.; Stewart, A.J. EEG Recommendations on Diagnosis and Management of Pituitary Pars Intermedia Dysfunction (PPID). Available online: https://sites.tufts.edu/equineendogroup/files/2021/12/2021-PPID-Recommendations-V11-wo-insert.pdf (accessed on 11 November 2021).
- Kirkwood, N.C.; Hughes, K.J.; Stewart, A.J. Prospective assessment of clinical signs and adrenocorticotrophin (ACTH) concentrations in horses transitioning to pituitary pars intermedia dysfunction (PPID). Vet. Sci. 2022, 9, 572.
- Miller, M.A.; Pardo, I.D.; Jackson, L.P.; Moore, G.E.; Sojka, J.E. Correlation of pituitary histomorphometry with adrenocorticotrophic hormone response to domperidone administration in the diagnosis of equine pituitary pars intermedia dysfunction. Vet. Pathol. 2008, 45, 26–38.
- Cordero, M.; McFarlane, D.; Breshears, M.A.; Miller, L.M.; Miller, M.A.; Duckett, W.M. The Effect of Season on the Histologic and Histomorphometric Appearance of the Equine Pituitary Gland. J. Equine Vet. Sci. 2012, 32, 75–79.
- McFarlane, D.; Miller, L.M.; Craig, L.E.; Dybdal, N.O.; Habecker, P.L.; Miller, M.A.; Patterson, J.S.; Cribb, A.E. Agreement in histologic assessments of the pituitary pars intermedia in aged horses. Am. J. Vet. Res. 2005, 66, 2055–2059.
- van der Kolk, J.H.; Heinrichs, M.; van Amerongen, J.D.; Stooker, R.C.; in de Wal, L.J.; van den Ingh, T.S. Evaluation of pituitary gland anatomy and histopathologic findings in clinically normal horses and horses and ponies with pituitary pars intermedia adenoma. Am. J. Vet. Res. 2004, 65, 1701–1707.
- Tatum, R.C.; McGowan, C.M.; Ireland, J.L. Efficacy of pergolide for the management of equine pituitary pars intermedia dysfunction: A systematic review. Vet. J. 2020, 266, 105562.
- Funk, R.; Stewart, A.; Wooldridge, A.; Kwessi, E.; Kemppainen, R.; Behrend, E.; Zhong, Q.; Johnson, A. Seasonal changes in plasma adrenocorticotropic hormone and α-melanocyte-stimulating hormone in response to thyrotropin-releasing hormone in normal, aged horses. J. Vet. Intern. Med. 2011, 25, 579–585.
- Frank, N.; Bailey, S.; Bertin, F.R.; de Laat, M.; Durham, A.; Kritchevsky, J.; Menzies-Gow, N. EEG Recommendations on Diagnosis and Management of Equine Metabolic Syndrome (EMS), Including Assessment of Insulin Status. Available online: https://sites.tufts.edu/equineendogroup/files/2020/09/200592_EMS_Recommendations_Bro-FINAL.pdf (accessed on 11 November 2021).
- Horn, R.; Bertin, F.R. Evaluation of combined testing to simultaneously diagnose pituitary pars intermedia dysfunction and insulin dysregulation in horses. J. Vet. Intern. Med. 2019, 33, 2249–2256.
- Bamford, N.; Bertin, F.-R.; El-Haige, C.; Stewart, A.; Bailey, S. Comparison of autumnal adrenocorticotropic hormone (ACTH) concentrations between apparently healthy horses and ponies. In Proceedings of the 4th Global Equine Endocrinology Symposium, Gut Ising, Bavaria, Germany, 6–10 January 2020.
- Durham, A.E.; Potier, J.; Huber, L. The Effect of Month and Breed on Plasma Acth Concentrations in Equids. Vet. J. 2022, 286, 105857.
- Diez de Castro, E.; Lopez, I.; Cortes, B.; Pineda, C.; Garfia, B.; Aguilera-Tejero, E. Influence of feeding status, time of the day, and season on baseline adrenocorticotropic hormone and the response to thyrotropin releasing hormone-stimulation test in healthy horses. Domest. Anim. Endocrinol. 2014, 48, 77–83.
- Rendle, D.; Litchfield, E.; Heller, J.; Hughes, K. Investigation of rhythms of secretion and repeatability of plasma adrenocorticotropic hormone concentrations in healthy horses and horses with pituitary pars intermedia dysfunction. Equine Vet. J. 2014, 46, 113–117.
- Stewart, A.J.; Yuen, K.Y.; Hinrichsen, S.; Horn, R.; Dryburgh, E.; Bertin, F.R. Effect of sample handling on adrenocorticotropic hormone (ACTH) concentrations following thyrotropin-releasing hormone (TRH) stimulation in horses. In Proceedings of the 4th Global Equine Endocrinology Symposium (GEES), Gut Ising, Bavaria, Germany, 6–10 January 2020.
- Hinrichsen, S.L.; Yuen, K.Y.; Dryburgh, E.L.; Bertin, F.-R.; Stewart, A.J. Short-Term Effects of Temperature and Thyrotropin-Releasing Hormone Stimulation on Adrenocorticotropin Stability in Horses. Animals 2022, 12, 324.
- Durham, A.E.; Copas, V.E.N. Investigation of the in vitro stability of ACTH in horses. Available online: https://sites.tufts.edu/equineendogroup/files/2012/11/MISC1_Investiageion_of_the_in_vitro_stability.pdf (accessed on 1 November 2021).
- Hu, K.; Stewart, A.J.; Yuen, K.Y.; Hinrichsen, S.; Dryburgh, E.L.; Bertin, F.R. The effect of freeze-thaw cycles on determination of immunoreactive plasma adrenocorticotrophic hormone concentrations in horses. J. Vet. Intern. Med. 2020, 34, 1350–1356.
- Meyer, J.C.; Hunyadi, L.M.; Ordóñez-Mena, J.M. The accuracy of ACTH as a biomarker for pituitary pars intermedia dysfunction in horses: A systematic review and meta-analysis. Equine Vet. J. 2021.
- Durham, A.; McGowan, C.; Fey, K.; Tamzali, Y.; Van der Kolk, J. Pituitary pars intermedia dysfunction: Diagnosis and treatment. Equine Vet. Educ. 2014, 26, 216–223.
- Beech, J.; McFarlane, D.; Lindborg, S.; Sojka, J.E.; Boston, R.C. α-Melanocyte—Stimulating hormone and adrenocorticotropin concentrations in response to thyrotropin-releasing hormone and comparison with adrenocorticotropin concentration after domperidone administration in healthy horses and horses with pituitary pars intermedia dysfunction. J. Am. Vet. Med. Assoc. 2011, 238, 1305–1315.
- Kam, Y.N.; McKenzie, K.; Coyle, M.; Bertin, F.R. Repeatability of a thyrotropin-releasing hormone stimulation test for diagnosis of pituitary pars intermedia dysfunction in mature horses. J. Vet. Intern. Med. 2021, 35, 2885–2890.
- Byrne, D.P.; Secombe, C.J.; Tan, R.H.H.; Perera, D.I.; Watts, S.P.; Wearn, J.G. Circannual variability in adrenocorticotropic hormone responses to administration of thyrotropin-releasing hormone in clinically normal horses in Australia. Vet. J. 2018, 238, 58–62.
- Thane, K.; Uricchio, C.; Frank, N. Effect of early or late blood sampling on thyrotropin releasing hormone stimulation test results in horses. J. Vet. Intern. Med. 2022, 36, 770–777.
- Bailey, S.R.; Menzies-Gow, N.J.; Harris, P.A.; Habershon-Butcher, J.L.; Crawford, C.; Berhane, Y.; Boston, R.C.; Elliott, J. Effect of dietary fructans and dexamethasone administration on the insulin response of ponies predisposed to laminitis. J. Am. Vet. Med. Assoc. 2007, 231, 1365–1373.
- Potter, K.; Stevens, K.; Menzies-Gow, N. Prevalence of and risk factors for acute laminitis in horses treated with corticosteroids. Vet. Rec. 2019, 185, 82.
- Borer-Weir, K.; Menzies-Gow, N.; Bailey, S.; Harris, P.; Elliott, J. Seasonal and annual influence on insulin and cortisol results from overnight dexamethasone suppression tests in normal ponies and ponies predisposed to laminitis. Equine Vet. J. 2013, 45, 688–693.
- Durham, A.E.; Fey, K.; McGowan, C.; Tamzali, Y.; van der Kolk, J. Dealing with Equine Pituitary Pars Intermedia Dysfunction (equine Cushing’s Disease) in Equine Practice. Consensus Recommendations (Boeringer-Ingelheim). Available online: https://files8.design-editor.com/93/9346614/UploadedFiles/F5F638B2-5C19-5586-913D-A8EA67DA722C.pdf (accessed on 17 April 2022).
- Copas, V.E.; Durham, A.E. Circannual variation in plasma adrenocorticotropic hormone concentrations in the UK in normal horses and ponies, and those with pituitary pars intermedia dysfunction. Equine Vet. J. 2012, 44, 440–443.
- Spelta, C.W. Equine pituitary pars intermedia dysfunction: Current perspectives on diagnosis and management. Vet. Med. (Auckl.) 2015, 6, 293.
- McFarlane, D.; Donaldson, M.T.; McDonnell, S.M.; Cribb, A.E. Effects of season and sample handling on measurement of plasma α-melanocyte-stimulating hormone concentrations in horses and ponies. Am. J. Vet. Res. 2004, 65, 1463–1468.
- Pease, A.; Schott, H.; Howey, E.; Patterson, J. Computed tomographic findings in the pituitary gland and brain of horses with pituitary pars intermedia dysfunction. J. Vet. Intern. Med. 2011, 25, 1144–1151.
- Hobbs, K.J.; Porter, E.; Wait, C.; Dark, M.; MacKay, R.J. Magnetic resonance imaging of the normal equine pituitary gland. Vet. Radiol. Ultrasound. 2022, 63, 450–455.
- Madrigal, R.; Andrews, F.; Rademacher, N.; McConnico, R.; Duplantis, D.; Eades, S. Large pituitary adenoma in an 8-year-old Arabian stallion. Equine Vet. Educ. 2018, 30, 295–300.
More
