The effectiveness and safety of the anti-cancer agent doxorubicin (Dox) (anthracycline group medicine) depend on the metabolism and retention of the drug in the human organism. Polymorphism of cytochrome p450 (CYP)-encoding genes and detoxifying enzymes such as CYP3A4 and CYP2D6 were found responsible for variations in the doxorubicin metabolism. Transmembrane transporters such as p-glycoproteins were reported to be involved in cancer tissue retention of doxorubicin. The metabolic transformation of Dox may follow several pathways, including two-electron reduction with the formation of doxorubicinol, one-electron reduction with the formation of semiquinone, and deglycosylation with the formation of aglycone. Several enzymes have been shown to be involved in this process. Doxorubicinol is considered the most dangerous metabolite of Dox degradation, as it may disturb iron and calcium balances.
1. CYP3A4 Polymorphism
CYP3A4*1B is one of the most studied polymorphisms of CYP3A4 in cancer patients. Current data on the enzyme activity and its impact on the chemotherapy effects are conflicting. Tavira et al. (2013) demonstrated an association between the expression of CYP3A4*1B variants and increasing drug concentration in blood serum
[1][30]. However, other studies reported a minimal influence of CYP3A4*1B on drug concentration
[2][31]. This contradicts what was previously thought to be the role of this enzyme in the Dox conversion. Decreased metabolic activity of CYP3A4 may be caused by the presence of the CYP3A4*22 polymorphism. Several studies have reported that expression of this gene variant leads to an increase in various drug concentrations
[3][32]. Interestingly, a meta-analysis study reported that CYP3A4*22 is a wide-spread polymorphism among Europeans (58.8%) and admixed Americans (82.4%)
[4][33]. The CYP3A4*15 polymorphism was also found in 73.8% of Africans, while CYP3A4*18 was found in 63.4% of East Asians
[4][33]. The role of the CYP3A4*15 polymorphism has not yet been clarified. The CYP3A4*18 polymorphism resulted in decreased enzyme function
[4][5][33,34].
Other genes, including X-pregnane receptor (PXR) polymorphism, were found associated with CYP3A4 expression and regulated responses to BC treatment
[5][34]. The expression of PXR mRNA in liver tissues of patients carrying clusters of PXR*1B haplotypes was found to be four times lower than that in people with the non-PXR*1B haplotype (*1A + *1C) clusters
[5][34]. The PXR*1B haplotype also correlated with significantly lower CYP3A4 (and p-glycoprotein ABCB1) expression in the liver. Notably, Dox clearance in BC patients with the PXR*1B haplotype was significantly lower compared to non-PXR*1B patients
[5][34]. Expression of the PXR*1B haplotype correlated with a lower Dox clearance, suggesting prolonged circulation of the drug and its higher therapeutic effects in Asian BC patients
[5][34]. However, the effect of the CYP3A4 polymorphism on the metabolism and effectiveness of Dox in different BC cohorts remains largely unclear and warrants further investigations.
2. CYP2D6 Polymorphism
The CYP2D6 gene is marked by a high allele heterogeneity which reflects abundant inter-individual variations. The gene variants were grouped according to levels of enzyme activity. The described association between CYP2D6 polymorphisms and enzyme activity is presented in
Table 1 according to the previously reported analysis
[4][33]. The difference in distribution of CYP2D6 alleles in various populations was assessed and reported
[4][33]. The CYP2D6*2 allele (normal-function allele) was found expressed in 56.3% of admixed Americans, 49.3% of the South Asians, 51.3% of Europeans, 29.5% of Africans, and 16.2% of East Asians. The alleles CYP2D6*3 and CYP2D6*6 (no-function alleles) were found less expressed in Europeans (4% and 6%, respectively), while the CYP2D6*10 allele (decreased function) was found almost exclusively in Africans, East Asians, and South Asians. The CYP2D6*1xN and CYP2D6*2xN alleles (increased function) were found in Europeans, Africans, and East Asians at a low frequency of 1.2–3.6%
[4][33]. Considering that Dox is a substrate of CYP2D6, the rate of Dox metabolism is expected to correlate with this enzyme’s activity: the higher the CYP2D6 activity, the less amount of Dox that remains in the circulation (reduced therapeutic effect). It has been estimated that about 50% of admixed Americans, Europeans, and South Asians are likely to have normal Dox metabolism
[4][5][33,34], and should therefore respond well to Dox-based anti-cancer therapies. However, this suggestion requires evidence-based confirmation. A meta-analysis study conducted in 2013 did not confirm the reliability of CYP2D6 genotyping as a guideline marker for anti-BC therapies
[6][35]. However, the included studies were analyzing the effects of tamoxifen, not Dox-treated patients
[6][35]. BC heterogeneity, confounding pre-selection of suitable patients for the treatment with tamoxifen, and differences in enzyme activity with Dox and tamoxifen as substrates may explain the observed contradictions. Analysis of associations between expression of all CYP2D6 variants in BC patients from different ethnic groups, their responses to Dox, and types of BCs has not been reported. The absence of data indicates an urgent need to estimate the level of CYP2D6 polymorphism in BC cohorts and its specific correlation with Dox metabolism and its therapeutic effects.
Table 1. CYP2D6 polymorphisms and the enzyme activity [4][5][6]. CYP2D6 polymorphisms and the enzyme activity [33,34,35].
3. P-Glycoprotein Polymorphism and Dox Blood Concentration and Clearance
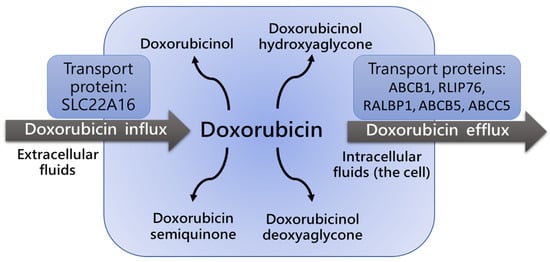
P-glycoproteins, including ATP-binding cassette (ABC) family members such as ABCB1 transporters (also known as Multi-Drug Resistance 1 (MDR1) proteins), are responsible for Dox cell influx and efflux (
Figure 12), and regulate both intra- and extracellular concentrations and bioavailability of the drug and its metabolites. A very limited number of studies estimated the impact of ABCB1 gene polymorphisms on Dox pharmacokinetics and pharmacodynamics
[5][7][8][9][10][11][34,36,37,38,39,40], although the role of the ABCB1/MDR1 transporter in the regulation of intracellular concentration of anti-cancer agents and their therapeutic effects were reported
[12][13][41,42]. The association between p-glycoprotein ABCB1 gene polymorphisms and changes in Dox concentration and clearance were reported
[7][9][12][13][36,38,41,42]. The most studied variants are C3435T, C1236T, and G2677T/A. The distribution of allelic variation was associated with ethnicity. For instance, the 3435C>T variant was found in 60–72% of Asians and 34–42% of Europeans
[7][8][36,37]. The distribution of ABCB1 haplotypes 1236C>T, 2677G/T, and 3435C>T was assessed in different races
[9][38]. Among Africans, the wild-type (CGC) allele was found to be predominant, compared to the presence of the TTT allele. In Europeans, CGC and TTT allele frequencies were found expressed at similar levels. However, the TTT haplotype prevailed among Asians and Indians
[9][38].
Figure 12. Influx and efflux of doxorubicin is defined by activity of ABCB1/MDR1 transport
[10][11][12][13][39,40,41,42]. ABCB1/MDR1 protein expression level and polymorphism determine the intensity of doxorubicin transport.
The role of C3435T polymorphism In the ABCB1 gene was recently investigated in patients with BC treated with Dox and docetaxel
[7][36]. Patients with the C3435TT genotype had higher AUC and greater overall survival compared with patients with the CC⁄CT genotype. However, the TT genotype was also associated with higher risk of neutropenia and diarrhea. This genotype was found in 14.4% of the 216 enrolled patients
[7][36]. It remains unclear which ABCB1 variants are linked to the most efficient effects of Dox in BC patients and which are associated with the poor survival outcomes and/or toxic effects of the drug.
A recent study indicated the influence of ABCB5, ABCC5, and RLIP76 polymorphisms on the pharmacokinetics of Dox in BC patients
[11][40]. Genetic analysis was performed using direct sequencing. The homozygous variant allele at locus ABCC5g + 7161G4A (rs1533682) was significantly associated with higher Dox clearance
[11][40]. Homozygosity of the reference allele at the ABCC5 locus g.-1679T4A was associated with significantly higher doxorubicinol blood concentration. No significant effect of ABCB5 polymorphisms (c.2T4C, c.343A4G, and c.1573G4A) on Dox pharmacokinetics was identified. RLIP76 gene polymorphisms were not reported. Therefore, Dox pharmacokinetics and pharmacodynamics may be influenced by ABCC5 gene polymorphisms
[11][40]. However, the role of tissue specificity in the expression of this variant remains to be determined. It is necessary to confirm the metabolic transformation of Dox and the enzyme activity in the liver as a requirement for the effective retention of Dox in circulation.