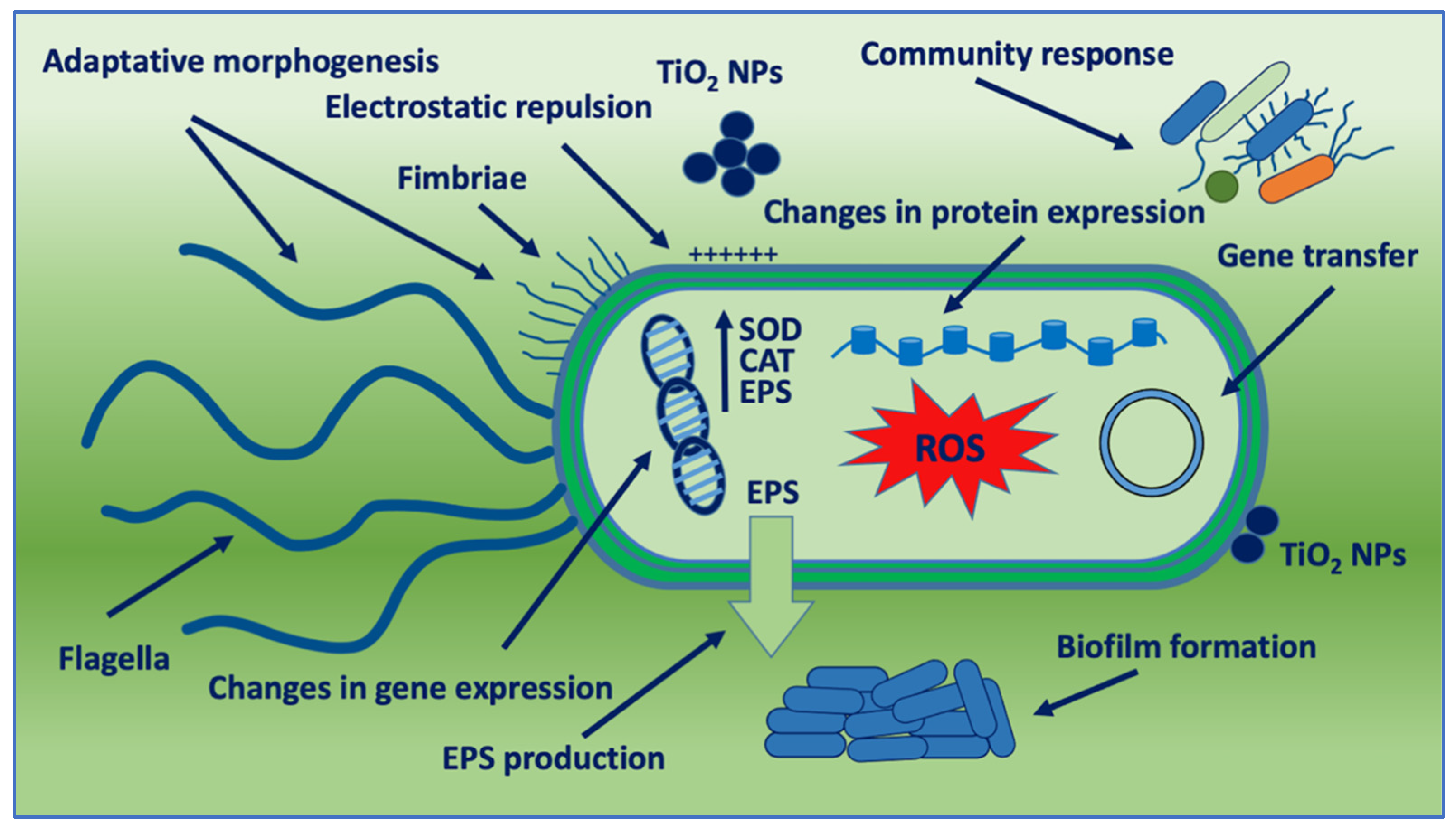
1. Introduction
Reported microbial resistance mechanisms to nanoparticles include efflux pumps, electrostatic repulsion, alteration of morphology, biofilm formation, extracellular matrices, gene transfer, metabolic responses, and mutations
[1]. These mechanisms are generated by the bacterial response to nanoparticle-induced stress and can be observed singly or collectively, and in dependence of single cells or bacterial communities and environmental conditions. In addition, acute and chronic cell or tissue exposure to nanoparticles can induce changes that influence bacterial opportunistic mechanisms favoring microbial virulence.
Apart from efflux systems, which do not seem to be involved in bacterial adaptation, the adaptation responses to the TiO
2 nanoparticles (NP
s) challenge will be illustrated below as nanoparticle impact on bacterial fitness (
FigureFigure 1). Efflux pumps participate in the development of resistance to ion release from metal nanoparticles under non-bactericidal concentrations, but TiO
2 NPs do not seem to release ions because of their very low dissolution rate and high stability
[2].
Figure 1. Schematic representation of bacterial adaptation mechanisms against TiO2 NPs. SOD: SuperOxide Dismutase; CAT: Catalase; and EPS: Extracellular Polymeric Substance.
2. Electrostatic Repulsion and Charge Modification
Some resistance mechanisms to nanoparticles involve electrostatic repulsion. Bacteria are able to regulate the electrical charge of their surface, which allows them to repel nanoparticles with different types of charge on their surface. The antimicrobial activity of the nanoparticles in some cases can be due to the interaction of nanoparticle charge with the electric charge present on the surfaces of the bacteria
[3], but some bacteria acquire mechanisms allowing resistance to the charge of nanoparticles
[4].
Planchon et al.
[5] studied the interaction between
Escherichia coli and TiO
2 NPs in natural and artificial waters and found that pH and dispersion status influenced the contact between the NPs and
E. coli. TiO
2 NP toxicity seemed to be due to the aggregation status of the nanoparticles and the physiological state of
E. coli at different pH. Compared to at pH 8, a better bacterial physiological state with better resistance to nanoparticle toxic effect was observed at pH 5, despite a stronger interaction between the cells and nanoparticles. At the same time, a bacterial subpopulation, apparently non-interacting with the nanoparticles, was found. Such heterogeneities in cell populations could be related to a strategy of the colony where some bacteria try to adsorb all the contaminants in the solution, including TiO
2 NPs, favoring bacterial resistance. Similar results were obtained from
ourthe study on the interaction between
Listeria monocytogenes and non-UV irradiated TiO
2 NPs. The influence of nanoparticles depended on nanoparticle concentrations and the biofilm-forming capability of the bacterial strains. Close contact of bacteria with TiO
2 NPs was extensively observed without decreased listeria vitality. A pH of 5.5 seemed to favor the close adhesion of nano-titania to bacterial cell surfaces, probably because of increased bacterial hydrophobicity. Bacteria-TiO
2 NP interaction led to an increased biofilm formation. It appeared that
L. monocytogenes exploited the large agglomerates of TiO
2 particles to massively adhere and promote bacterial aggregation and biofilm production, in order to prolong their survival and dissemination
[6].
It is known that the hydrophobicity of both bacteria and surfaces could play a role in their initial interaction with each other depending on several factors such as temperature, nutritional content, or bacterial features
[7]. A higher interaction between nanoparticles and bacteria is expected to enhance the toxic effects of the nanoparticles. The absence of UV light irradiation during the interaction at acidic pH can explain the low or no significant toxicity of TiO
2 NPs. On the contrary, nanoparticle adsorption on bacterial surfaces seems to promote bacterial survival strategies.
3. Adaptative Morphogenesis
It has been reported that nano-resistance begins with changes in the shape of bacteria and modulation of membrane protein expression
[8]. Generally, adaptative morphogenesis was observed in chronic exposure to nanoparticles. Repeated exposure of a commensal
E. coli strain to a low dose of nano-titania for 400 days in the dark led to filamentation, thickening of the cell wall, and biofilm formation, accompanied by decreased sensitivity to oxidative stress and multiple antibiotics. Enhanced bacterial motility was observed with flagellar assembly, and fimbria biosynthesis increased. These adaptive traits were associated with increased pathogenicity, as confirmed by a higher death rate of the macrophages
in vitro and more severe bacterial infection in mice
in vivo. The adaptive evolution was attributed to free radical production by nano-titania in the dark.
ThHerei
s studyn, it is one of only a few evaluating free radical production in the dark. TiO
2 NPs were able to generate low levels of ROS, specifically hydroxyl radicals, in dark conditions such as in the gut, probably due to nanoparticle surface defects, such as oxygen vacancies. This oxidative stress induced a commensal-to-pathogen transition of
E. coli, raising concern mainly for intestinal microbiota
[9].
E. coli showed also an adaptative response to palladium oxide-modified nitrogen-doped TiO
2 (TiON/PdO) under UV light irradiation
[10]. After repeated exposure to photo-disinfection,
E. coli adapted its response by regulation of chemotaxis and flagellar assembly followed by increased superoxide radical degradation. After photocatalysis of TiON/PdO nanoparticles, a mutant strain was obtained showing a small colony size and irregular margin morphology. Metabolic processes of the mutant, such as oxidative phosphorylation, TCA cycle, glycolysis, pyruvate, fatty acid, and glutathione, were decreased. Motility was enhanced through the up-regulation of genes in the flagellar assembly pathway during the stress response.
E. coli response to photocatalysis was adapted through an enhanced ability for superoxide radical degradation.
Following the discovery that biofilms formed from activated sludge exposed to 5 and 50 mg/L nano-titania in the dark had increased biomass and selectively enriched pathogens, Zhu et al.
[11] examined the protein response and protein phosphorylation modification of
E. coli K12 exposed to nano-titania. Using the integrative system biology analyses of proteomics and phosphoproteomics, they demonstrated that
E. coli cultivated with TiO
2 NPs up-regulated iron acquisition and regulated protein phosphorylation states associated with transcription, translation, and biofilm formation. Bacteria showed increased siderophores and exopolysaccharide content together with enhanced resistance to transcriptional inhibitory antibiotics. Some up-regulated proteins were associated with increased curli production and cellulose biosynthesis, which are important components of the extracellular matrix of
E. coli supporting biofilm formation.
E. coli was therefore shown to adapt to sublethal TiO
2 NP concentrations by adaptative morphogenesis leading to bacterial survival by promoting biofilm formation.
4. Community Response
The bactericidal activity of TiO
2 NPs is mainly due to ROS generation during the interaction with bacteria that develop different strategies to counteract this challenge
[12]. Bacterial adaptation to TiO
2 NPs producing ROS was observed mainly in multispecies microbial aggregates under chronic nanoparticle exposure.
Engineered TiO
2 NPs are released into biological wastewater treatment plants and are recognized as environmental stressors. Mathur et al.
[13] have demonstrated that a consortium of different bacteria in wastewater is able to reduce damage from oxidative stress by TiO
2 NPs. The viability of the consortium is higher than that of single isolates. In particular, some bacteria of the consortium, such as
Exiguobacterium acetylicum and
Pseudomonas nitroreducens, having a higher capacity to produce SOD enzyme, contributing to the survival of other bacteria. Extracellular polymeric substance (EPS) production was also most expressed in the bacterial consortium compared to single bacteria. Capsular EPS provides a defense against the attachment of TiO
2 NPs and ROS diffusion to the bacterial cells preventing membrane integrity loss. In addition, enhanced release of EPS corresponded to increased biofilm production, with most of the nanoparticles and ROS unable to access bacterial cell surfaces. Thus, the consortium of cells was shown to have better abilities to counteract the toxic effects of TiO
2 NPs, whilst also maintaining the ability to reduce organics in sewage.
Periphytic biofilm, a typical autotropic multispecies microbial aggregate, also showed adaptation to TiO
2 NP impact. While there is no evident toxic effect of nanoparticle exposure on periphytic biofilm in terms of biomass, chlorophyll content, and ATPase activity, the microbial communities were protected from ROS production and accumulation. Moreover, periphytic biofilms changed their community composition in the presence of TiO
2 NPs by increasing the relative abundance of phototrophic and high-nutrient metabolic microorganisms
[14].
Changes in the bacterial abundance in multicellular bacterial communities were found also in studies on TiO
2 impact on the growth and activity of bacterial communities of three Swedish lakes. Exposure to different concentrations of TiO
2 NPs, and in the presence of particular environmental conditions that make nanoparticles stable, significantly reduced bacterial abundance. Despite the reduction of bacterial abundance following nanoparticle exposure, the overall bacterial activity did not, in most cases, change significantly, which was due to a strongly enhanced activity per cell in the higher TiO
2 NP concentration exposure group. This indicated the presence of bacterial groups that are more resistant to TiO
2 NP toxicity or are even stimulated in the presence of TiO
2 NPs
[15].
Perturbation of microbial communities was reported also in soil bacteria exposed to TiO
2 NPs over time. Short-term TiO
2 NP exposure revealed significant effects on enzyme activity and bacterial community structure and composition in clay soil with high organic matter. Response alterations were observed in the taxa belonging to Acidobacteria and Verrucomicrobia, and functional pathways related to carbohydrates degradation. As exposure time increased, the bacterial community recovered after long-term exposure of 60 days, suggesting that the bacterial evolution and adaptation could overcome the TiO
2 NP selection after long-term exposure
[16].
5. Metabolic Response
The presence of environmental stressors, such as nanoparticles, can influence bacterial gene expression and facilitate resistance mechanisms. The release of TiO
2 NPs into biological wastewater treatment plants has drawn significant attention because microorganisms used for pollutant removal are potentially threatened by the TiO
2 NPs due to their biotoxicity. A study conducted in a chemostat reactor exploring the behaviour of ammonia oxidizer bacteria under chronic TiO
2 NP exposure indicated that
Nitrosomonas europea was able to adapt to the TiO
2 stressor. After 40 days of incubation,
N. europea cultures appeared to recover cell growth inhibition, membrane integrity, nitrification rate, and ammonia monooxygenase activity. The recovery capacities of the bacteria were associated with the activation of several metabolic activities, such as processes involved in membrane repair and metabolic and stress-defense pathways. Changes in these metabolic processes induced cellular adaptation and recovery, providing the selection of resistant bacterial cells
[17].
The impact of nano-titania on the physiological function of
Shewanella oneidensis, a metal reducer bacterium, has been evaluated.
S. oneidensis secretes flavin mononucleotide that was rapidly converted into riboflavin that transforms metals and serves as a method of respiration for
S. oneidensis in limited oxygen content. After exposure to varying concentrations and types of TiO
2 NPs, minimal changes in vitality were observed, whereas significant changes in bacterial growth, biofilm formation, and riboflavin secretion of
S. oneidensis occurred. These changes were the result of the proximity of the nanoparticles causing altered gene expression, which influenced bacterial activities such as biofilm growth and riboflavin secretion. Bacterial growth showed a dose-dependent increase whereas biofilm production revealed a slower biofilm growth. In addition, extracellular riboflavin increased as a function of nanoparticle concentration. These metabolic changes were due to the modification of gene expression induced by the TiO
2 NPs. In particular, increased expression of riboflavin correlated with omcA expression, which encodes for an outer membrane c-type cytochrome that plays a small role as a terminal reductase for metals. This alteration indicated that
S. oneidensis flavin secretion is activated as a response to a system stressor
[18].
An unexpected TiO
2 NP resistance phenotype was found in a study evaluating the interaction of nanoparticles with an LPS-truncated
E. coli K12 mutant. The exposure of bacteria carrying this core-free LPS to nanoparticles in the dark increased the action of nanoparticles, with the stripping of outer membranes, increased osmotic stress, and efficient vesicle-facilitated release of damaged membrane components. In addition, vesicles were observed acting as electrostatic baits for TiO
2 NPs, mitigating TiO
2 NP toxicity. Surprisingly, the TiO
2 NP activity on the altered LPS structure favored a further membrane destabilization that seemed to be able to generate an antagonistic response to nanoparticles
[19].
In some cases, a stress response to nanoparticles could favor antibiotic activity, as found during the evaluation of the impact of TiO
2 NPs on the activity of antimicrobials, quorum sensing (QS), and efflux pump genes expression in Multidrug resistant (MDR)
Pseudomonas aeruginosa isolates. Nano-titania not only exerted antibacterial activity against
P. aeruginosa and high reduction of biofilm formation but, when used alone or in combination with antibiotics, provided a significant down-regulation of the efflux pump genes (MexY, MexB, and MexA) and QS-regulated genes (lasR, lasI, rhll, rhlR, pqsA, and pqsR). This effect allowed for a better response to antibiotics against MDR
P. aeruginosa [20]. Nevertheless, it cannot be ruled out that photoactivated TiO
2 NPs could induce bacterial antibiotic tolerance that could evolve into resistance. It was observed that the
E. coli DH5α strain treated with lethal photo-activation showed bacterial stress responses that improve antibiotic tolerance by several mechanisms, such as efflux pumps, biofilm formation, and increased mutation rates. The bacteria with higher antibiotic tolerance could evolve into antibiotic resistance faster with subsequent antibiotic selection
[21].
6. Gene Transfer
Nanoparticles, acting as environmental stressors, can cause a spontaneous rise in mutation and trigger genome plasticity, which can greatly facilitate resistance to antimicrobial agents and the evolution of strains with increased fitness. By altering bacterial physiology, and especially competence, NPs may influence the dissemination of antibiotic resistance in bacteria. Nano-titania is able to significantly modify the transformation efficiency of
Bacillus subtilis in biofilm growth conditions
[22]. Transformation is defined as the uptake of foreign DNA and its subsequent integration into the bacterial chromosome or replication as an independent plasmid. The first step in the transformation is the “competence”, which involves the take up of DNA through the bacterial surface and then the complete entry of DNA in the cell. After
B. subtilis exposure to nano-titania, the competence appeared to be significantly decreased. Two oligopeptide ABC transporters, OppABCDF and AppDFABC, are differentially expressed in response to nanoparticles. The Opp and App transporters are responsible for the import of the extracellular peptide factors, which initiate the competence process. These processes involved in the induction of competence were affected as a consequence of a physiological adaptation.
Regarding the spread of antibiotic resistance genes mediated by nanoparticles, it was observed that at various nanomaterial concentrations, bacterial density, matting time, and matting temperature, nano-titania can significantly promote the conjugation of the RP4 plasmid in
E. coli. A mathematical model to quantitatively describe the conjugation process and evaluate the effects of TiO
2 NPs on the spread of antibiotic resistance genes revealed that the nanoparticles inhibited bacterial growth and promoted conjugation simultaneously with a potential environmental risk
[23]. The spread of antibiotic resistance genes has been demonstrated also by phage infection mediated by TiO
2 NPs, which exhibited the ability to promote bacteriophage attachment on cell surfaces as the constructed phage gM13 that infects
E. coli TG1 strain. Increased membrane permeability induced by nano-titania appeared to facilitate the infectious entry of phage gM13 into periplasmic space. Following TiO
2 NP photoexcitation, extracellular ROS production was shown to facilitate phage entry by increasing the peroxidation of phospholipids and damaging the integrity of the bacterial membrane. Moreover, the expression of pilus-related genes was improved when
E. coli TG1 was exposed to TiO
2 NPs and photoexcitation. This enhanced pili-related gene expression promoted the synthesis of bacterial pili, thereby increasing the phage invasion sites and improving the transduction efficiency
[24].
7. Impact on Intestinal Microbiota
TiO
2 NPs introduced while consuming food can exert an influence on the human microbiome. During their passage through the small intestine, they come in contact with proteins and peptides that can interact with the NPs forming agglomerates, as well as changing their charge
[25]. Moreover, the contact of TiO
2 NPs with commensal bacteria can influence the resident microbiota by inhibiting the growth and activity of gastrointestinal bacteria, mainly in bacteria of the probiotic type. The bacterial communities composing the intestinal microbiota can develop adaptative strategies to survive, such as biofilm formation or metabolic regulation
[26]. Taylor et al.
[27], by using three different nanoparticles including nano-titania in a colon model, demonstrated changes in multiple characteristics of bacteria phenotypes, such as hydrophobicity, the sugar content of EPS, electrophoretic mobility, and the production of short-chain fatty acids. The most relevant phenotypic transformation induced by TiO
2 NP exposure was the hydrophobicity leading to an increased trend in biofilm formation. Pinget et al.
[28], after oral administration of TiO
2 NPs, reported changes in the release of bacterial metabolites of commensal bacteria
in vivo, and biofilm promotion
in vitro–although minimum NP impact on the composition of gastrointestinal microbiota in mice was found.
Metabolomic and proteomic responses of
E. coli to nano-titania stress observed by Planchon et al.
[29] demonstrated differences between bacteria fully covered with TiO
2 NPs and the population that remained free from nanoparticles. Several proteins appeared down-regulated whereas the proteins associated with energy metabolism were up-regulated. The proteins most affected by the exposure to nanoparticles are those associated with the integrity of the membrane, together with proteins involved in the stress response, DNA protection during starvation, or those which promote protein folding. The up-regulated proteins were involved in energy metabolism, especially glycolysis and the TCA cycle. Moreover, the synthesis of amino acids or proteins involved in biosynthetic processes was modified. The authors concluded that the exposure of
E. coli cells to nano-titania led to a heterogeneous response with part of the bacterial population able to adapt to TiO
2 NP stress and survive, while the remainder died because they were unable to adapt to this stress.
Waller et al.
[30] demonstrated that bacterial exposure to TiO
2 NPs in an
in vitro human colon reactor model caused changes to the composition of the microorganisms, as well as lowering the colonic pH. The addition of industrial-grade and food-grade TiO
2 NPs to the colon model resulted in a bacterial response linked to the microbial composition and phenotypic and biochemical changes with a reduced transition of the microbial community from Proteobacteria abundance to Firmicutes. Moreover, a reduced system pH and conductivity, with probable disruption of the anaerobic digestive process, was observed. These alterations representing bacterial community adaptation to TiO
2 NPs can lead to the potential onset of deleterious conditions over continuous, long-term exposure.
At the same time, literature data are available that reveal a limited influence or no significant direct impact of TiO
2 NPs on human gastrointestinal microbiota. Low TiO
2 NP concentrations, equivalent to those found in chewing gums and candies, showed low effects on the intestinal bacterial community known as microbial ecosystem therapeutic-1 (MET-1), and no changes in gas production and fatty acid methyl ester profiles, suggesting no significant influence on bacterial metabolism and microbiota composition
[31]. Similar results were obtained by Agans et al.
[32], using an
in vitro Human Gut Simulator (HGS). Cultures exposed to TiO
2 NPs displayed only a modest reduction in microbial community density with no impact on community diversity and evenness, even though the NPs were found to loosely interact with microbial cells.
8. Impact on Bacteria-Cell Interaction
TiO
2 NP impact on bacterial virulence also plays a role in bacteria-cell interactions. Cells or tissues interacting with TiO
2 NPs can undergo changes that can make them more susceptible to bacterial adhesion or invasion. It was reported that TiO
2 particles, that exhibit no sign of toxicity, were able to perturb the cholesterol gradient on HeLa cell membranes
[33]. In particular, cholesterol in the inner plasma membrane leaflet was reduced, while there was an increased amount of cholesterol in the outer plasma membrane leaflet. The enhanced asymmetry in the cholesterol distribution caused an increase in
Staphylococcus aureus infection on HeLa cells, as
S. aureus requires cholesterol for proper membrane attachment and virulence. Cell exposure to low TiO
2 NP concentrations led to the up-regulation of the cholesterol transporter proteins that facilitate the transport of cholesterol across membranes. Thus, nano-titania, rather than preventing bacterial cell infection, promoted bacterial infectivity by regulation of cellular genes that modify the structural features of membranes.
L. monocytogenes also showed increased invasiveness in intestinal cells pretreated with nano-titania at low doses. In a study performed by the authors
of th
is reviewerein, the
in vitro exposure of human intestinal cells to non-activated TiO
2 NPs before
L. monocytogenes infection significantly increased the efficiency of bacterial invasion and survival. Pretreatment of HT-29 cells with 1 μg/cm
2 of TiO
2 NPs, comparable to the real amount of TiO
2 ingestion through food, induced higher invasiveness compared to untreated cells. Cytoskeletal changes, probably induced by TiO
2 NP treatment, enhanced bacterial internalization. In addition, increased bacterial entry led to higher intracellular bacterial survival
[34].
In these cases, TiO2 NPs are not intrinsically anti-bacterial and, when ingested by cells, they do not exert cellular toxicity by the generation of free radicals or chemical damage to cell structures. Nanoparticles at low concentrations and non-UV irradiated act in a subtle manner inducing cell membrane or intracellular changes leading to an increased bacterial invasion.