Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Luís R. Silva and Version 2 by Sirius Huang.
Anthocyanins are among the best-known phenolic compounds and possess remarkable biological activities, including antioxidant, anti-inflammatory, anticancer, and antidiabetic effects. Despite their therapeutic benefits, they are not widely used as health-promoting agents due to their instability, low absorption, and, thus, low bioavailability and rapid metabolism in the human body. Research suggests that the application of nanotechnology could increase their solubility and/or bioavailability, and thus their biological potential.
- bioactive compounds
- anthocyanins
- drug delivery system
- nanoparticles
1. Introduction
Given the toxicity of many synthetic drugs, the search for and development of new effective drugs is essential [1][4]. Anthocyanins are a target of many studies in this regard [2][3][4][5][6][7][2,159,160,161,162,163].
However, these phytochemicals are very sensitive to external factors (e.g., pH variations, water, temperature) and have a short half-life [8][164]. Therefore, it is imperative to develop new delivery systems that do not exhibit toxicity and are able to increase the stability of anthocyanins and make them kinetically and thermodynamically stable, as well as improve their solubility and pharmaceutical properties [9][165].
Among the various alternatives studied, those related to nanotechnology and nanoencapsulation have attracted the greatest interest in many fields, such as medicine, namely in the prevention, diagnosis, and treatment of numerous diseases through active or passive targeting [10][11][12][13][14][166,167,168,169,170]. They are preferred over micro-delivery systems because the latter are more unstable in the physiological environment due to their large particle size, low zeta potential, and low encapsulation efficiency [15][171]. Therefore, various approaches to nanoencapsulation are currently being investigated for the different routes of administration, including intravenous, nasal, oral, parenteral, intraocular, and dermal topical applications [16][172]. In addition, most of these approaches can also easily cross the blood–brain barrier, which increases the therapeutic potential of the molecule(s) [14][17][170,173]. This technique can be applied because anthocyanins are able to generate van der Waals and hydrogen bonds as well as hydrophobic interactions with these nanocarriers, which increases their stability [9][18][19][24,26,165]. The latter has a particle size between 1 and 1000 nm and may consist of organic components (e.g., polymer and lipid-based nanoparticles, such as nanoemulsions, liposomes, and nanoparticles of solid lipid), inorganic coatings (e.g., metallic nanostructures of gold and titanium dioxide, such as nano-quantum dots and nanodiamonds), or a combination of both. Most of them are biodegradable and non-toxic [15][20][171,174].
Biopolymers are the most commonly used for nanoencapsulation because they incorporate various types of hydrophobic and hydrophilic molecules (e.g., drugs, proteins, phytochemicals, and other molecules), minimizing their undesirable effects and enhancing their benefits [21][22][175,176]. Among them, carbohydrates derived from alginate and chitosan, natural gums, and protein derivatives are the most popular [8][23][164,177]. They are very effective in encapsulating anthocyanins and contribute greatly to shifting the flavylium cation structure, protecting them from negative environmental effects, thus promoting their stability in the gastrointestinal tract and increasing their bioavailability [4][24][25][20,160,178]. Thus, several studies have already shown that encapsulation of anthocyanins with chitosan and derivatives can improve their physical and oxidative stability, preserve their antioxidant activity, and promote a slower degradation during simulated gastrointestinal digestion and environmental storage [8][26][27][28][29][22,27,164,179,180]. Pectin is also considered a promising coating because of its distinct ability to protect anthocyanins from different pH ranges and temperatures, as well as from damage by ascorbic acid [30][31][23,181]. In addition, it promotes a slower release of anthocyanins [32][182]. Zhao et al. [33][183] developed a nanoliposome of anthocyanins from blueberries coated with pectin and demonstrated a slower release of anthocyanins in simulated gastric fluid (≤35.9%), but faster release in simulated intestinal fluid, caused by the degradation of vesicles by the enzyme pancreatin. Liposomal micelles were also found to be effective in increasing the stability and resistance of blueberry anthocyanins in the gastrointestinal tract, resulting in a bioavailability greater than 90% [34][184]. In addition, the combination of pectin and chitosan has also been a subject of numerous studies. So far, they have already shown a promising ability to protect anthocyanins from degradation by milk and white fluorescent light, as well as from different pH and temperature levels, to maintain their antioxidant capacity and to improve retention during simulated ingestion and cellular uptake into human Caco-2 intestinal cells [35][36][37][13,28,185]. Similar results were obtained with gum arabic [38][39][186,187], α-lactalbumin [40][48], β-lactoglobulin [2], β-glucan [41][21], inulin and oligofructose carbohydrates [42][5], chondroitin sulfate [18][24], and casein conjugated with carboxymethyl cellulose [4][160]. The combination of whey protein with glucose also showed a remarkable ability to protect anthocyanins from high temperatures (80 °C) at acidic pH [43][188], while the use of ovalbumin conjugated with dextran appears to provide added value for the protection of anthocyanins from hydrogen peroxide-induced oxidative damage, as its conjugation showed a remarkable ability to increase anthocyanins’ chemical stability, cellular uptake, and intestinal absorption in human Caco-2 intestinal cells [19][26]. Peptides are also promising materials for nanoencapsulation. For example, C6M1, a peptide composed of 18 amino acids, was previously reported to maintain the free radical scavenging ability of cyanidin 3-O-glucoside and to be resistant to higher pH values, temperature, and concentrations of metal ions [6][162]. Apoferritin nanocages exhibit similar capabilities and also show the ability to facilitate the transport of this anthocyanidin through the cell monolayer of Caco-2 cells [44][189]. Finally, the use of EUDRAGIT® L100 and polyethylene glycol 2000 can also improve the bioavailability of anthocyanins from açaí berries [45][190].
Nanoencapsulation involves two primary structures: the first is the nanospheres core structure, in which the polymer matrix is dispersed and/or adsorbed by the bioactive compounds, and the second is the nanocapsule, which may consist of water or oil and a polymer shell [15][46][171,191]. When nanoparticles reach the outer cell membrane, they can interact with components of the plasma or extracellular membrane and enter the cell by endocytosis [19][47][48][26,192,193]. In addition, some biopolymers allow controlled release of the molecules that they coated at specific times and cells/organs [49][50][9,194].
The most promising in vitro and in vivo studies on the effects of nanoencapsulated anthocyanins on human health are summarized and discussed below (Figure 1).
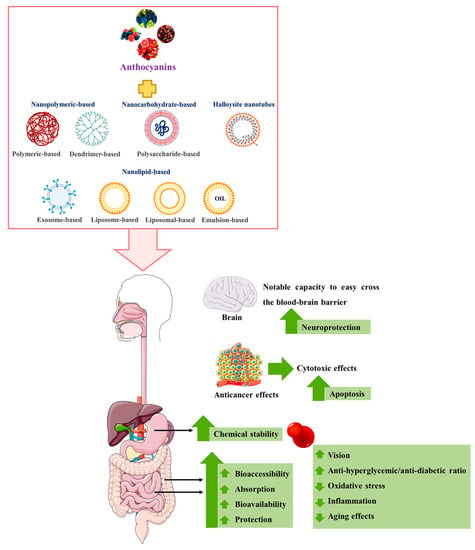
Figure 1. Main in vitro and in vivo effects of anthocyanins-loaded nanocarriers. ↑: increase; ↓: decrease. (Portions of Figure 1 were drawn using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/) and BioRender.com (https://biorender.com/) (both accessed on 6 August 2022) and from Guimarães [51][195]).
2. In Vitro Studies
2.1. Nano-Polymeric Coatings
The nanoencapsulation of anthocyanins with polymeric coatings has been commonly performed and investigated in in vitro conditions [48][52][53][193,196,197]. Among the various options, the use of the synthetic polymer poly(lactic-co-glycolic acid)-acid is very popular because it has tremendous potential to form stable complexes, is non-toxic and biodegradable, and the associated results are highly reproducible worldwide [54][198]. In addition, they display good tissue penetration and are easily manipulated [48][193]. This synthetic polymer showed great efficiency in coating pelargonidin, at concentrations of 10, 20, and 30 µM, increasing its ability to inhibit the production of reactive oxygen species in L6 muscle cells exposed to the pesticide cypermethrin after 12 and 24 h of exposure [53][197], as well as protecting these cells from nuclear damage by activating poly(ADP-ribose) polymerase and the p53 pathway, at a concentration of 9 µg/mL [54][198]. In addition, this coating can also enhance the ability of pelargonidin, at a concentration of 9 µM, to reduce the expression of GLUT 4, insulin receptor substrate 1, peptidase inhibitor 3, glycerol kinase, and protein kinase, and to induce cytochrome complex-mediated apoptosis in alloxan-induced hyperglycemic L6 skeletal muscle cells [52][196]. Moreover, anthocyanins encapsulated with this polymer also showed a higher capacity to protect human neuroblastoma cells SH-SY5Y from neurodegeneration against Aβ1–42-induced toxicity than those not encapsulated. Indeed, encapsulated anthocyanins at concentrations between 50 and 200 µM can remarkably reduce Alzheimer’s disease markers amyloid precursor protein, beta-site amyloid precursor protein cleaving enzyme, neuroinflammatory markers phospho-nuclear factor kappa B, tumor necrosis factor alpha, and inducible nitric oxide synthase after 12 h of exposure, and the neuroapoptotic markers B-cell lymphoma 2 and associated X-protein (Bax) and caspase-3 protein expressions, and showed notable scavenging properties and ability to abrogate reactive oxygen species production via the p38-MAPK/JNK pathway, as well as increase the expression of endogenous nuclear factor erythroid 2-related factor 2 and heme oxygenase [55][199].
Nevertheless, it is important to underline that some polymeric coatings can induce allergic reactions and that the complete degradation of some polymeric coatings, such as poly(lactic-co-glycolic acid) in lactic and glycolic acids, takes months; therefore, their accumulation can lead to disturbances in the microenvironmental pH [55][199]. Given this knowledge, it is urgent to determine the polymer degradation rate and long-term safety of polymeric drug delivery systems intended for clinical use.
2.2. Exosome Coatings
Milk exosomes are another promising strategy once they can take on a nanosize and can be easily customized depending on what they encapsulate [56][200]. Moreover, they are non-toxic, present a reduced immune response, and can confer protection on circulating genetic material; like other nanoencapsulation coatings, they feature remarkable biocompatibility and tumor targeting [57][201]. In addition, due to their high stability at lower pH values, milk exosomes can be used as carriers for oral drug delivery, enabling a wide range of preventive and therapeutic applications [20][174]. Recently, Barkallah et al. [56][200] demonstrated that exosomes loaded with 10 µg/mL delphinidin are non-toxic to human aortic endothelial cells, and can correct their nitric oxide levels and reduce angiogenesis, after 1 day of treatment. Moreover, Aqil et al. [57][201] have previously shown that exosomes in combination with anthocyanins at a concentration of 75 µM increase the potential of these phenolics to arrest the growth of A2780, A2780/CP70, OVCA432, and OVCA433 ovarian cancer cells, decrease the P-glycoprotein expression, and reduce the effective cisplatin concentration required to inhibit cisplatin-resistant ovarian cancer cells, after 3 days of treatment. In addition, exosomes composed of anthocyanins from blueberries also showed higher antiproliferative effects than the unencapsulated anthocyanins on colon cancer cell lines HCT-116 and HT-29 at concentrations between 25 and 200 µM, after 24 h of treatment [58][202], and on human lung cells A549 and H1299, breast cells MDA-MB-231 and MCF7, pancreatic cells PANC1 and Mia PaCa2, prostate cells PC3 and DU145, colon cells HCT-116, and ovarian cells OVCA432, at concentrations ranging from 20 to 100 µM, after 72 h of treatment [20][174].
However, it is important to take into account that exosomes present a limited transfection efficiency.
2.3. Nanolipid Coatings
The nanoencapsulation of anthocyanins with lipids has also been extensively studied owing to their great biocompatibility, small size, high surface-to-volume ratio, and low fusibility [23][177]. In addition, their synthesis does not require the use of many solvents or aggregation with other nano delivery systems and can be manufactured on a large scale with high reproducibility and provide remarkable protection against enzymatic degradation, allowing the creation of a complex with unique physicochemical properties that can deliver the therapeutic molecule directly to target tissues or cells, minimizing its concentration and frequency of treatment [47][192]. The lipid chosen to coat the active molecules depends on many factors, such as the type of bioactive molecules, cosurfactants and surfactants, lipids, and cryoprotectants, as well as the target of the loaded bioactive molecule(s). Nowadays, many studies focus on the nasal delivery of drugs and phytochemicals, e.g., for the treatment of Alzheimer’s disease. Nanoencapsulation of anthocyanins from elderberries, at a concentration of 0.5 mg/mL, with lipids showed a remarkable ability to modulate mitochondrial functionality, by enhancing complexes I and II of the mitochondrial respiratory chain and preserving the mitochondrial membrane potential in the presence of rotenone thanks to their ability to target mitochondria and protect these cells from rotenone and glutamate-induced toxicity [47][192].
Recently, lipid-derived nano-niosomes systems have also attracted considerable attention because they increase chemical stability and are economical and practical [42][5]. Anthocyanins encapsulated in niosomes, from black carrots, at concentrations of 6.25 and 100 µg/mL, showed the ability to be released after 10 h and decreased the viability of neuroblastoma cells Neuro 2A, after 2 days of treatment [59][203], while anthocyanins extracted from Zea mays and Clitoria ternatea (2–2000 µg/mL) showed a remarkable ability to increase collagen production in human gingival fibroblasts and permeate through the esophageal mucosa [7][163]. In addition, anthocyanin-added nano-niosomes (1%, 2%, 5%, and 10%, v/v) were reported to be non-toxic to normal HT22 hippocampal neuronal cells after 1 day of exposure, and fully internalized in BV2 microglial cells [60][204].
However, one of the major concerns of using lipids as nanocarriers is their cytotoxic potential due to their ability to create non-specific uptake [47][192].
2.4. Nano-Polysaccharide Coatings
Certain types of polysaccharides are also used to encapsulate anthocyanins, as some of them have a charged residue and can therefore be easily linked to the opposite charge of the phospholipid residue, thus preventing phospholipid hydrolysis in the presence of enzymes and under acidic pH conditions [35][61][13,205]. Among them, chitosan and pectin are the most commonly used because both are non-toxic, environmentally friendly, biodegradable, and have high biocompatibility [36][28]. In addition to their potential advantages, they appear to be a promising, effective drug delivery system to the colon. In in vitro studies, nanoencapsulation of anthocyanins at concentrations of 5, 10, 20, and 40 µg/mL with pectin and chitosan showed greater potential to protect normal rat kidney (NRK) cells from acrylamide-induced damage than non-encapsulated anthocyanins and allowed their controlled release via the gastrointestinal tract [49][9]. They decrease reactive oxygen species, matrix metalloproteinases, and glutathione levels and provide protection to normal human hepatocyte L02 cells from palmitic acid-induced damage at concentrations ranging from 1.6 to 8.0 µM after 24 h of exposure [22][176]. Nevertheless, it is important to emphasize that chitosan dissolves only in certain dilute acidic solutions. Chondroitin sulfate is another promising alternative, as it is able to enhance the ability of black soybean anthocyanins at concentrations of 4, 7.5, and 15 µM to scavenge reactive oxygen species in human colon cells HCT-116, after 2 days of treatment [62][206] and to suppress human cervical cancer HeLa cells more effectively compared to anthocyanins alone, both at 100 µM after 1 day [24][20]. The combination of chitosan and chondroitin sulfate-loaded black rice anthocyanins at concentrations of 10 and 50 µg/mL also showed remarkable effects on human colon cancer cells HCT-116 by promoting negative changes in mitochondria and, consequently, apoptosis after four hours of exposure [63][207]. Finally, hyaluronic acid, a natural polysaccharide, together with 20 µg/mL cornflower anthocyanins, can enhance CD44+ apoptosis in colon cancer cells HT29, after 1 h of treatment [64][208].
Despite this, the employment of polysaccharide coatings shows some drawbacks, such as their cost, undesirable aggregation, and burst release promoted by their small particle size and large surface [65][209].
2.5. Liposome Coatings
Nanoencapsulation of anthocyanins with liposomes has also been investigated, with most studies having shown that they can be used as nanocarriers for drug delivery due to their self-assembling and amphiphilic properties, flexibility, biocompatibility, biodegradability, longer duration of circulation, and low immunogenicity [33][183]. In addition, since they are composed of an aqueous core and a spherical hydrophobic shell, they can transport hydrophilic, hydrophobic, and amphiphilic molecules, as well as easily cross the blood–brain barrier [17][173]. Regarding their encapsulation with anthocyanins, Homayoonfal et al. [25][178] showed that they can enhance the antioxidant capacity of anthocyanins in a very remarkable and effective manner, as well as increase the metabolite activity and replication of human mesenchymal MSC and fibroblast FBL stem cells, at concentrations of 0.5 mg/mL and 10.4 µg/mL, respectively, after 1 week of treatment. Moreover, it has been also reported that there is a positive correlation between the size of nanoliposome particles and the encapsulation efficiency [5][161].
Nevertheless, it is important to note that liposomes can trigger undesirable immune responses, present a moderate loading capacity, and possibly crystallize after prolonged storage times [65][209].
2.6. Halloysite Nanotubes Coatings
The use of halloysite nanotubes is also increasing worldwide, due to their unique properties that include particle size, high surface area, and surface-to-volume ratio, and very high dispersion rate [48][193]. Regarding anthocyanins, they have shown to be effective in suppressing the growth of human breast cancer MCF-7 and human colon cancer HT-29 at a concentration of 500 µg/mL compared to non-encapsulated anthocyanins, after one and 2 days of exposure [66][8]. Nonetheless, it is important to consider that halloysite nanotubes are inorganic and present toxicity and poor biocompatibility [48][193].
2.7. Dendrimers Coatings
Dendrimers are another promising alternative, as they can reduce toxicity and improve water solubility, as well as possessing pharmacokinetic properties and biodistribution [65][209]. In addition, dendrimers can also be adjusted in size and shape, in order to favor surface area, loading, and delivery efficiency. They are usually composed of poly(amidoamine), poly(L-lysine), polyamides, polyethers, polyesters, poly(2,2-bis(hydroxyl methyl) propionic acid, and polypropylenimine. Together with anthocyanins, dendrimers can increase, dose-dependently, the cytotoxicity of neuro-2A brain neuroblastoma cell, after 24 h of treatment [50][194].
However, it is important to note that dendrimers present a complex synthetic route and can only carry low amounts of active molecules.
2.8. Nanoemulsions Coatings
Nanoemulsions are another interesting drug delivery system, since their production is economic and they show high loading capacity and efficiency to carrier hydrophobic molecules, as well as a notable capacity to protect bioactive molecules against degradation. In addition, they can be easier manipulated to ameliorate drug release. Nonetheless, their use usually requires conjugation with nonionic surfactants to provide better stability [16][172]. Nonionic surfactants are recommended because they are safer than their ionic equivalents and are usually accepted for oral administration [16][172]. Recently, Nazareth et al. [9][165] reported that nanoemulsions of anthocyanins (400–1050 µg/mL) could be a promising alternative to conventional antibiotics. They demonstrated a remarkable ability to inhibit biofilm formation in Pseudomonas aeruginosa and Yersinia enterocolitica, and anti-quorum sensing activity against Chromobacterium violaceum, in a dose-dependent manner.
Nevertheless, it is important not to forget that some nanoemulsions present some non-compatibility, low viscosity, and can irritate skin.
3. In Vivo Studies
In vivo studies focusing on the employment of anthocyanins in nanocarriers for nano delivery are still scarce. Nonetheless, some up-to-date reports show promising data involving anthocyanins coated with nano-polymeric, dendrimers, carbohydrates, polysaccharides, and nano-niosomes with notable antioxidant, neurological and cardiovascular properties, and anticancer effects with no apparent adverse effects and, thus, with the potential to be marketed, as mentioned below.
3.1. Nano-Polymeric Coatings
However, the use of polymer-based nanocarriers loaded with anthocyanins, at doses of 0.5 and 1 mg/g body weight, has already been reported. In these conditions, anthocyanins had shown the ability to protect fish models exposed to the pesticide cypermethrin from DNA degradation by increasing the penetrability of anthocyanins in liver, kidney, muscle, brain, and ovarian/testicular tissue, and decreasing cytotoxicity by modulating the antioxidant enzymes superoxide dismutase, catalase, and lipid peroxidase, after 7 days of treatment [53][197]. Moreover, complete tumor ablation was verified in MCF-7-bearing nude mice treated with anthocyanins at 250 µg/mL loaded into polymeric nanoparticles, at doses of 0.5 and 1 mg/g body weight, after 26 days of treatment [10][166].
3.2. Dendrimers Coatings
Anthocyanins encapsulated in exosomes (5 mg anthocyanins and 50 mg exosome protein/kg body weight administered orally by gavage five times a week for a period of 3 weeks) also showed a remarkable ability to reduce tumor size in mice transplanted with A549 human lung cancer cells, with no apparent adverse effects [20][174]. In addition, they can also reduce the proliferation of colorectal cancer in ApcMin/+ mice inoculated with enterotoxigenic Bacteroides fragilis bacteria and treated for 4 weeks, 3 days per week, with a dose of 8.6 mg/kg/day, by increasing the activity phase II enzymes glutathione S-transferase Mu 1 and uridine 5′-diphospho-glucuronosyltransferase family 1 member A6 and decreasing the expression of aryl hydrocarbon receptor nuclear translocator 1, aryl hydrocarbon receptor, and cytochrome P450 family 1 subfamily A member 1 [58][202].
3.3. Carbohydrate Coatings
Carbohydrate-based derivatives, especially chitosan, have been intensively studied, mainly due to their high loading efficiency, water solubility, and biocompatibility [67][29]. For example, Chatterjee et al. [21][175] reported that the use of anthocyanins loaded with chitosan (at a dose of 600 mg/kg body weight/day) from black carrot resulted in the remarkable ability to increase the activity of superoxide dismutase and catalase enzymes in rats. In addition, they can also enhance the potential of cyanidin 3-O-glucoside (5 mL/20 g body weight) to attenuate the oxidative stress induced by selenite sodium in rats treated four times daily for 1 week, enhance the transepithelial transport of liposomes to a depth of 40 mm in the cornea of rats, prolong the residence time on the cornea, and improve permeability in the corneal epithelium, thus increasing superoxide dismutase and catalase activity and decreasing glutathione activity and lipid peroxidation in the lens [61][205]. Moreover, they can also confer to this anthocyanin a higher ability to balance the B-cell lymphoma-2/leukemia-2 ratio and reduce UVB-induced epidermal damage, by decreasing lipid peroxidation, malondialdehyde, and 8-hydroxy-2′-deoxyguanosine and increasing visual and histological appearance and skin hydration, after 1 day of treatment [68][210]. In addition, Zhao et al. [49][9] reported that the use of chitosan combined with pectin can provide the ability of blueberry anthocyanins, at a concentration of 320 µg/mL, to increase the lifespan and reproductive capacity and improve the flexible locomotion behavior of Caenorhabditis elegans from damage induced by acrylamide, heat shock, oxidative stress, and ultraviolet light, and reduce ageing effects.
However, one of the major drawbacks of using carbohydrates as nanocarriers is related to their toxicity.
3.4. Polysaccharide Coatings
On the other hand, the use of polysaccharides nanocarried with anthocyanins from black soybean for 5 days showed efficacy in reducing the tumor size of mice previously injected with human cancer cells HCT-116, increasing the number of apoptotic cells, and also contributing to the release of drugs at target sites [62][206]. In particular, cornflower anthocyanins loaded into the hyaluronic acid were found to be highly effective in increasing myelosuppression in mice transplanted with human colon cancer cells HT29 and treated for 13 days [64][208]. In addition, Hanafy [69][14] reported that encapsulation of these phytochemicals with bovine serum albumin can ameliorate cardiomyopathy, completely remove glycogen from tissues, and reduce malondialdehyde and collagen fibers in a fibrotic mouse model treated twice a week for 4 weeks.
3.5. Nano-Niosomes Coatings
Nano-niosomes are another promising strategy, mainly thanks to their non-toxicity, greater stability, biocompatibility, capacity of penetration, and potential to control and target bioactive molecule(s) delivery [59][203]. So far, their encapsulation with anthocyanins has already shown remarkable healing activity in oral cavity wounds of rats [7][163]; the ability to improve insulin resistance and glucose intolerance, by increasing the anti-hyperglycemic/anti-diabetic ratios with no apparent toxicity; and lowering animal weight and plasma levels of insulin, glucose, leptin, and total cholesterol in obese mice at 300 mg/kg of body weight [1][4]. Moreover, nanoencapsulation of anthocyanins with nano-niosomes showed pronounced neuroprotection. For example, nano-niosomes can give anthocyanins a remarkable ability to cross the blood–brain barrier, reach it, and accumulate in mice with Alzheimer’s disease treated for 14 days, thus preventing tau hyperphosphorylation and reducing the expression of amyloid beta, beta-site amyloid precursor protein cleaving enzyme 1, and advanced glycation end products receptor, and neuroinflammatory and oxidative stress markers via the GSK-3β/CDK5 pathway [60][204]. Moreover, in the Aβ1–42 mouse model of Alzheimer’s disease, they may enhance the ability of anthocyanins (12 µg/g) to ameliorate memory impairment, as well as protect their pre- and postsynaptic proteins from Aβ1–42-induced synaptic dysfunction, regulate the p-PI3K/p-Akt/p-GSK3β pathway, prevent the hyperphosphorylation of tau protein at serines 413 and 404, and inhibit apoptosis during 14 days of treatment [70][211]. Although they seem to be a hopeful strategy, it is still difficult to find an appropriate concentration able to balance the relationship between the entrapment efficiency and the affinity of the cell membrane.
