Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Amin Shamsabadipour and Version 2 by Catherine Yang.
Nanomaterials have demonstrated a wide range of applications and recently, novel biomedical studies are devoted to improving the functionality and effectivity of traditional and unmodified systems, either drug carriers and common scaffolds for tissue engineering or advanced hydrogels for wound healing purposes. Due to the high thermal stability and mechanical strength of Fe2O3, they have been combined with several polymers and employed for various nano-treatments for specific human diseases.
- iron oxide nanoparticles
- nanomaterials
- nanotreatment
- nanocarrier
1. Drug Delivery Application of Fe2O3-Based Nanocomposites
In biomedical settings, pH-responsive systems may benefit from inducing physiochemical alterations due to the wide range of pH values seen in the human body [1][2][3][27,50,51]. Gastric passage elevates pH in oral medication delivery systems, including insulin administration, causing acrylic-based polymers to expand and discharge the medicine [4][52]. A negatively charged surface would be formed in pH 7.4 settings, causing the nanoparticle to discharge the loaded medicine. This is another advantage of functionalized nanoparticles with positively charged surfaces that trigger anionic drug loading at lower pH [5][53]. By covering Eudragit-S100 polymer using ibuprofen-loaded Fe2O3 magnetic mesoporous silica nanocomposites tablets, Xing et al. [6][54] create a dual-stimulus-responsive platform. Nanoparticles with tailored releasing capability were created by the magnetic characteristics of Fe2O3 when used in conjunction with an externally applied magnetic field. More medication was delivered into modeled proximal intestinal fluid when the pH-sensitive polymer was used, as opposed to modeled stomach fluid.
pH-responsive mechanisms may also be used at the cellular level. DNA and other therapeutic compounds are damaged when the pH of early endosomes, sorting endosomes, and multivesicular bodies drops rapidly following endocytosis [7][55]. Injury caused by intracellular distribution may be avoided using polymers that safeguard endosomal compartments. A group led by Alexander et al. [8][56] using γ-Fe2O3@polymerized 2-(dimethyl amino) ethyl methacrylate developed dual-responsive core-shell nanoparticles for plasmid DNA delivery throughout CHO-K1 cells. Researchers found that, compared to polyethyleneimine, magnetic core-shell nanoparticles might experience bidirectional pH-dependent temperature-induced aggregation and enhanced efficacy of gene transport with no extra cytotoxic effects. Chemotherapy, which is the typical cancer treatment strategy, can assault both diseased and normal cells due to the application of nonspecific targeting medicines [9][57]. By increasing the specificity of drug-loaded NPs and ameliorating permeability and retention (EPR) events, intelligent pH-responsive platforms can help reduce complications [10][11][58,59].
Tumors develop rapidly, accumulating lactic acid and lowering the pH of these areas (pH 4–6) in comparison to physiological status (pH 7.4) [2][12][50,60]. The highly expanded surface area, appropriate porosity, and capacity to be coated by polymers as core-shell nanoparticles make Fe2O3 NPs such an ideal carrier for pH-responsive drug delivery platforms, moreover, there is a potential for the reduction of Fe2O3 based nanocarriers for loading biomolecules (Figure 12) [13][14][15][16][17][18][61,62,63,64,65,66]. These characteristics provide sufficient room for loading antitumor medications. Regarding the delivery of doxorubicin, Sheng et al. [19][67] created polyethylene glycol-functionalized γ-Fe2O3 nanoparticles, then tested for pH influences on drug distribution and alternating magnetic field treatment. They observed that total discharges were 32 and 63% at pH levels of 7.2 and 5.5, respectively. Daunorubicin hydrochloride was loaded into a γ-Fe2O3/ZnO nanocomposite by Maiti et al. [15][63], and then drug delivery was measured at pH 5.5 and 7.5. When used as a model for tumor locations, the acidic setting aided in the drug delivery. To make things even better for medication distribution, mesoporous ZnO can load large amounts of medicines. With curcumin anticancer medication, Patil and colleagues created functionalized chitosan-coated γ-Fe2O3 nanomaterials activated by a change in pH [20][68]. They found that the discharge frequency at pH 6.0 was approximately 20% higher than at pH 7.4, suggesting the nanocarrier’s capacity for cancer treatment. Various proportions of oxidized pectin/chitosan were loaded in nano γ-Fe2O3 to improve the antitumor properties of the 5-FU medication by Li et al. [21][69] pH levels below 7 and temperatures greater than 36.5 °C demonstrated the system’s pH and thermo-sensitivity by showing a larger swelling frequency. The MMT test against L929 and MCF-7 cell cultures was used to determine the synthesized composite’s biocompatibility and cancer-killing abilities. Due to this, 5-FU may be more effectively targeted, and its anticancer capabilities are enhanced when the oxidized pectin/chitosan/γ-Fe2O3 NPs are used.
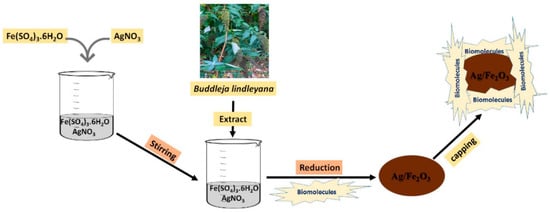
Figure 12.
The reduction procedures of the Ag/Fe
2
O
The exceptional electrochemical redox capabilities of magnetic nanoparticles may be attributed to their substantial electrocatalytic characteristics in a single structure and their composites. It may be formed by optimizing factors, including the transmission of ions by solid NPs because of their high penetration, which accelerates internal dispersion’s phase changes [22][32]. With the help of their redox capabilities, magnetic nanomaterials can gather and concentrate the desired electrochemical data for quick reading [23][70].
The most often employed NPs in this sector are γ-Fe2O3 (oxidized state, Fe3+) and Fe3O4 (two oxidized states, Fe2+ and Fe3+) in MNMs-based composites, respectively [24][25][71,72]. Magnetic nanoparticles and targets discovered by cyclic voltammetry influence the electrodes’ predicted redox potential [26][73].
Poly (ethylene glycol) and poly(ε-caprolactone) disulfide bonds (PEG SS PCL) were used to create biodegradable reduction-responsive micelles for the administration of super-paramagnetic iron oxide (SP IO) nanoparticles and doxorubicin (a chemotherapeutic drug). In the main chain of disulfide bonds, the amphiphilic deblock copolymer has redox reaction characteristics. Doxorubicin (DOX) was loaded 32% throughout magnetic nanomicelles. Consolidation of self-assembled PEG-PCL micelles with oleic acid and water was achieved using coarse-grained molecular dynamics (CG-MD) modeling. In comparison, the hydrophobic and hydrophilic proportions of each copolymer block were identical, and each oleic acid was similarly attached to magnetic nanomaterials [27][74].
To modulate camptothecin pharmaceutical discharge from the mesoporous silica pathways and achieve a dynamic dual-mode MRI contrast, researchers employed superparamagnetic iron oxide nanoparticles (SPION) coated by acid, oxidation stress, and redox-sensitive manganese oxide (MnOx). The efficiency of responsive therapy has been tested throughout pancreatic tumor cells and tumor-bearing animals, and results have supported the performance of Non-vehicle MnOx-SPION@MSn@CPT fighting cells [28][75]. To further improve biodistribution and efficacy for convection-enhanced delivery (CED) of BG to GBM, iron oxide superparamagnetic nanomaterials were developed. The nanomaterials (NPCP-BG-CTX) consist of a magnetic core surrounded by a surface coating of redox-responsive chitosan-PEG copolymer altered by BG covalent attachment and peptide chlorotoxin tumor (CTX). NPAC-BG-CTX demonstrated precise in vitro BG transport in human GBM cells under decreased intracellular circumstances, allowing for controlled and targeted BG discharge. MGMT activity and TMZ toxicity were significantly reduced in cells administered with NPCP-BG-CTX. Animal studies in mice with primary human GBM xenografts showed that CED from NPCP-BGCTX had good in vivo distribution volume (Vd). When NPCP-BG-CTX and NPCP-CTX were used in conjunction, the mean survival rate in both medicated and unprotected animals increased by three times [29][76].
Enzymes are widely used by internal stimulus-responsive nanocarriers since they are involved in a wide range of biochemical and physiological mechanisms. Enzyme transcription at specific places is critical in a variety of pathological processes. In addition to indicating a sick condition, it may also be modified to transport medications with more safety to the appropriate region and serves as a possible screening tool [30][33]. In contrast to other stimuli, enzymatic processes are more powerful, efficient, and accurate, providing extra benefits by intracellularly participating in various metabolic reactions. The transcription of enzymes, including proteases, phospholipases, hydrolases, and glucosidase, increases in a variety of malignancies that use growth factors to increase cell populations. These specific enzymes detect and split intelligent nanocarriers comprised of substances such as Fe2O3 to deliver the payload, namely the medicine, to the targeted place. Multifunctioning is typically supported by adding more than one molecule to the surface of a material [31][77]. The overexpression of MMP-14 in MMTV-PyMT cells was shown to be much more hazardous than the downregulation of MMP-14 throughout fibroblasts. CLIO-ICT (a platform for iron oxide nanocarrier) was produced by Ansari et al. [32][78], to be used as theragnostic nanomaterials for magnetic resonance imaging (MRI) and medication administration. CLIO-ICT was created by linking an azademethylcolchicine (ICT) conjugator to an MMP-14-cleavable region. MMP-14 overexpression caused considerable toxicity among MMTV-PyMT cells, as seen by comparison to fibroblasts with modest frequencies of MMP-14 transcription.
Furthermore, in MR imaging of MMTV-PyMT tumor-bearing mice given by CLIO-ICT, preferential accumulation of nanomaterials in the cancerous site was detected. An enzyme-response, multifunctional DOX-SMNP combination was produced employing clicking chemical properties. Due to the upregulation of a specific enzyme in these live cells, the chemotherapeutic drugs released by this SMNPs-combined medication are selectively controlled. For the detection of intracellular drug delivery and tumor cell scanning, this drug-loaded nanoparticle combination demonstrates a potential mixture of fluorescence and magnetic resonance imaging methods performed in real time. Prospective uses of the multipurpose drug-coated nanostructures include tumor-targeted medication delivery and concurrent diagnostic or detection of therapeutic effects [33][79].
2. Fe2O3 in Magnetic-Responsive Drug Delivery Systems
Site-specific drug distribution may be achieved by directing IONs caused by a confined external magnetic field, which makes use of the magnetic characteristics of iron oxide nanomaterials. Several illnesses, including tumors and inflammation, have been shown to benefit from this strategy [34][80]. To govern the nanomaterials’ passage through the circulation and their concentration at the intended places, the magnetic reaction of IONs is highly associated with their physicochemical qualities; more precisely, the saturation magnetization of the manufactured nanostructures should be significant [35][81]. Some scientific teams are creating magnetic nanocarriers with unique structural properties that have examined this site-directed function. There was a publication in 2012 by the group of Wagstaff et al. [36][82] about how to make gold-covered iron oxide nanomaterials loaded with a platinum-containing chemotherapeutic agent (cisplatin). Coprecipitation and oxidation of iron oxide nanocrystals resulted in the formation of maghemite during this investigation. A technique known as “incremental hydroxylamine seeding” was used to cover the nanomaterials with gold [37][83]. Subsequently, thiolation was used to coat the particles. Earlier, the same group discovered substances such as thiolated polyethylene glycol (PEG) linkers [38][39][84,85]. Eventually, robust coordination linkages involving the PEG linker were used to load cisplatin onto the magnetic nanocarriers. Using a naked magnet, the nanocarriers were tested in vitro and shown to have a 110-fold enhancement in cytotoxic effects on human ovarian cancer cell lines A2780, as well as particular cell development suppression.
Unterweger et al. [40][86] created IONs coated with dextran/hyaluronic acid, which have cisplatin-carrying IONs66. The loading of medications, including cisplatin, and the targeting of overexpressed CD44 receptors throughout malignant cells, were made possible by applying hyaluronic acid (HA) [41][87]. Following the dextran-coated IONs amination, they were covered by lower-molar mass HA (acquired via enzymatic breakdown of HA) [42][88]. After forming a polymer-metal combination with HA, cisplatin was successfully incorporated into the resultant nanocarriers, which were noted for their effective drug entrapment (43.2 ± 0.2%). A beginning 30-minute explosive discharge was accompanied by an ongoing discharge for 48 h, which was described by the researchers as being typical for surface-bound drugs in their study. A substantial enhancement in drug delivery has also been evidenced in the availability of hyaluronidases, which are extensively discovered in tumors and make these nanostructures especially encouraging for tumor-targeted drug delivery [43][89]. This is made even more promising by their regulation through magnetic power illustrated by the uptake of nanomaterials containing a neodymium magnet. PVA-coated iron oxide nanostructures loaded with doxorubicin have also been explored by Nadeem et al. [44][90], who investigated the magnetic manipulation of IONs for specific drug delivery purposes. These nanocarriers exhibited good management for magnetically directed medication delivery by providing a magnetic flux. The antineoplastic medication mitoxantrone was adsorbed on the HSA shell of iron oxide nanostructures (average inorganic diameter of around 7 nm) synthesized by Zaloga et al. [45][91] using lauric acid (LA) and HSA coatings. Over 72 h, such nanocarriers showed improved durability and linear discharge pharmacokinetics. Using an in vitro magneto-guided approach, researchers were able to convincingly demonstrate the site-specific curative impact of these nanocarriers [46][92].
In a recent study, Jeon et al. [47][93] demonstrated the synthesis of polymerized β-cyclodextrin covered IONs that, via host-guest association, were capable of loading polymerized paclitaxel (a chemotherapeutic medication renowned for its limited solubility in water). Due to the strong magnetism of the produced nano assemblies, they may be used as magnetically directed drug delivery platforms. The improved antitumor efficacy of these platforms was shown in vivo throughout CT26-bearing mice due to magnetically-mediated targeting.
Normal cells suffer adverse effects when the body’s temperature is elevated during tumor hyperthermia therapy. An external magnetic field would allow for targeted hyperthermia, which reduces the damage to healthy tissues and reduces the needed medication dosage [48][49][94,95]. External signals may act as a guide for transporting the medicine to the tumor location and initiating pharmaceutical discharge [50][96]. Since malignant cells are less resistant to temperature rise than healthy ones, utilizing MNPs in the carrier generates a thermal flow that aids in the destruction of diseased cells [51][52][97,98]. An external source’s proximity to the targeted region influences the magnetic-responsive drug delivery platform’s precision; as tissue profundity increases, the required intensity magnetic flux decreases [53][99]. As a consequence, this technique is confined to surface organs and so cannot be used to treat pulmonary or hepatic tumors [54][100]. Aside from that, frequencies greater than 8–16 kA.m−1 generate eddy currents that are damaging to normal organs [55][56][101,102]. Owing to their super magnetic capabilities, biocompatibility, high specialized region, appropriate nano-sized particulates, and lower toxic effects, Fe2O3 nanomaterials are one of the most promising alternatives for magnetic-responsive devices according to the researchers [57][58][59][31,103,104] (Figure 23). Improved drug loading potential, increased carrier durability, and opportunity for future functionalization might be achieved by coating iron oxide NPs using appropriate polymers [19][60][61][62][63][67,105,106,107,108]. In vitro experiments using human hepatocarcinoma SMMC-7721 cells, Yan et al. [64][109] employed a combination of Fe2O3 nanomaterials and magnetic fluid hyperthermia (MFH) and found that apoptosis was dose-dependent, and proliferation was inhibited. Due to their absorption capabilities, Fe2O3 nanomaterials have magnetic sensitivity, which causes the magnetic fluid temperature to rise by around 7 °C. An AC magnetic field was used to study the emission of heat from the nanohydroxyapatite matrix-encased γ-Fe2O3 NPs. Investigations on the cytotoxic effects of human (sarcoma osteogenic) SAOS-2 cell lines in vitro demonstrated minimal toxicity, indicating that they may be employed in magnetic hyperthermia [65][110].
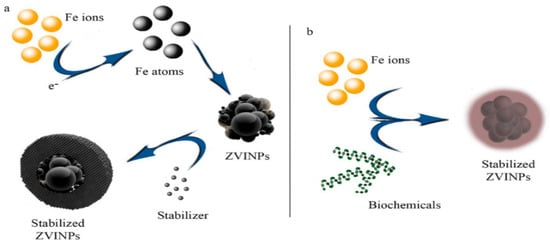
Figure 23.
Chemical (
a
) and biological (
Due to low viral concentration in the targeted tissue, gene therapy faces a key drawback: delayed vector deposition [66][111]. The specificity can be improved by attaching targeted ligands to viral particles; however, this is insufficient [67][112]. The difficulty may be solved by introducing an exterior alternating current magnetic field to gene vectors. For this technique of gene delivery, Scherer et al. [68][113] attached gene vectors to superparamagnetic nanoparticles. Transduction effectiveness was shown to be great in vivo, and the delivery time of genes was lowered to minutes, supporting the idea that magnetic field guidance may be used to overcome fundamental obstacles.
3. Wound Healing Applications of Fe2O3-Based Nanocomposite
Wound healing is a highly systematic procedure of repairing defected tissue, which is consisted of four consecutive, but overlapping, biological phases: hemostasis, inflammation, proliferation, and remodeling [69][70][192,193]. Any disruption by both extrinsic and intrinsic factors in any of the aforementioned phases may cause each phase to be prolonged and result in a dissatisfying result, leading to chronic wound status. The colonization of contaminating pathogens at the spot of damage during the natural wound-healing process is the most commonly occurring issue with complete wound healing [71][194]. At the spot of a wound, the bacteria that constituted the skin microbiota protect against pathogen colonization. When pathogenic bacteria hit the critical level and generate a large amount of biofilm, the healing process slowed. Staphylococcus aureus is the most widely recognized colonizing pathogen influencing the early stages of wound healing, while Pseudomonas aeruginosa and Escherichia coli are commonly seen in chronic wounds and impact downward layers of the skin [72][195]. This pathogen-associated bare skin infection may cause acute inflammatory reactions and delayed wound healing. To prevent bacterial infections and assist in the natural wound-healing process, a suitable antimicrobial wound dressing must be used.
However, the proliferation of antimicrobial-resistant bacteria slows the healing process, entailing the development of new wound-dressing substances that are non-toxic or resistant to employment and ameliorating the efficiency of the wound-healing procedure.
In other words, the primary goal of wound dressing is to keep the wound free of extrinsic contamination. It also keeps the wound hydrated, promoting regeneration and preventing the wound’s origin from being exposed [73][196]. As a result, wound-healing materials must be biocompatible, semi-permeable to water and oxygen, hypoallergenic, and economical. As a result, wound dressing necessitates the use of technologically progressed dressing substances instead of traditional wound-dressing materials such as cotton and wool.
The new materials can preserve the wound environment and also transfer active compounds to facilitate the wound-healing process [74][197].
Diverse wound-dressing products, including ointments, hydrogels, and antibacterial agents combined with polymers, are recently accessible and are made primarily of biodegradable substances including chitosan, hyaluronic acid, collagen, silicon, cellulose, and gelatin [75][76][77][78][79][80][198,199,200,201,202,203].
Due to their capability to prevent bacterial proliferation, quinolones [81][204], cephalosporins [82][205], polymyxin B [83][206], neomycin [83][206], and tetracyclines [84][207] are the most typically utilized antibiotics in wound dressing.
However, repeated and ineffective antibiotic administration would hasten the emergence of antibacterial resistance. To prevent such resistance, treatments based on alternative antibiotics, non-antibiotic materials, and a combination of antibiotics and non-antibiotic materials such as essential oils, honey, and nanomaterials (e.g., Ag and Au) have been recommended for wound dressings [84][207].
The usage of nanomaterials in wound healing is growing quickly and is being studied in clinical trials [69][85][86][87][192,208,209,210] (Figure 310).
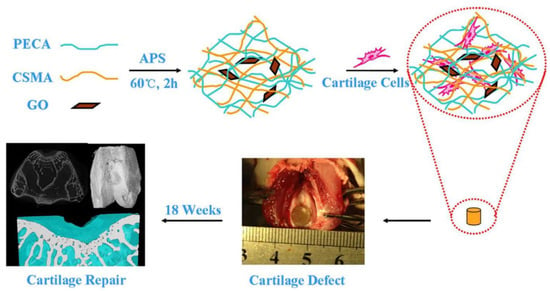
Figure 310. CSMA/PECA/GO hybrid scaffold in bone tissue engineering application (cartilage repairing) [87].
Due to their inherent nature, metal and metal oxide nanomaterials are more effective in the wound-healing treatment and antibacterial action than traditional materials. Metallic nanoparticles’ size, structure, surface modification, zeta potential, porosity, and thermal stability typically influence their efficacy in biological applications [88][89][90][91][217,218,219,220]. Considering their favorable physiochemical properties and antibacterial activity, Silver (Ag) [37][83], gold (Au) [38][84], and zinc oxide (ZnO) [39][85] are the most investigated metallic nanoparticles. Other metals and metal oxides, such as iron oxide (Fe2O3), are also being studied in this regard [72][195].
Harandi et al. [92][45] studied the antimicrobial activity of Fe2O3 nanoparticles as coating for polyvinyl alcohol-prebiotic gum arabic-polycaprolactone (PVA-GA-PCL) electrospun nanofibers for wound dressing applications. Their findings revealed spherical Fe2O3 NPs with a mean size diameter of 100 nm that contained LAB and demonstrated significant antimicrobial potential against pathogens such as E. coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Candida albicans. These results demonstrated both the biocompatibility of coated nanofibers with mouse embryonic fibroblast cell lines and their antimicrobial and antibiofilm efficacy against pathogens. C. Albicans exhibited the greatest growth inhibition by Fe2O3 NPs-LAB@PVA-GA-PCL. Intriguingly, combining probiotic Lactobacillus and prebiotic GA as a symbiotic hybrid revealed synergistic antimicrobial effects, implying the therapeutic potential for wound healing applications.
Another study by Raisi et al. [93][46] fabricated a nanocomposite dressing (NCD) made up of carboxymethyl chitosan (CMC) with various content of Fe2O3 nanoparticles (0, 2.5, 5, and 7.5 wt%) by a method called freeze-drying (FD) technique to study its potential for wound healing applications. The results showed that the wound dress was porous with micron-sized interconnections. Indeed, the results show that as the amount of Fe2O3 nanoparticles increases, so does the porosity. Tensile strength was 0.32 MPa for the pure sample and 0.85 MPa for the sample with the highest percentage of magnetic nanoparticles, respectively.
Furthermore, the cytotoxicity of this nanocomposite revealed no cytotoxicity toward fibroblast cell growth and suitable biocompatibility. The results indicated that NCD had exceptional biodegradability, biocompatibility, and mechanical properties. As a result, NCD made of CMC and Fe2O3 nanoparticles were proposed as a promising candidate for wound healing applications.
The positive effects of Fe2O3 NPs on the rapid blood coagulation in wounds with excessive bleeding were proved by a study by Rubtsov et al. [94][221] in 2019. They synthesized a novel nanoparticle-hydrogel composite as a potential hemostatic wound-healing agent composed of Poly (sodium acrylate) cross-linked by aluminum ions and nanoscale boehmite (γ-AlOOH) and Fe2O3 as fillers.
