Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Conner Chen and Version 2 by Conner Chen.
Cannabis (Cannabis sativa L.) is an outstanding source of bioactive natural products, with more than 150 different phytocannabinoids isolated; however, studies of their bioactivity have historically concentrated on the so-called “big four” [∆9-THC (1a)tetrahydrocannabinol, CBD (2a)cannabidiol, CBGcannabigerol (3a) and CBC (4a)cannabichromene]. Among the remaining products, which have traditionally been referred to as “minor cannabinoids”, cannabinol (CBN, 5a) stands out for its important repercussions and implications on the global scientific landscape.
- cannabinol
- cannabinoids
- phytocannabinoids
- cannabinome
1. Introduction
There is extensive historical evidence that cannabis (Cannabis sativa L.) has been used for different purposes, among them industrial [1], ornamental [2], and pharmaceutical (e.g., treating rheumatic pain, constipation, gout, and gynecological disorders) [3] applications. Nowadays, the intake of marijuana is permitted in many countries of the world for the treatment of different pathologies [4], including nausea caused by chemotherapy, anorexia in patients suffering from AIDS, and pain management [5][6].
The biological activity of cannabis is strictly related to phytocannabinoids, the hallmark secondary metabolites of this remarkable plant [7]. This class of meroterpenoids is characterized by great chemical diversity among its constituents; however, a general phytocannabinoid structure is easily identifiable due to its characteristic hybrid nature; since this class derives from the merging of polyketides and amevalonate biosynthetic pathway, a resorcinyl core decorated with p-oriented isoprenyl residues and an alkyl side chain is easily identifiable [8].
Despite this impressive variety, early studies in this field focused almost exclusively on the narcotic principle of marijuana, ∆9-tetrahydrocannabinol (∆9-THC, 1a, Figure 1), eventually expanding to the other related compounds, which are cannabidiol (CBD, 2a, Figure 1), cannabigerol (CBG, 3a, Figure 1), and cannabichromene (CBC, 4a, Figure 1), that together form a group of compounds often referred to as “the major cannabinoids” or “big four”[9]. As understanding of the biological mechanisms underlying ∆9-THC narcotic properties developed, the endocannabinoid system (ECS) was discovered [10], and its complexity, homeostatic role, and potential for drug discovery prompted a reconsideration of the other three major cannabinoids derived from cannabis. In addition to these studies, CBD (2a) was developed into a standardized extract (Sativex) and as an active pharmaceutical ingredient (API) (Epidiolex). The latter is the drug of choice for the treatment of certain rare genetic forms of epilepsy, while Sativex (a combination of ∆9-THC and CBD) is used to treat spasticity associated with multiple sclerosis [5]. Likewise, CBG (3a) and CBC (4a) found their way into the literature as well [11][12].
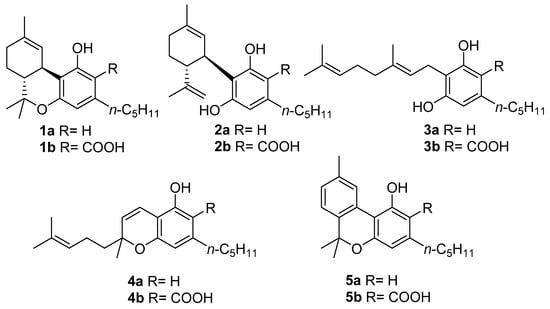
Figure 1. The structures of the “big four” phytocannabinoids [∆9-THC (1a), CBD (2a), CBG (3a) and CBC (4a)] and their acidic precursors (1b–4b), CBN (5a) and its acidic form CBNA (5b).
Most of the studies on phytocannabinoids, both chemical and biological, have focused on the “big four” due to their high extraction yield from vegetable sources or easy accessibility through total synthesis [11][12][13][14][15][16][17]. However, cannabis plants are also capable of producing more than 150 other compounds referred to as “minor cannabinoids” [18], which have significant structural differences and specific biological properties [19][20]; among these compounds, one stands out in particular, namely cannabinol (CBN, 5a, Figure 1).
CBN (5a) is one of the most famous phytocannabinoids in C. sativa, and although several phytocannabinoids have been identified in different plants and fungi, CBN (5a) has only been identified in cannabis. In the cannabinoid family, CBN (5a) is unique in several ways, the main one being its origin: whereas the acidic precursors of major cannabinoids (1b, 2b,4b, Figure 1) are generated by the result of different cyclizations of the terpenyl moiety of cannabigerolic acid (CBGA, 3b, in turn obtained from the condensation of olivetolic acid with geranylpyrophosphate) mediated by particular cyclases [21], a biosynthetic pathway for cannabinolic acid (CBNA, 5b, Figure 1), and so of CBN (5a) itself, has not been identified; the latter is a degradation artifact of ∆9-THC (1a) which, as result of air oxidation, undergoes aromatization at the level of the menthyl moiety [22]. However, small amounts of CBNA (5b) have been found in some hemp samples [23], suggesting that, in particular conditions, oxidative degradation may also occur to tetrahydrocannabinolic acid (THCA, 1b, Figure 1), the acidic precursor of ∆9-THC (1a), as well as before decarboxylation.
CBN (5a) was probably considered a “minor” phytocannabioid owing to its unfortunate and confusing discovery: it was the first phytocannabinoid to be isolated from hashish in the late 19th century, but its structure was not fully solved until 1940 due to some issues related to both nomenclature misunderstandings and the nature of the plant material used for the extraction. This confusing situation—associated with its limited availability and the discovery of more interesting bioactive phytocannabinoids—severely hindered its characterization from a biological and pharmacological standpoint.
2. History
Marijuana is perhaps one of the oldest plants grown by mankind, so much that people can say that Cannabis sativa L. and humanity share a close and intertwined history [24]. From the perspective of time, CBN (5a) probably plays the most important role of all the cannabinoids: due to its exceptional stability and direct chemical relationship with the psychoactive constituent Δ9-THC (1a), the natural product has been assumed to be the most relevant marker [25] for the identification of narcotic cannabis in archaeological plant samples. CBN’s (5a) extraordinary stability has been demonstrated by the discovery of plant material (seeds) dating back to 750 BC, found in a tomb in the Xinjiang-Uighur autonomous region (China), still containing high levels of the molecule [26]. Also attributed to CBN (5a) are the great uncertainty and ambiguity that plagued the first studies on the psychotropic properties of C. sativa: a significant limitation of the initial researches was related to the poor quality of the plant material investigated, which was mostly imported, directly or through Egypt, from India [27]. As a result of the long journey between the collection site and the European laboratories, usually lasting months, combined with uncontrolled and careless storage conditions, a sharp reduction in Δ9-THC (1a) levels in favor of CBN (5a) was achieved, leading to a relative falsification of the biological observations [28]. Wood coined the term “cannabinol” at the end of the 19th century in order to describe the “red oil”, a dense resin containing both CBN (5a) and other major phytocannabinoids as well [29]; these compounds are brownish and colorless oils or white solids, whereas the ruby red color likely resulted from quinoid structures [30] forming during the oil purification. Obtaining this resin required a difficult and complex preparation optimized by Wood himself, Spivey, and Easterfield at Cambridge University, and entailed the distillation of ethanolic or ethereal Cannabis extract at reduced pressure (2 mm) and collecting the vapors at a temperature of between 100 and 220 °C (corresponding to a bath temperature of 170–300 °C) [29]. Initially considered to be a pure compound, “red oil” was acetylated by Easterfield resulting in a crystalline compound with an optically inactive nature (6a, Figure 2). Its natural phenol (5a) was then referred to as “cannabinol”, taking the name used previously for the narcotic red oil and making CBN (5a) the first phytocannabinoid isolated [31].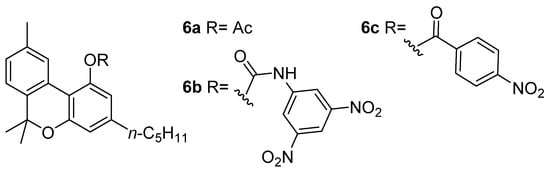
Figure 2. The structures of CBN adducts used for its isolation and characterization: CBN-acetate (6a), CBN-3,5-dinitrophenylurethane (6b) and CBN-4-nitrophenyl ester (6c).
References
- Karche, T.; Singh, M.R. The Application of Hemp (Cannabissativa L.) for a Green Economy: A Review. Turk. J. Bot. 2019, 43, 710–723.
- Hesami, M.; Pepe, M.; Baiton, A.; Salami, S.A.; Jones, A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants 2022, 11, 2383.
- Pisanti, S.; Bifulco, M. Medical Cannabis: A Plurimillennial History of an Evergreen. J. Cell. Physiol. 2019, 234, 8342–8351.
- De Souza, M.R.; Henriques, A.T.; Limberger, R.P. Medical Cannabis Regulation: An Overview of Models around the World with Emphasis on the Brazilian Scenario. J. Cannabis Res. 2022, 4, 33.
- Fraguas-Sánchez, A.I.; Torres-Suárez, A.I. Medical Use of Cannabinoids. Drugs 2018, 78, 1665–1703.
- Romero-Sandoval, E.A.; Fincham, J.E.; Kolano, A.L.; Sharpe, B.N.; Alvarado-Vázquez, P.A. Cannabis for Chronic Pain: Challenges and Considerations. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2018, 38, 651–662.
- Hesami, M.; Pepe, M.; Alizadeh, M.; Rakei, A.; Baiton, A.; Phineas Jones, A.M. Recent Advances in Cannabis Biotechnology. Ind. Crops Prod. 2020, 158, 113026.
- Nguyen, G.-N.; Jordan, E.N.; Kayser, O. Synthetic Strategies for Rare Cannabinoids Derived from Cannabis sativa. J. Nat. Prod. 2022, 85, 1555–1568.
- Atakan, Z. Cannabis, a Complex Plant: Different Compounds and Different Effects on Individuals. Ther. Adv. Psychopharmacol. 2012, 2, 241–254.
- Di Marzo, V. New Approaches and Challenges to Targeting the Endocannabinoid System. Nat. Rev. Drug Discov. 2018, 17, 623–639.
- Anokwuru, C.P.; Makolo, F.L.; Sandasi, M.; Tankeu, S.Y.; Elisha, I.L.; Agoni, C.; Combrinck, S.; Viljoen, A. Cannabigerol: A Bibliometric Overview and Review of Research on an Important Phytocannabinoid. Phytochem. Rev. 2022, 21, 1523–1547.
- Pollastro, F.; Caprioglio, D.; Del Prete, D.; Rogati, F.; Minassi, A.; Taglialatela-Scafati, O.; Munoz, E.; Appendino, G. Cannabichromene. Nat. Prod. Commun. 2018, 13, 1934578X1801300.
- Iversen, L. The Pharmacology of Delta-9-Tetrahydrocannabinol (THC); Oxford University Press: Oxford, UK, 2018; Volume 1.
- Sholler, D.J.; Schoene, L.; Spindle, T.R. Therapeutic Efficacy of Cannabidiol (CBD): A Review of the Evidence From Clinical Trials and Human Laboratory Studies. Curr. Addict. Rep. 2020, 7, 405–412.
- Maiocchi, A.; Barbieri, J.; Fasano, V.; Passarella, D. Stereoselective Synthetic Strategies to (−)-Cannabidiol. ChemistrySelect 2022, 7, e202202400.
- Nachnani, R.; Raup-Konsavage, W.M.; Vrana, K.E. The Pharmacological Case for Cannabigerol. J. Pharmacol. Exp. Ther. 2021, 376, 204–212.
- Bloemendal, V.R.L.J.; van Hest, J.C.M.; Rutjes, F.P.J.T. Synthetic Pathways to Tetrahydrocannabinol (THC): An Overview. Org. Biomol. Chem. 2020, 18, 3203–3215.
- Hanuš, L.O.; Meyer, S.M.; Muñoz, E.; Taglialatela-Scafati, O.; Appendino, G. Phytocannabinoids: A Unified Critical Inventory. Nat. Prod. Rep. 2016, 33, 1357–1392.
- Caprioglio, D.; Amin, H.I.M.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Minor Phytocannabinoids: A Misleading Name but a Promising Opportunity for Biomedical Research. Biomolecules 2022, 12, 1084.
- Walsh, K.B.; McKinney, A.E.; Holmes, A.E. Minor Cannabinoids: Biosynthesis, Molecular Pharmacology and Potential Therapeutic Uses. Front. Pharmacol. 2021, 12, 777804.
- Tahir, M.N.; Shahbazi, F.; Rondeau-Gagné, S.; Trant, J.F. The Biosynthesis of the Cannabinoids. J. Cannabis Res. 2021, 3, 7.
- Garrett, E.R.; Gouyette, A.J.; Roseboom, H. Stability of Tetrahydrocannabinols II. J. Pharm. Sci. 1978, 67, 27–32.
- Bastola, K.; Hazekamp, A.; Verpoorte, R. Synthesis and Spectroscopic Characterization of Cannabinolic Acid. Planta Med. 2007, 73, 273–275.
- Pain, S. A Potted History. Nature 2015, 525, S10–S11.
- Lavrieux, M.; Jacob, J.; Disnar, J.-R.; Bréheret, J.-G.; Le Milbeau, C.; Miras, Y.; Andrieu-Ponel, V. Sedimentary Cannabinol Tracks the History of Hemp Retting. Geology 2013, 41, 751–754.
- Russo, E.B. History of Cannabis and Its Preparations in Saga, Science, and Sobriquet. Chem. Biodivers. 2007, 4, 1614–1648.
- Appendino, G. The Early History of Cannabinoid Research. Rendiconti Lincei. Scienze Fisiche e Naturali 2020, 31, 919–929.
- Pertwee, R.G. Cannabinoid Pharmacology: The First 66 Years: Cannabinoid Pharmacology. Br. J. Pharmacol. 2006, 147, S163–S171.
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. XL.—Charas. The Resin of Indian Hemp. J. Chem. Soc. Trans. 1896, 69, 539–546.
- Caprioglio, D.; Mattoteia, D.; Taglialatela-Scafati, O.; Muñoz, E.; Appendino, G. Cannabinoquinones: Synthesis and Biological Profile. Biomolecules 2021, 11, 991.
- Wood, T.B.; Spivey, W.T.N.; Easterfield, T.H. III.—Cannabinol. Part I. J. Chem. Soc. Trans. 1899, 75, 20–36.
- Madan, H.G.; Randall, W.B.; Shaw, S.; Simpson, M.; Newton Spivey, W.T. Obituary notices: Sir Joseph Henry Gilbert, Ph.D., M.A., L.L.D., Sc.D., F.R.S., 1817–1901. J. Chem. Soc. Trans. 1902, 81, 625–636.
- Walton, R. Marihuana, America’s New Drug Problem; Lippincott: New York, NY, USA, 1938.
- Brian, R. Davis “Easterfield, Thomas Hill”, Dictionary of New Zealand Biography, First Published in 1996. Te Ar—The Encyclopedia of New Zealand. Available online: https://teara.govt.nz/en/biographies/3e1/easterfield-thomas-hill (accessed on 15 September 2022).
- Mills, J.H. Cannabis Britannica: Empire, Trade, and Prohibition, 1800–1928; Oxford University Press: Oxford, UK, 2005; ISBN 978-0-19-927881-7.
- Cahn, R.S.; Ingold, C.; Prelog, V. Specification of Molecular Chirality. Angew. Chem. Int. Ed. Engl. 1966, 5, 385–415.
- Cahn, R.S. 326. Cannabis Indica Resin. Part IV. The Synthesis of Some 2: 2-Dimethyldibenzopyrans, and Confirmation of the Structure of Cannabinol. J. Chem. Soc. Resumed 1933, 1400–1405.
- Mechoulam, R. Todd’s Achievement. Nature 1997, 386, 755.
- Adams, R.; Pease, D.C.; Clark, J.H. Isolation of Cannabinol, Cannabidiol and Quebrachitol from Red Oil of Minnesota Wild Hemp. J. Am. Chem. Soc. 1940, 62, 2194–2196.
- Work, T.S.; Bergel, F.; Todd, A.R. The Active Principles of Cannabis Indica Resin. I. Biochem. J. 1939, 33, 123–127.
- Jacob, A.; Todd, A.R. 119. Cannabis Indica. Part II. Isolation of Cannabidiol from Egyptian Hashish. Observations on the Structure of Cannabinol. J. Chem. Soc. Resumed 1940, 649–653.
- Adams, R.; Baker, B.R.; Wearn, R.B. Structure of Cannabinol. III. Synthesis of Cannabinol, 1-Hydroxy-3-n-Amyl-6,6,9-Trimethyl-6-Dibenzopyran 1. J. Am. Chem. Soc. 1940, 62, 2204–2207.
More
