Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Soo-Jin Park and Version 2 by Camila Xu.
Supercapacitors (SCs) have attracted considerable attention among various energy storage devices due to their high specific capacity, high power density, long cycle life, economic efficiency, environmental friendliness, high safety, and fast charge/discharge rates. SCs are devices that can store large amounts of electrical energy and release it quickly, making them ideal for use in a wide range of applications. They are often used in conjunction with batteries to provide a power boost when needed and can also be used as a standalone power source. They can be used in various potential applications, such as portable equipment, smart electronic systems, electric vehicles, and grid energy storage systems.
- carbon-based materials
- metal oxides
- conductive polymers
1. Introduction
In recent years, the world has experienced an increase in development, leading to energy shortages and global warming. These problems have underscored the need for supercapacitors as green energy storage devices. Supercapacitors can store large amounts of energy and deliver excellent power, making them ideal for various applications. Supercapacitors are an increasingly attractive option in the race to develop new and improved energy storage technologies due to their high-power density and long cycle life. As the supercapacitor market grows, so does the need for improved fabrication processes and electrode materials. Supercapacitors have several advantages over other energy storage devices. They can charge and discharge quickly, making them well-suited for various applications. In addition, supercapacitors are environmentally friendly and have a long lifetime. Supercapacitors are expected to grow in the coming years as the world looks for ways to address energy shortages and global warming.
Identifying clean and renewable new energy sources and developing efficient energy storage technologies and devices for low-carbon and sustainable economic development have become important [1][2][3][4][1,2,3,4]. Common electrochemical energy storage and conversion systems include batteries, capacitors, and supercapacitors [5]. The three energy storage systems complement each other in practical applications and meet different needs in different situations. Although the three systems have different energy storage and conversion mechanisms, they are all based on similar electrochemical thermodynamics and kinetics, i.e., the process of supplying energy occurs at the phase boundary of the electrode/electrolyte interface with independent electron and ion transport [6]. Recent advances in smart electronic devices have spurred a corresponding increase in the use of supercapacitors.
A supercapacitor is a promising energy storage device between a traditional physical capacitor and a battery. Based on the differences in energy storage models and structures, supercapacitors are generally divided into three categories: electrochemical double-layer capacitors (EDLCs), redox electrochemical capacitors (pseudocapacitors), and hybrid capacitors (Figure 1) [7]. Figure 1 summarizes the basic energy storage principles of supercapacitors with the classification as the basic framework and examines the research progress of electrode materials commonly used in recent years.
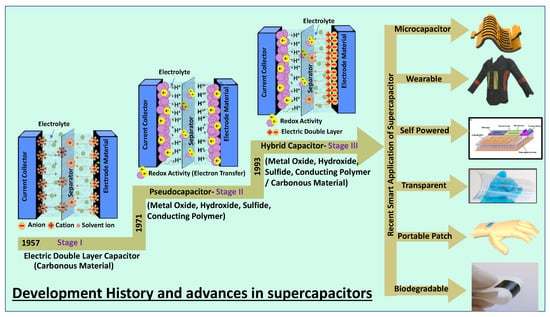
Figure 1.
Classification of supercapacitors based on various electrode materials and their advanced applications.
Supercapacitors are being researched extensively in smart electronics applications such as flexible, biodegradable, transparent, wearable, flexible, on-chip, and portable energy storage. In comparison with conventional capacitors, supercapacitors use materials with a high specific surface area as electrodes [8][9][8,9]. A higher specific surface area and thinner dielectrics result in greater specific capacitance and energy density. In comparison with the rated capacitance of traditional capacitors in the range between micro and milli- Farads, the capacitance of a supercapacitor unit can reach thousands of Farads. In contrast with batteries, the charge storage mechanism of supercapacitors is based on the surface reaction of the electrode material, and there is no diffusion of ions inside the material. Therefore, supercapacitors have a better power density under the same volume. Another electrochemical characteristic that is different between supercapacitors and batteries is that the charge on the electrodes of a typical supercapacitor always increases (or decreases) linearly, resulting in voltage rise (or fall) during the charge and discharge process.
As shown in Figure 2, during the charge and discharge process, the cyclic voltammetry (CV) curve of the supercapacitor (Figure 2a) remains rectangular, whereas the current is almost constant. In addition, its galvanostatic charge-discharge (GCD) curve (Figure 2c) is usually inclined with a constant slope. A battery exhibits Faradaic reactions during the charge and discharge process, and its CV curve shows a clear redox peak; it maintains a constant voltage except when it is near 100% charged/discharged (TOC/EOD) (the GCD curve shows a relatively flat charge-discharge platform).
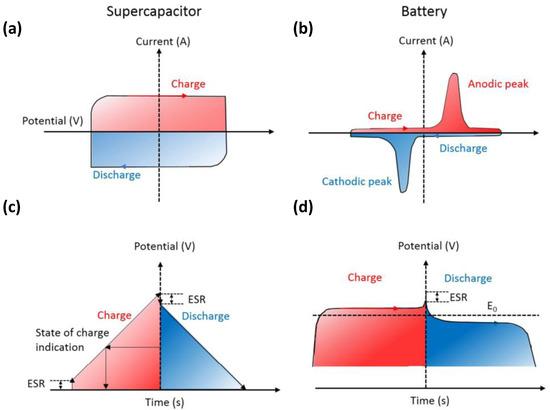
Figure 2.
(
a
,
b
) cyclic voltammetry (CV) curves and (
c
,
d) galvanostatic charge-discharge (GCD) curves of supercapacitors and batteries. Reproduced with permission [10] Copyright © 2022, Chemical Reviews.
) galvanostatic charge-discharge (GCD) curves of supercapacitors and batteries. Reproduced with permission [10] Copyright © 2022, Chemical Reviews.
Supercapacitors have many other advantages, such as being environmentally friendly, having a long service life, being able to operate in wide temperature ranges, and being good at retaining charge even when large currents are applied; they are widely used in consumer electronics, smart meters, and transport [7][8][9][10][7,8,9,10]. Supercapacitors have shown that they can perform very well in some applications, but there are still several shortcomings that are relevant in some applications. These include high energy density requirements and very long working times. Notably, the areas in which supercapacitors can store charge are limited to the surfaces (or near the active surface area) of the electrodes; they have lower energy density than batteries.
2. Ceramic-Based Hybrid Supercapacitors
Most metal oxides used in pseudocapacitors are often hindered by the severe aggregation of nanoparticles, low electron–proton transport, and weak conductivity between nanoparticles, leading to a lower specific capacitance in practical applications. In order to effectively address these problems, the development of hybrid electrodes by combining metal oxides with carbon materials with a high specific surface area, such as activated carbon, graphene, CNTs, and carbon aerogels, has attracted widespread attention. Various metal oxide/carbon material composite electrodes for supercapacitors are summarized in Table 1 [11][12][13][14][15][16][17][18][19][20][21][22][23][24][25][26][27][28][29][30][31]2 [181,182,183,184,185,186,187,188,189,190,191,192,193,194,195,196,197,198,199,200,201].Table 12.
Summary of various metal oxide/carbon composite electrodes for supercapacitors.
| Material | Potential Window/V | Electrolyte | Specific Capacitance/F g−1 (Scan Rate or Current Density) |
Retention/% (Cycles) |
Ref. |
|---|---|---|---|---|---|
| ZrO2 carbon nanofibers | 0–1 | 6 M KOH | 140 (1 A g−1) | 82.6 (10,000) | [32][175] |
| RuNi2O4/rGO composites | 0–1 | 0.5 M Na2SO4 | 792 (1 A g−1) | 93 (10,000) | [33][176] |
| NiO/activated carbon composites | 0–0.4 | 2 M KOH | 568.7 (0.5 A g−1) | 90.6 (5000) | [34][177] |
| Ni0.25Co0.25oxide/carbon nanofibers | −1–0 | 6 M KOH | 431.2 (1 A g−1) | 94 (2000) | [35][178] |
| MnO/Fe2O3/carbon nanofibers | 0–1 | 6 M KOH | 437 (1 A g−1) | 94 (10,000) | [36][179] |
| ZnO/MnO/carbon nanofibers | 0–1.6 | 6 M KOH | 1080 (1 A g−1) | 96 (800) | [37][180] |
| Au-Mn3O4/GO nanocomposites | −0.2–1 | 0.5 M H2SO4 | 475 (1 A g−1) | 94 (10,000) | [11][181] |
| Bi2O3/MWCNT composites | −1.2–0.2 | 6 M KOH | 437 (1 A g−1) | 88.7 (3000) | [12][182] |
| NiO/MnO2/MWCNT composites | 0–0.55 | 2 M KOH | 1320(1 A g−1) | 93.5 (3000) | [13][183] |
| Carbon nanosheets/MnO2/NiCo2O4 composites | 0–1 | 1 M KOH | 1254 (1 A g−1) | 81.9 (5000) | [14][184] |
| ZrO2/C nanocomposites | 0–1 | 1 M H2SO4 | 214 (1.5 A g−1) | 97 (2000) | [15][185] |
| NiO/porous amorphous carbon nanostructure | 0–1.6 | 6 M KOH | 508 (1 A g−1) | 78 (3000) | [16][186] |
| Defective mesoporous carbon/MnO2 nanocomposites | −0.8–0.8 | 1 M Na2SO4 | 292 (0.5 A g−1) | 79 (2000) | [17][187] |
| Activated carbon/MWCNT/ZnFe2O4 composites | −0.1–0.6 | 3 M KOH | 609 (1 A g−1) | 91 (10,000) | [18][188] |
| NiO/C@CNF composites | −0.1–0.5 | 3 M KOH | 742.2 (1 A g−1) | 88 (5000) | [19][189] |
| N-doped carbon quantum dots/Co3O4 nanocomposites | −0.4–0.6 | 6 M KOH | 1867 (1 A g−1) | 96 (500) | [20][190] |
| Mn3O4/Fe3O4@Carbon composites | −0.4–1.2 | 1 M NaCl | 178 (1 A g−1) | 95 (1000) | [21][191] |
| rGO/CNTs/MnO2 composites/// | 0–1.8 | 1 M Na2SO4 | 332.5 (0.5 A g−1) | 89.2 (10,000) | [22][192] |
| (HPC)/polyaniline (PANI) nanowire | 0–1.8 | 1 M H2SO4 | 1080 (1 A g−1) | 91.6 (5000) | [23][193] |
| Cyclodextrin polymer-functionalized polyaniline (CDP)/porous carbon composites(PC) | −0.2–0.8 | 6 M KOH | 437 (0.1 A g−1) | 81 (5000) | [24][194] |
| MnO2/Graphene Oxide/Polyaniline composites | 0–1 | 1 M Na2SO4 | 512 (0.25 A g−1) | 97 (5100) | [25][195] |
