Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by DHRUVI PATEL.
Amphiphilic block copolymers (with a variety of hydrophobic blocks and hydrophilic blocks; often polyethylene oxide) self-assemble in water to micelles/niosomes similar to conventional nonionic surfactants with high drug loading capacity.
- block copolymers
- self-assembly
- polymer micelle
1. Introduction
Amphiphilic block copolymers are constituted of two or more different polymer size blocks, often incompatible, chemically linked in a linear or branched fashion. The blocks can be a neutral polymer (hydrophilic or hydrophobic) or polyelectrolyte (anionic, cationic or zwitterionic) [1,2,3][1][2][3]. A variety of structures can be obtained from such different constituting blocks that are schematically shown in Figure 1.
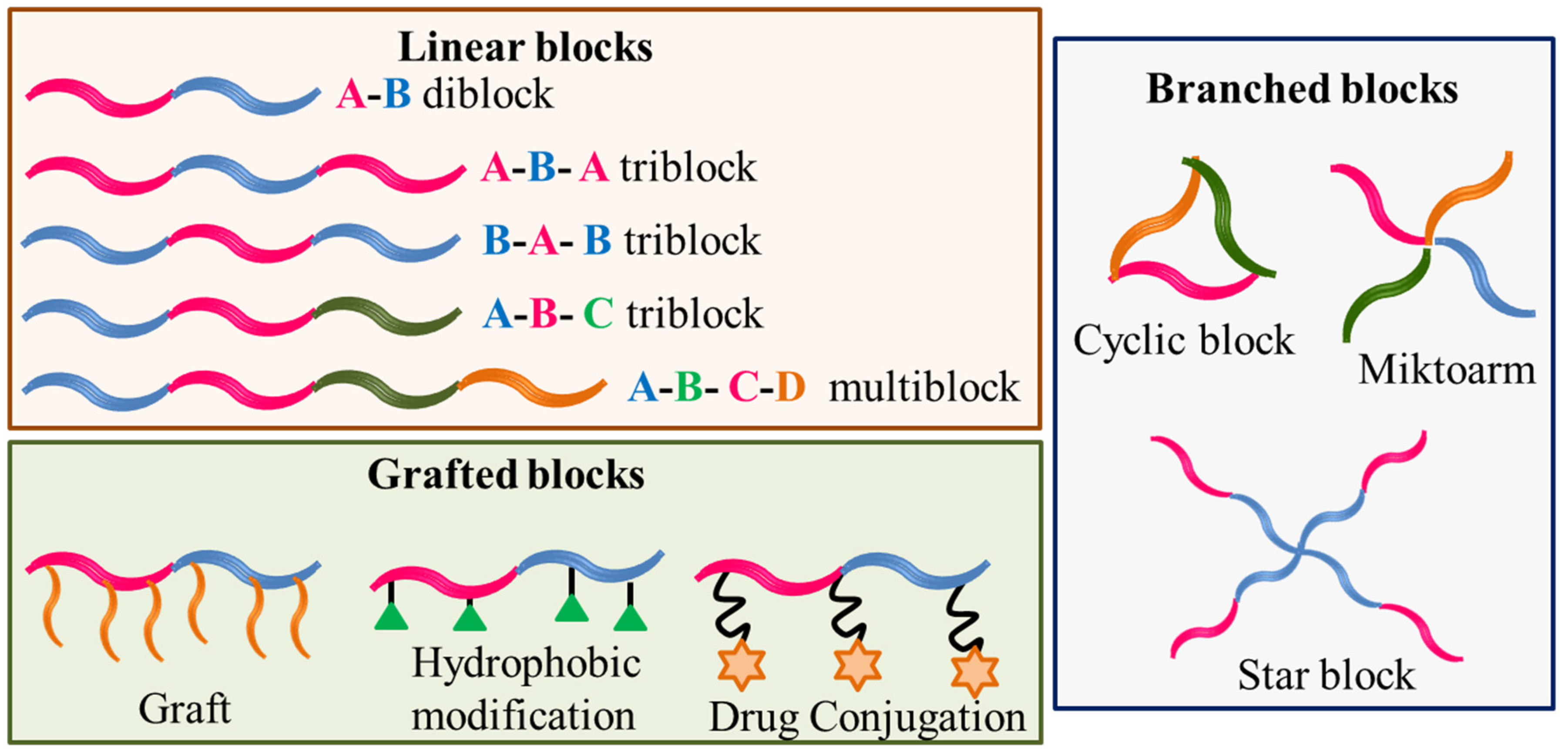
Figure 1. Schematic designer representations of some block copolymer structures.
2. Schizophrenic Micelles
Double hydrophilic block copolymers (DHBCs) constitute a new class of aqueous multiphase systems and have speedily increased significance with unique behaviour. These can be used for a series of applications in stabilization of colloidal dispersions, crystal growth modification, and polyelectrolyte complexes as drug carrier systems [71,72,73][4][5][6]. Figure 92 shows some commonly used DHBCs which may constitute a responsive block in DHBC.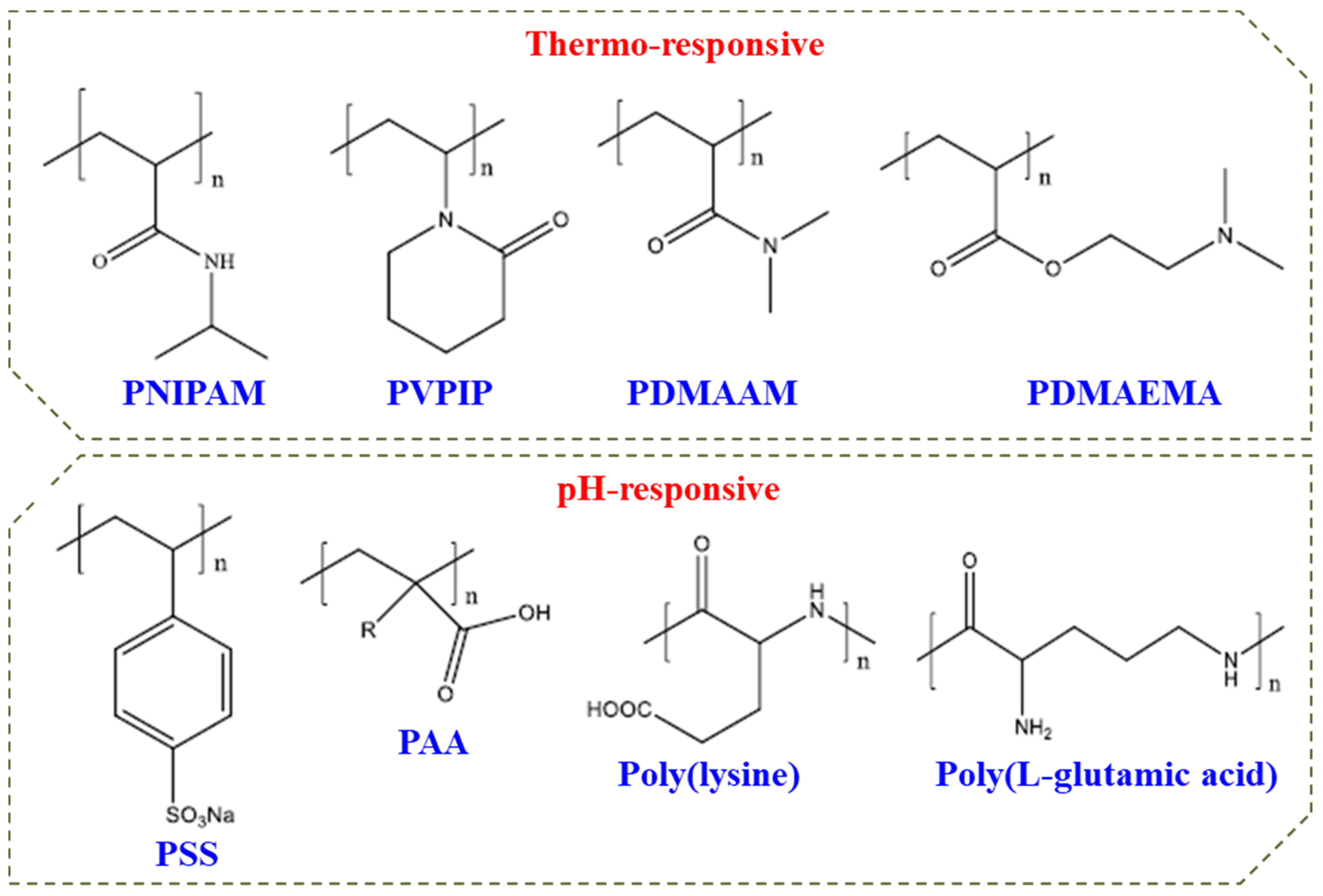
Figure 92. Commonly used polymers in DHBCs.
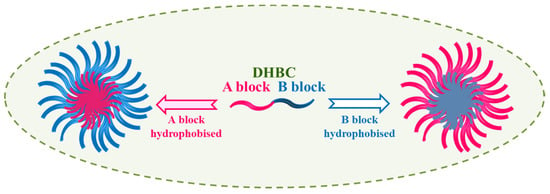
Figure 103. Illustration of Schizophrenic micelles formation.
3. Polyion Complex Micelles (PICMs)
Polyelectrolytes (polyacids or polybases) are water-soluble charged polymers that have peculiar aqueous solution behaviour where they dissociate to form a macroion and counterion. The counter ion is condensed onto the macroion or remains as hydrated in bulk solution. The solution behaviour of polyelectrolytes is greatly altered in the presence of salts and also by pH and temperature. Oppositely charged polymers in aqueous solution can form nanoscale water soluble/insoluble complexes or coacervates in solution due to columbic attraction. The formation of soluble/insoluble polyion complexes (PICs) depends on several factors such as the chemical structure, charge, flexibility of the macroions, their mixing ratio concentration, as well as on solution conditions such as pH, temperature, and ionic strength [93,94,95,96][26][27][28][29]. Polyion complex micelles (PICMs) have an insoluble core of complexed polyelectrolytes and hydrated shell of hydrophilic block when aqueous solution of a neutral-polyelectrolyte block copolymer interacts with an oppositely charged polymer or surfactant (Figure 114).
Figure 114. PICMs self-assembly from oppositely charged polyelectrolytes.
4. Polymerization Induced Self-Assembly (PISA)
Polymerization-induced self-assembly (PISA) is a cost-effective one-pot approach that requires simple procedures to produce polymeric nanoparticles proficiently of various sizes/shapes (sphere, worm-like micelles and vesicles) at high solid concentration as high as 50% (Figure 125).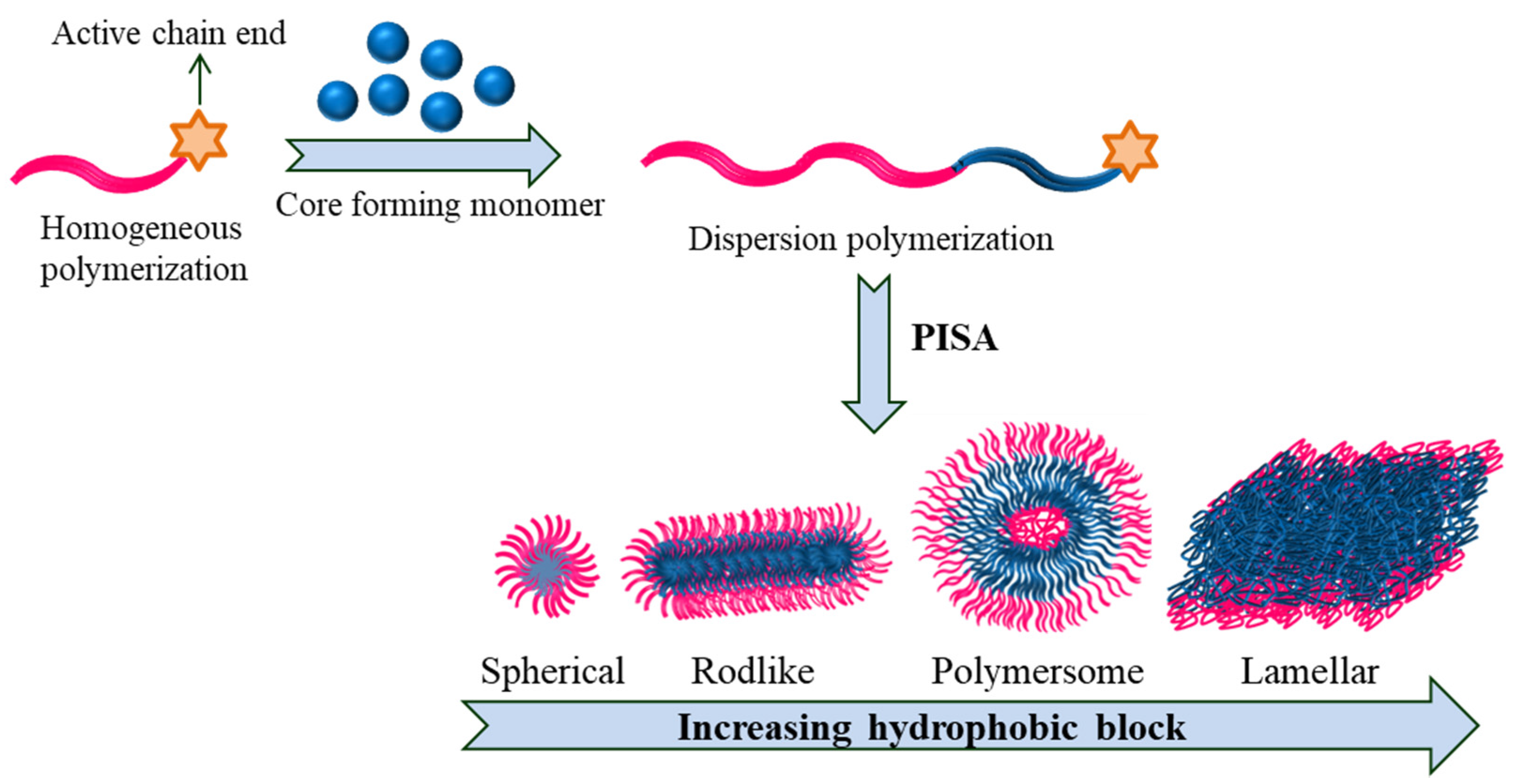
Figure 125. PISA depicting varied shape transition in block copolymers.
5. Crystallization-Driven Self-Assembly (CDSA)
Living crystallization-driven self-assembly (CDSA) has developed as a growth route to form colloidally stable nanoparticles and more complex hierarchical assemblies with the desired size and low size dispersity from crystallizable polymers. The origin of the low dispersity has often been described as initiation being faster than propagation, and termination is absent. When crystal packing forces dominate, a morphological transition is triggered and may lead to elongated nanostructures such as cylinders, nanoribbons, fibres, etc. that depend on crystallization temperature and time, solvent quality, and polymer composition. Thus, the crystallization entails an accurate control of their molecular weight, and their distribution and stereochemistry, and so crystallizable polymers can be used as the immiscible core-forming block to create nanostructures with additional structural characteristics [111,112,113,114,115][44][45][46][47][48].6. Cross-Linked, Functionalised and Stimuli-Responsive Micelles
Polymer micelles can be cross-linked using different reagents where both the core and shell can be cross-linked. Furthermore, these micelles can be functionalized at the terminal end of the hydrophilic tail (Figure 8). These possibilities provide better opportunity for such micelles to act as nanocarriers. The core cross-linking often increases micelle stability and such cross-linked micelles retain their structure even at low concentration below critical micelle concentration (CMC) of micelle, thereby forming polymeric amphiphile. These micelles can be isolated and re-dissolved as stable nanoparticles and thus prolong the circulating time. The most vulnerable approach is the core cross-linking. This can be achieved using a polymerizable group in the hydrophobic moiety of the block copolymer or adding a polymerizable monomer that stays in the micelle core and then is polymerized using a certain initiator. Sometimes, the decrease in free volume of the micelle core may adversely affect the drug loading capacity. Likewise, the shell of micelles can be cross-linked and the core of the shell cross-linked micelle can disintegrate through degradation/good solvent resulting in nanocontainers as reported by several researchers [67,68,69,70,116][49][50][51][52][53]. Another way to alter micelle morphology/characteristics is to functionalize the chain ends of the soluble shells. The chemical functionalization includes the covalent linkage between chain end and an agent that could be ligand. The ligand receptor interaction being highly selective helps in targeting the release of the solubilized drugs to the site of interest. The presence of different additives and salts that can be fine-tuned micellization and micelle characteristics can be also employed for improved solubilization and release. Furthermore, stimuli-responsive block copolymer can form core-shell aggregate which can be used for drug loading. The drug can be released under the influence of external stimuli such as pH, temperature, magnetic response. There are excellent reviews describing cross-linked, functionalised and stimuli- responsive micelles in the context of drug delivery systems [117,118,119,120][54][55][56][57].7. Mixed Micelles
Two or more block copolymers may interact synergistically and form mixed polymeric system with improved features that can be employed as vehicles for drug delivery systems [121,122][58][59]. The mixed micelles can have improved physical stability, enhanced solubility and drug bioavailability, and provide better functionality by simple mixing of the constituting amphiphilic copolymers. Polymer mixed micelles from block copolymers as well as polymer-surfactant mixed systems have been of great interest over the past few decades due to their applications in industries and biomedical fields. Several research papers on mixed micelles assembled from block copolymers and their use for drug delivery have appeared in the past and have been critically reviewed. The presence of incompatible blocks drives the block copolymers to a separate phase, but the covalent bond between them prevents phase separation at a macroscopic length scale and occurs at a nanometer length scale, thus producing a rich array of nanostructures in solid state and as well as in selective solvents. These structures exhibit tunable and enhanced mechanical, electrical and chemical properties and thus are significant from a technological view point. Thus, complete information on nanostructures coupled with precise synthesis of block copolymers is highly desired for practical purposes. Additionally, the theoretical studies based on the self-consistent field theory (SCFT) have been used to examine the micro scale phase separation and self-assembly of block copolymers [123,124,125][60][61][62].8. Polymer-Drug Conjugates
Polymer-drug conjugates a contain covalent bond between a water-soluble polymer and a drug. This idea was propounded by Rings Dorf in the mid-1970s. Polymer-drug conjugates behave like a prodrug that resides inactively before cutting of the conjugated bond and release of the active drug (Figure 136). The resulting drug conjugates readily self-assemble in solution and can potentially be used in drug delivery [126,127,128,129,130,131,132][63][64][65][66][67][68][69].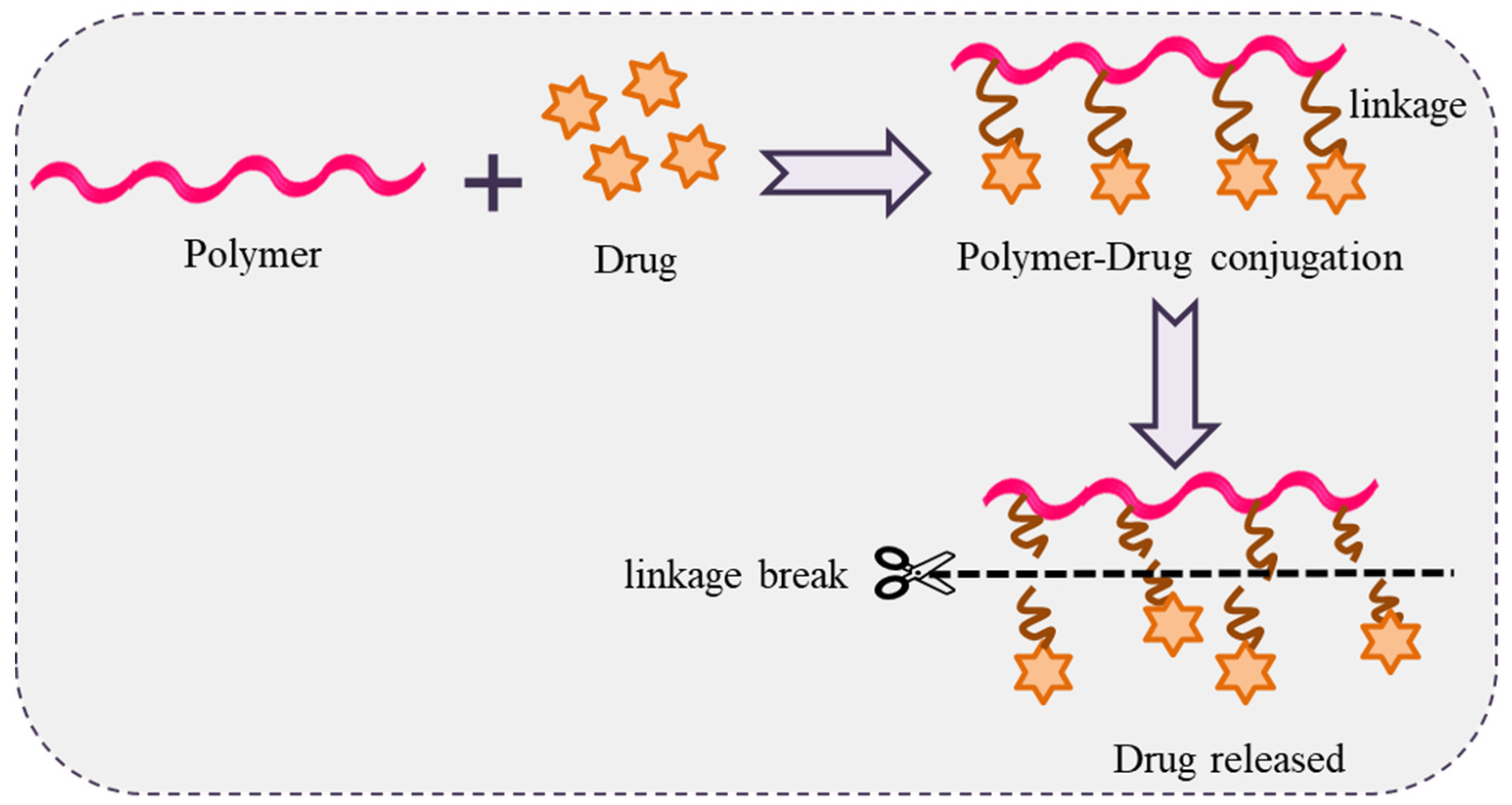
Figure 136. Schematic representation of polymer-drug conjugation with drug release.
References
- Mai, Y.; Eisenberg, A. Self-assembly of block copolymers. Chem. Rev. 2012, 41, 5969–5985.
- Wang, Z.; Chan, C.; Zhao, T.; Parker, R.; Vignolini, S. Recent Advances in Block Copolymer Self-Assembly for the Fabrication of Photonic Films and Pigments. Adv. Opt. Mater. 2021, 9, 2100519.
- Cabral, H.; Miyata, K.; Osada, K.; Kataoka, K. Block Copolymer Micelles in Nanomedicine Applications. Chem. Rev. 2018, 118, 6844–6892.
- Chen, W.; Du, J. Ultrasound and pH Dually Responsive Polymer Vesicles for Anticancer Drug Delivery. Sci. Rep. 2013, 3, 2162–2171.
- Papadakis, C.M.; Müller-Buschbaum, P.; Laschewsky, A. Switch it inside-out: “Schizophrenic” behavior of all thermoresponsive UCST-LCST diblock copolymers. Langmuir 2019, 35, 9660–9676.
- Butun, V.; Armes, S.; Billingham, N.; Tuzar, Z.; Rankin, A.; Eastoe, J.; Heenan, R. Synthesis and aqueous solution properties of a well-defined thermo-responsive schizophrenic diblock copolymer. Macromolecules 2001, 34, 1503.
- Kocak, G.; Tuncer, C.; Bütün, V. pH-Responsive polymers. Polym. Chem. 2017, 8, 144–176.
- Checot, F.; Rodrıguez-Hernandez, J.; Gnanou, Y.; Lecommandoux, S. pH-responsive micelles and vesicles nanocapsules based on polypeptide diblock copolymers. Biomol. Eng. 2007, 24, 81–85.
- Atanase, L.I.; Riess, G. Micellization of pH-stimulable poly(2-vinylpyridine)-b-poly(ethylene oxide) copolymers and their complexation with anionic surfactants. J. Colloid Interface Sci. 2013, 395, 190–197.
- Harada, A.; Kataoka, K. Polyion complex micelle formation from double hydrophilic block copolymers composed of charged and non-charged segments in aqueous media. Polym. J. 2018, 50, 95–100.
- Jundi, A.; Buwalda, S.; Bakkourb, Y.; Garric, X.; Nottelet, B. Double hydrophilic block copolymers self-assemblies in biomedical applications. Adv. Colloid Interfac. Sci. 2020, 283, 102213–102225.
- Smith, A.; Xu, X.; Kirkland-York, S.; Savin, D.; McCormick, C. Schizophrenic Self-Assembly of Block Copolymers Synthesized via Aqueous RAFT Polymerization: From Micelles to Vesicles. Macromolecules 2010, 43, 1210–1217.
- Can, A.; Zhang, Q.; Rudolph, T.; Schacher, F.; Gohy, J.; Schubert, U.; Hoogenboom, R. Schizophrenic thermoresponsive block copolymer micelles based on LCST and UCST behavior in ethanol-water mixtures. Eur. Polym. J. 2015, 69, 460–471.
- Colfen, H. Double-Hydrophilic Block Copolymers: Synthesis and Application as Novel Surfactants and Crystal Growth Modifiers. Macromol. Rapid Commun. 2001, 4, 220–252.
- Guragain, S.; Bastakoti, B.P.; Malgras, V.; Nakashima, K.; Yamauchi, Y. Multi-Stimuli-Responsive Polymeric Materials. Chem. Eur. J. 2015, 21, 13164–13174.
- Zhuang, J.; Gordon, M.; Ventura, J.; Li, L.; Thayumanavan, S. Multi-stimuli responsive macromolecules and their assemblies. Chem. Soc. Rev. 2013, 42, 7421–7436.
- Zhang, Y.; Chen, S.; Pang, M.; Zhang, W. Synthesis and micellization of a multi-stimuli responsive block copolymer based on spiropyran. Polym. Chem. 2016, 7, 6880–6885.
- Appold, M.; Mari, C.; Lederle, C.; Elbert, J.; Schmidt, C.; Ott, I.; Stühn, B.; Gasser, G.; Gallei, M. Multi-stimuli responsive block copolymers as a smart release platform for a polypyridyl ruthenium complex. Polym. Chem. 2016, 8, 890–900.
- Corten, C.; Kretschmer, K.; Kuckling, D. Novel multi-responsive P2VP-block-PNIPAAm block copolymers via nitroxide-mediated radical polymerization. Beilstein J. Org. Chem. 2010, 6, 756–765.
- Hu, J.; Liu, S. Responsive Polymers for Detection and Sensing Applications: Current Status and Future Developments. Macromolecules 2010, 43, 8315–8330.
- Tan, S.; Zhao, D.; Yuan, D.; Wang, H.; Tu, K.; Wang, L. Influence of indomethacin-loading on the micellization and drug release of thermosensitive dextran-graft-poly(N-isopropylacrylamide). React. Funct. Polym. 2011, 71, 820–827.
- Liu, S.; Billingham, N.C.; Armes, S.P. A Schizophrenic Water-Soluble Diblock Copolymer. Angew. Chem. Int. Ed. 2001, 40, 2328.
- Liu, S.; Armes, S.P. Micelle Formation and Inversion Kinetics of a Schizophrenic Diblock Copolymer. Langmuir 2002, 19, 4432.
- Yusa, S.; Yokoyama, Y.; Morishima, Y. Synthesis of oppositely charged block copolymers of polyethylene glycol via reversible addition-fragmentation chain transfer radical polymerization and characterization of their polyion complex micelles in water. Macromolecules 2009, 42, 376–383.
- Nakai, K.; Nishiuchi, M.; Inoue, M.; Ishihara, K.; Sanada, Y.; Sakurai, K.; Yusa, S. Preparation and characterization of polyion complex micelles with phosphobetaine shells. Langmuir 2013, 29, 9651–9661.
- De Santis, S.; Ladogana, R.; Diociaiuti, M.; Masci, G. PEGylated and thermosensitive polyion complex micelles by self-assembly of two oppositely and permanently charged diblock copolymers. Macromolecules 2010, 43, 1992–2001.
- Amaral, S.; Tawara, M.; Fernandez-Villamarin, M.; Borrajo, E.; Martinez-Costas, J.; Vidal, A.; Riguera, R.; Fernandez-Megia, E. Tuning the Size of Nanoassembles: A Hierarchical Transfer of Information from Dendrimers to Polyion Complexes. Angew. Chem. 2018, 57, 5273–5277.
- Fuoss, R.; Sadek, H. Mutual interaction of polyelectrolytes. Science 1949, 110, 552–554.
- Nakahata, R.; Yusa, S. Preparation of water-soluble polyion complex (PIC) micelles covered with amphoteric random copolymer shells with pendant sulfonate and quaternary amino groups. Polymers 2018, 10, 205.
- Ohno, S.; Ishihara, K.; Yusa, S. Formation of Polyion Complex (PIC) Micelles and Vesicles with Anionic pH-Responsive Unimer Micelles and Cationic Diblock Copolymers in Water. Langmuir 2016, 32, 3945–3953.
- Takahashi, R.; Sato, T.; Terao, K.; Yusa, S. Reversible Vesicle-Spherical Micelle Transition in a Polyion Complex Micellar System Induced by Changing the Mixing Ratio of Copolymer Components. Macromolecules 2016, 49, 3091–3099.
- Kim, H.; Zheng, M.; Miyata, K.; Kataoka, K. Preparation of polyion complex micelles using block copolymers for siRNA delivery. Methods Mol. Biol. 2016, 1364, 89–103.
- Penfold, N.; Yeow, J.; Boyer, C.; Armes, S. Emerging Trends in Polymerization-Induced Self-Assembly. ACS Macro Lett. 2019, 8, 1029–1054.
- Phan, H.; Taresco, V.; Penelle, J.; Couturaud, B. Polymerisation-Induced Self-Assembly (PISA) as a straightforward formulation strategy for Stimuli-Responsive Drug Delivery Systems and Biomaterials: Recent Advances. Biomater. Sci. 2020, 9, 38–50.
- Zhang, W.; Kadirkhanov, J.; Wang, C.; Ding, S.; Hong, C.; Wang, F.; You, Y. Polymerization-induced self-assembly for the fabrication of polymeric nano-objects with enhanced structural stability by cross-linking. Polym. Chem. 2020, 11, 3654–3672.
- D’Agosto, F.; Rieger, J.; Lansalot, M. RAFT-Mediated Polymerization-Induced Self-Assembly. Angew. Chem. 2020, 59, 8368–8392.
- Liu, C.; Hong, C.; Pan, C. Polymerization techniques in polymerization-induced self-assembly (PISA). Polym. Chem. 2020, 11, 3673–3689.
- Cao, J.; Tan, Y.; Chen, Y.; Zhang, L.; Tan, J. Expanding the Scope of Polymerization-Induced Self-Assembly: Recent Advances and New Horizons. Macromol. Rapid Commun. 2021, 42, 2100498–2100510.
- An, N.; Chen, X.; Yuan, J. Non-thermally initiated RAFT polymerization-induced self-assembly. Polym. Chem. 2021, 12, 3220–3232.
- Damsongsang, P.; Hoven, Y. Core-functionalized nanoaggregates: Preparation via polymerization-induced self-assembly and their applications. New J. Chem. 2021, 45, 12776–12791.
- Ganda, S.; Stenzel, M. Concepts, fabrication methods and applications of living crystallization-driven self-assembly of block copolymers. Prog. Polym. Sci. 2020, 101, 101195–101205.
- Noack, S.; Schanzenbach, D.; Koetz, J.; Schlaad, H. Polylactide-Based Amphiphilic Block Copolymers: Crystallization-Induced Self-Assembly and Stereocomplexation. Macromol. Rapid Commun. 2018, 40, 1800639–1800650.
- MacFarlane, L.; Shaikh, H.; Garcia-Hernandez, J.; Vespa, M.; Fukui, T.; Manners, I. Functional nanoparticles through π-conjugated polymer self-assembly. Nat. Rev. Mater. 2021, 6, 7–26.
- MacFarlane, L.; Zhao, C.; Cai, J.; Qiu, H.; Manners, I. Emerging applications for living crystallization-driven self-assembly. Chem. Sci. 2021, 12, 4661–4682.
- Finnegan, J.; Pilkington, E.; Alt, K.; Rahim, A.; Kent, S.; Davis, T.; Kempe, K. Stealth Nanorods via the Aqueous Living Crystallisation-Driven Self-Assembly of Poly(2-oxazoline)s. Chem. Sci. 2021, 12, 7350–7360.
- Ringsdorf, H. Structure and properties of pharmacologically active polymers. J. Polym. Sci. Symp. 1975, 51, 135–153.
- Shi, A.C. Self-Consistent Field Theory of Block Copolymers, Developments in Block Copolymer Science and Technology; John Wiley & Sons: Hoboken, NJ, USA, 2004; pp. 265–294.
- Chantawansri, T.L.; Bosse, A.W.; Hexemer, A.; Ceniceros, H.D.; Garcia-Cervera, C.J.; Kramer, E.J.; Fredrickson, G.H. Self-consistent Field Theory Simulations of Block Copolymer Assembly on a Sphere. Phys. Rev. E 2007, 75, 031802.
- Procházka, K.; Limpouchová, Z.; Štěpánek, M.; Šindelka, K.; Lísal, M. DPD Modelling of the Self-and Co-Assembly of Polymers and Polyelectrolytes in Aqueous Media: Impact on Polymer Science. Polymers 2022, 14, 404.
- Deng, Z.; Liu, S. Emerging trends in solution self-assembly of block copolymers. Polymer 2020, 207, 122914.
- Ge, Z.; Xie, D.; Chen, D.; Jiang, X.; Zhang, Y.; Liu, H.; Liu, S. Stimuli-Responsive Double Hydrophilic Block Copolymer Micelles with Switchable Catalytic Activity. Macromolecules 2007, 40, 3538–3546.
- Manga, M.; Cayre, O.; Biggs, S.; Hunter, T. Influence of pH-Responsive Monomer Content on the Behavior of Di-Block Copolymers in Solution and as Stabilizers of Pickering Latex Particle Emulsifiers. Front. Chem. 2018, 6, 301–313.
- Lyubimov, I.; Daniel, J.; Beltran, V.; Jayaraman, A. PRISM Theory Study of Amphiphilic Block Copolymer Solutions with Varying Copolymer Sequence and Composition. Macromolecules 2017, 50, 7419–7431.
- Zhang, Q.; Lin, J.; Wang, L.; Xu, Z. Theoretical modeling and simulations of self-assembly of copolymers in solution. Prog. Polym. Sci. 2017, 75, 1–30.
- Duncan, R. Polymer conjugates as anticancer nanomedicines. Nat. Rev. Cancer 2006, 6, 688–701.
- Greco, F.; Vicent, M.J. Combination therapy: Opportunities and challenges for polymer-drug conjugates as anticancer nanomedicines. Adv. Drug. Deliv. Rev. 2009, 61, 1203–1213.
- Kopeček, J. Polymer-drug conjugates: Origins, progress to date and future directions. Adv. Drug. Deliv. Rev. 2013, 65, 49–59.
- Feng, Q.; Tong, R. Anticancer nanoparticulate polymer-drug conjugate. Bioeng. Trans. Med. 2016, 1, 277–296.
- Russell, J.; Baker, S.L.; Colina, C.M.; Figg, C.A.; Kaar, J.L.; Matyjaszewski, K.; Simakova, A.; Summerlin, B.S. Next Generation Protein-Polymer Conjugates. AIChE J. 2018, 64, 3230–3245.
- Murthy, K.S.; Ma, Q.; Clark, C.G., Jr.; Remsen, E.E.; Wooley, K.L. Fundamental design aspects of amphiphilic shell-cross-linked nanoparticles for controlled release applications. Chem. Commun. 2001, 8, 773–774.
- Xi, S.; Wang, L.; Liu, J.; Chapman, W. Thermodynamics, microstructures, and solubilization of block copolymer micelles by density functional theory. Langmuir 2019, 35, 5081–5092.
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202.
- Lee, J.S.; Feijen, J. Polymersomes for drug delivery: Design, formation and characterization. J. Cont. Release 2012, 161, 473–483.
- Torchilin, V. Micellar nanocarriers: Pharmaceutical perspectives. Pharm. Res. 2007, 24, 1–16.
- Guiraud, S.; Alimi-Guez, D.; Wittenberghe, L.; Scherman, D.; Kichler, A. The Reverse Block Copolymer Pluronic 25R2 Promotes DNA Transfection of Skeletal Muscle. Macromol. Biosci. 2011, 11, 590–594.
- Zhao, L.; Du, J.; Duan, Y.; Zang, Y.; Zhang, H.; Yang, C.; Cao, F.; Zhai, G. Curcumin loaded mixed micelles composed of Pluronic P123 and F68: Preparation, optimization and in-vitro characterization. Colloids Surf. B Biointerfaces 2012, 97, 101–108.
- Hassanzadeh, S.; Feng, Z.; Pettersson, T.; Hakkarainen, M. A proof-of-concept for folate-conjugated and quercetin-anchored Pluronic mixed micelles as molecularly modulated polymeric carriers for doxorubicin. Polymer 2015, 74, 193–204.
- Patel, D.; Rathod, S.; Tiwari, S.; Ray, D.; Kuperkar, K.; Aswal, V.; Bahadur, P. Self-Association in EO-BO-EO Triblock Copolymers as a Nanocarrier Template for Sustainable Release of Anticancer Drugs. J. Phys. Chem. B 2020, 124, 11750–11761.
- Patel, D.; Kuperkar, K.; Bahadur, P. Temperature stimulated self-association and micellar transition for star shaped normal and reverse EO-PO block copolymers and their mixed systems as potential use for anticancer drug solubilization. Soft Matter 2022, 18, 4543–4553.
More
