Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Leila Elyasi.
Picein is one of the herbal agents that has been investigated in only a few studies. Picein is the active ingredient of several herbs and can be thus extracted from different types of herbs, which makes it more available. It has shown to have anti-inflammatory properties in cellular and plant studies.
- neurodegenerative diseases
- neuroprotective agents
- picein
1. Background
Neurodegenerative diseases (NDDs) are one of the main concerns of modern medicine and are responsible for the majority of the 50 million cases of dementia worldwide [1]. NDDs are progressive diseases that result in a great disease burden and as most NDDs are age-related diseases [2], their incidence and disease burden are expected to increase annually due to an aging population and triple by 2050 [3]. Alzheimer’s disease (AD) is the most commonly known NDD, although there are also other types of NDDs. These include, but are not limited to, Parkinson’s disease (PD), multiple sclerosis (MS), amyotrophic lateral sclerosis (ALS), and Huntington’s disease [4]. Despite the different clinical, laboratory, and neuroimaging features, as well as the neuropathology and management of NDDs, they all have the common feature of neurodegeneration (gradual loss of specific neuronal populations), which makes them progressive diseases that result in the death of patients after several years of suffering (for the patient as well as their caregivers). Despite advances in medicine, there is still no cure for NDDs and available medications can only relieve the patient’s symptoms and improve brain function deficits (memory, movement, and cognition). Sometimes the treatments are inefficient due to the multiplicity and complexity of the patient’s symptoms [5,6][5][6]. Therefore, disease-modifying treatments, which can efficiently slow down or halt disease progression, have been identified as the main treatment goal in NDDs, for which several trials have been performed, although the progression rate seems slow [7,8][7][8]. Most recent research has focused on neuroprotective agents to target the pathophysiology of NDDs [9].
Considering the unmet need for effective curative treatment of NDDs, in thires paper, we earchers provide a short review of the bioactive compounds and herbal agents with potentially neuroprotective properties that have been examined in preclinical and clinical studies of NDDs with the main focus on an agent that has been scarcely evaluated, Picein.
2. Antioxidative and Neuroprotective Properties of Picein
Picein (PubChem ID: 92123) is a phenolic (non-salicylic) glycoside extracted from different plant species with the formula C14H18O7 [73][10]. In our previous study, we researchers showed the anti-inflammatory and neurodegenerative properties of NB [72][11]. However, the literature is scant and not centered in this regard. Below, wresearchers present the herbs from which picein can be extracted and evaluate their possible antioxidative, anti-inflammatory, and neuroprotective properties. Figure 1 shows a summary of the plants from which picein is extracted.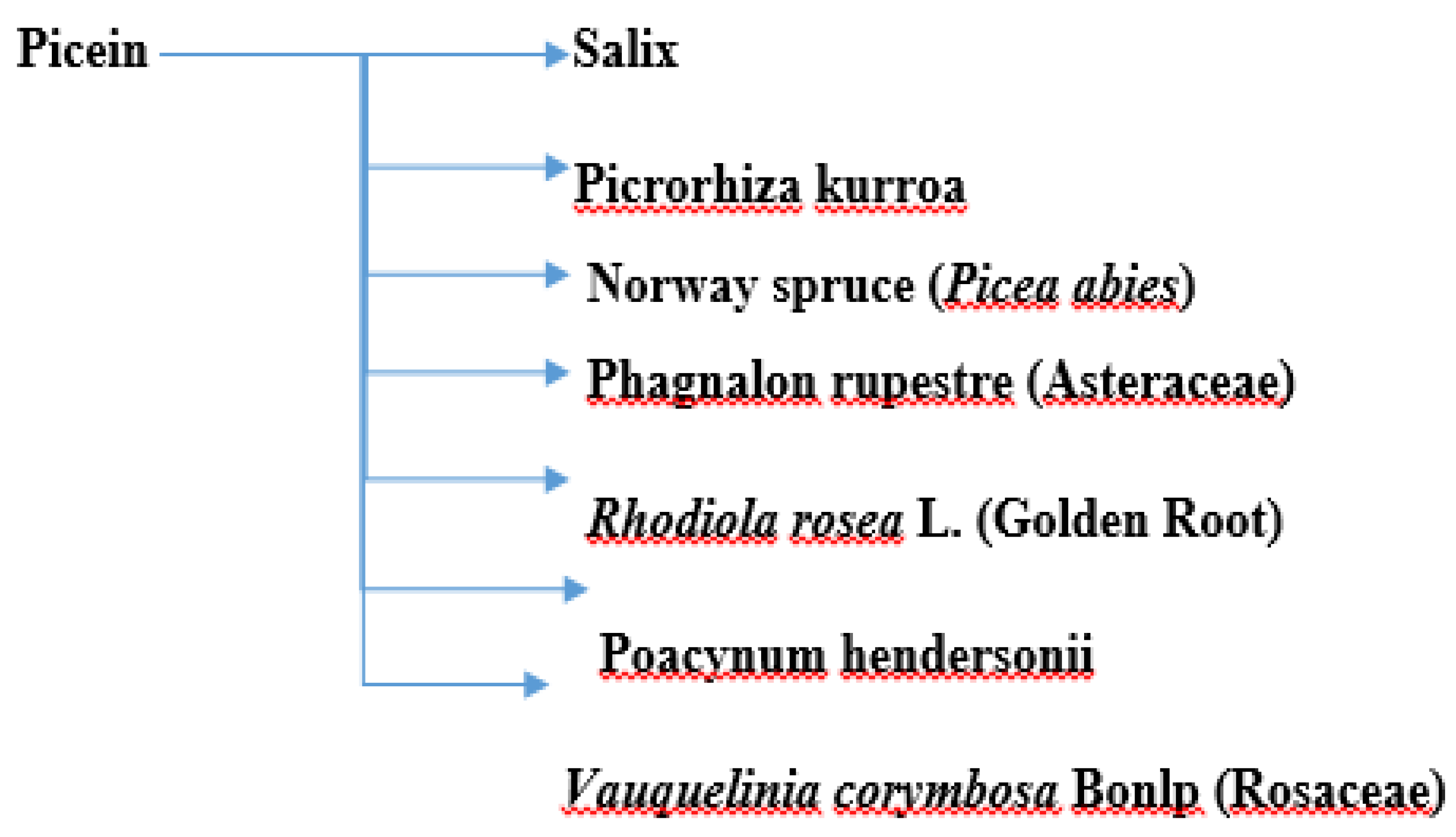
Figure 1. A summary of plants containing picein.
2.1. Willow (Salix)
One of the earliest records of treating inflammation and pain with herbal plants refers to the extracts of willow, Salix Sp., which belongs to the Salicaceae family. Analgesic, anti-inflammatory, antioxidative, anticancer, antidiabetic, antimicrobial, anti-obesity, antimigraine, cytotoxic, hepatoprotective, and neuroprotective activities have been documented for the Salix genus [74,75,76][12][13][14]. Salix is an aspirin precursor for which salicylic acid is extracted from water that is extracted from willow bark and leaves. It has also been used by animals and humans for its analgesic, antipyretic, and anti-inflammatory properties [77,78][15][16]. The antioxidative activity of Salix Sp. has also been identified and is mainly attributed to salicin [79][17], the mechanism of which is said to be the downregulation of the inflammatory mediators, tumor necrosis factor-α, and nuclear factor-kappa B [80][18]. Besides salicin, flavonoid and other phenolic compounds of Salix have also been identified to have anti-inflammatory properties and synergistic effects with coffee against scavenging free radicals and the inhibition of lipid peroxidation [81,82][19][20]. It is worth mentioning that Salix comprises several species such as S. purpurea, S. daphnoides clone 1095, S. alba clone 1100, S. triandra, S. viminalis, and S. herbacea. Each of these contains different concentrations of salicin, different compositions of phenols, and other compounds, for instance, acetylsalicortin was only found in S. alba [83][21]. Furthermore, an evaluation of 91 genotypes of the common species Salix purpurea showed that all were rich in salicylic glycosides (salicin, salicortin and tremulacin, flavanones, maringenin, chalcone isosalipurposide, and catechin), whereas picein and populin were only identified in 10% of them [84][22]. Heiska et al. extracted 1.61–31.08 mg/g d.m. from willow bark (Salix myrsinifolia) [85][23]. However, the comparison of the S. daphnoides and S. purpurea extracts showed the possible presence of picein in the former preparation [86][24]. The extracted compounds and their concentrations may not only vary based on the Salix genotype but also on the analytical (such as spectroscopy or chromatography) and extraction methods, as well as the plant part used, the age of the Salix, and the seasonal changes in their concentrations [87,88,89,90][25][26][27][28]. Dou et al. extracted picein from a hot water extract (HWE) of willow bark by dissolving the freeze-dried HWE (5.2 mg) in pyridine (10 mL). Then, 0.7 mL of this solution and 0.1 mL xylitol (0.1 mg/mL) were treated with 0.2 mL BSTFA at 50 °C for 40 minutes, which resulted in a relative content ratio of 7.60 and a response factor of 3.04 [91][29]. The neuroprotective effect of Salix has been shown in previous studies [74][12]; however, wresearchers could not find any study other than one by members of ourthe team that directly addressed the neuroprotective effect of the picein compound of Salix [72][11]. In tResearchis study, we sers showed that treatment of NB SH-SY5Y cells with picein decreased the menadione-induced ROS levels and recovered the mitochondrial activity to normal, indicating the potential of picein to be used as a neuroprotective agent (Figure 2). More details on this will be discussed in Section 5.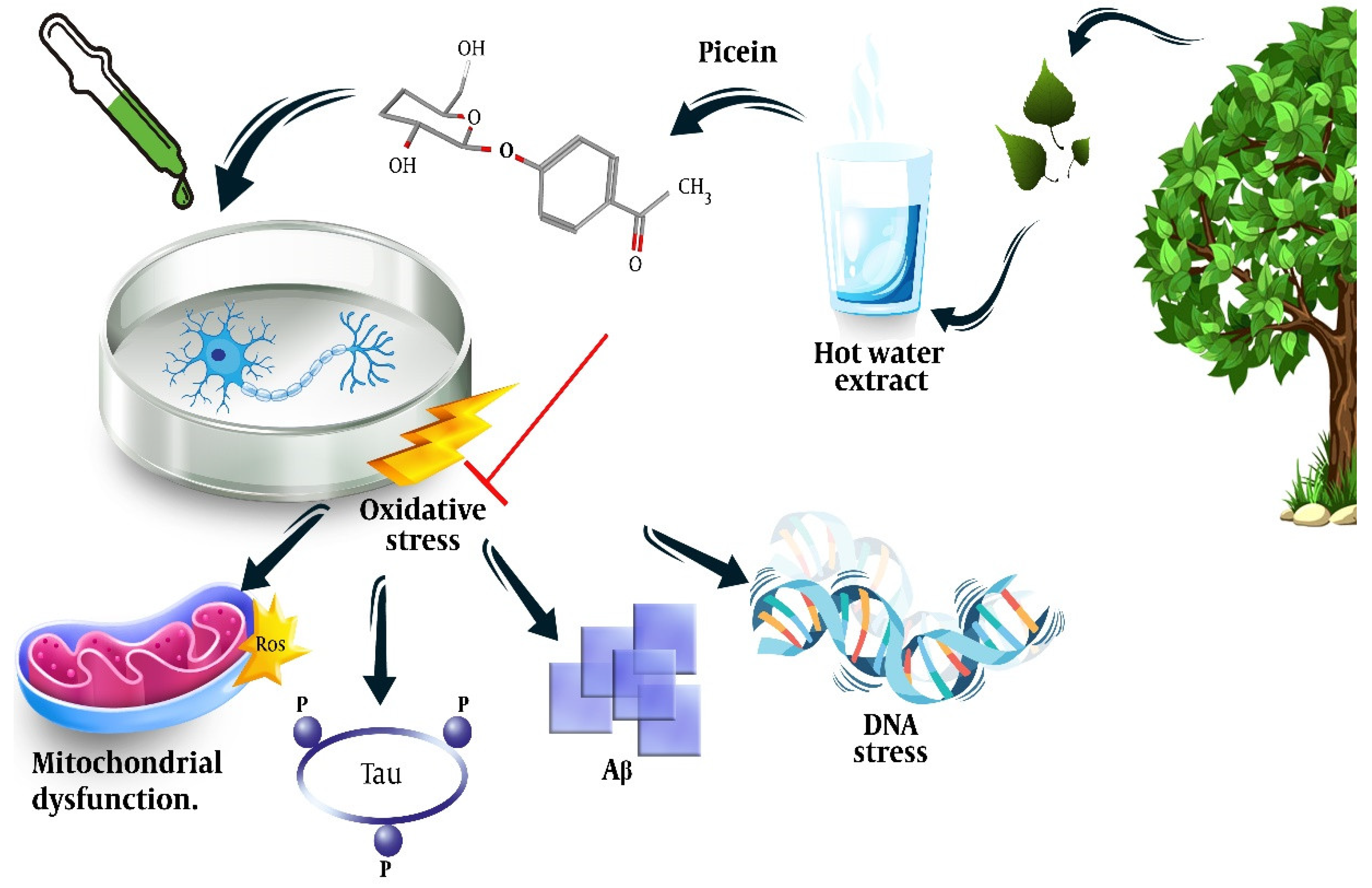
2.2. Picrorhiza kurroa
Picrorhiza kurroa, also known as Kutki, belongs to the Scrophulariaceae family and is a well-known herb in Ayurvedic medicine that is used for asthma, jaundice, fever, malaria, snake bite, and liver disorders, and is suggested to have antimicrobial, antioxidant, antibacterial, antimutagenic, cardioprotective, hepatoprotective, antimalarial, antidiabetic, anti-inflammatory, anticancer, anti-ulcer and nephroprotective properties [96,97][35][36]. A study by Morikawa et al. showed that picein extracted from the rhizomes of Picrorhiza kurroa had favorable results on the promotion of collagen synthesis (at 10–30 μM) without cytotoxicity observed at effective concentrations [98][37]. Studying the antioxidative activity of picein extracted as one of the two phenol glycosides of this herb identified no such effect for picein, whereas the other compound of these leaves, luteolin-5-O-glucopyranoside, showed significant antioxidative properties [99][38]. WResearchers believe that evidence is scant about the effects of picein extracted from this plant and further studies are required in this regard.2.3. Norway Spruce (Picea abies)
Picein and its aglucone piceol (4-hydroxy acetophenone) are phenolic compounds found in different parts of Norway spruces (Picea abies) [100][39]. In Norway spruce, picein contained 0.09–0.2% of the dry weight of non-mycorrhizal short roots [101][40] and 1.8–2.2% of dry weight in the spruce needles (which contained a 0.4–1.1% piceol concentration) [102,103][41][42]. Picein is considered the precursor of piceol and the effects observed from this phenolic compound are mainly through piceol release. Picein has also been suggested as an indicator of plant stress [104,105][43][44]. Ganthaler and colleagues evaluated the concentrations of different compounds in Norway spruce forest trees and showed that picein and stilbene concentrations were associated with the tree’s defensive power against fungal pathogens [106][45] and budworm [107][46]. Bahneweg and colleagues also showed increased picein in response to the fungal provenance of Norway spruce by Sirococcus conigenus [108][47]. The antimicrobial activity of picein against both Gram-positive and Gram-negative bacteria (Staphylococcus aureus, S. epidermidis, S. typhimurium, Escherichia coli, Bacillus cereus, Klebsiella pneumoniae, Enterococcus faecalis, and Pseudomonas aeruginosa) has also been detected, with minimum inhibitory concentration values of 16–64 mg/L [109][48]. Another similar tree is White spruce (Picea glauca), which also contains (the acetophenone glucoside) picein (the second most abundant metabolite with averages exceeding 10% of the foliar dry weight) that exerts favorable results against spruce budworm (Choristoneura fumiferana), a highly damaging forest insect pest [110][49]. Although the above-mentioned studies have confirmed the favorable effects of picein and piceol on the protection of Norway and White spruces against tree harm, the picein extracted from these plants has not been examined in cellular or animal studies to indicate the efficacy of this compound on other cells.2.4. Other Plants
In addition to spruce, picein has been mentioned as a phenolic compound of several other plants, for instance, the aerial parts of Phagnalon rupestre (Asteraceae) [111][50], Ebenus pinnata [112][51], and Rhodiola rosea L. (Golden Root) [113,114][52][53]. However, the properties of this compound have not been investigated. OurThe literature search also found a few cellular studies on the random effects of plant-derived picein. Morikawa and colleagues showed that picein and several other compounds extracted from the flowers of Poacynum hendersonii could moderately promote adipogenesis of 3T3-L1 cells (a cell line derived from mice) [115][54]. Picein extracted from the aerial parts of Vauquelinia corymbosa Bonlp (Rosaceae) has shown favorable enzymatic activity against yeast and rat small intestinal α-glucosidases, which shows this compound is a potential source of the α-glucosidase inhibitor and is suitable for the development of new antidiabetic drugs [116][55]. Lai and colleagues have also shown that the guinea pig liver cytosolic beta-glucosidase can hydrolyze the plant glucoside, L-Picein, and its product, piceol [117][56], which suggests an ability to use this compound as an oral agent for this animal. However, further studies are required in this regard.3. The Potential Role of Picein in the Treatment of Alzheimer’s Disease
As described in Section 2, Aβ42 peptide and NFT, composed of hyperphosphorylated Tau protein, are the two major neuropathological hallmarks of AD. Aβ aggregation is regarded as the precursor for the neurotoxicity of AD, which leads to increased assembly of the toxic and plaque-promoting Aβ42 peptide [118][57]. Enzymes, including BACE1 (a transmembrane aspartic acid protease) and γ-secretase, are involved in the generation of the Aβ peptide by APP cleavage [119,120][58][59] and their activity is directly associated with Aβ formation. Accordingly, pharmaceutical companies have performed and continue to perform clinical trials on the potential of the BACE1 inhibitors (MK8931, AZD-3293, JNJ-54861911, E2609, and CNP520) for the treatment of AD and downregulation of Aβ levels [120,121,122][59][60][61]. However, the initially promising BACE1 inhibitors failed in later stages of clinical trials because of the side effects, insufficient potency, or poor pharmacokinetics [123,124][62][63]. Further studies on novel classes of BACE1 inhibitors are being performed to investigate their potential as novel candidates for AD treatment [125][64]. In a previously mentioned study [72][11], wresearchers investigated and reported on in silico and in vitro studies of picein. The results of the in-silico studies suggested BACE1 as a possible molecular target for picein. In addition, it was found to be structurally very similar to another molecule (gastrodin) that has shown to play a role in the reduction of memory deficit and neuropathology in a mouse model of AD [126][65]. Additionally, our the in vitro studies demonstrated the potent neuroprotectant role of picein by neutralizing ROS generated by a neurotoxin [72][11]. These encouraging preliminary results strongly indicate that picein may have well-desired dual features, offering both a potential BACE1 inhibitory function and a potent neuroprotective effect. In the in silico studies, wresearchers used a pharmacophore mapping approach to predict the putative biomolecular targets of picein in silico with the PharmMapper web server [127][66]. BACE1 was predicted as the major molecular target of picein and molecular docking of picein to the BACE1 active site was successfully performed with AutoDock 4 [128][67]. The predicted binding affinity and Ki value (dissociation constant) of picein at the BACE1 active site were −5.94 kcal/mol and 44.03 µM, respectively, suggesting a modest affinity to the target. Key interactions of the docked pose of picein with the active site residues of BACE1 are shown in Figure 3A. These included seven hydrogen bond interactions as well as several hydrophobic contacts with the surrounding residues.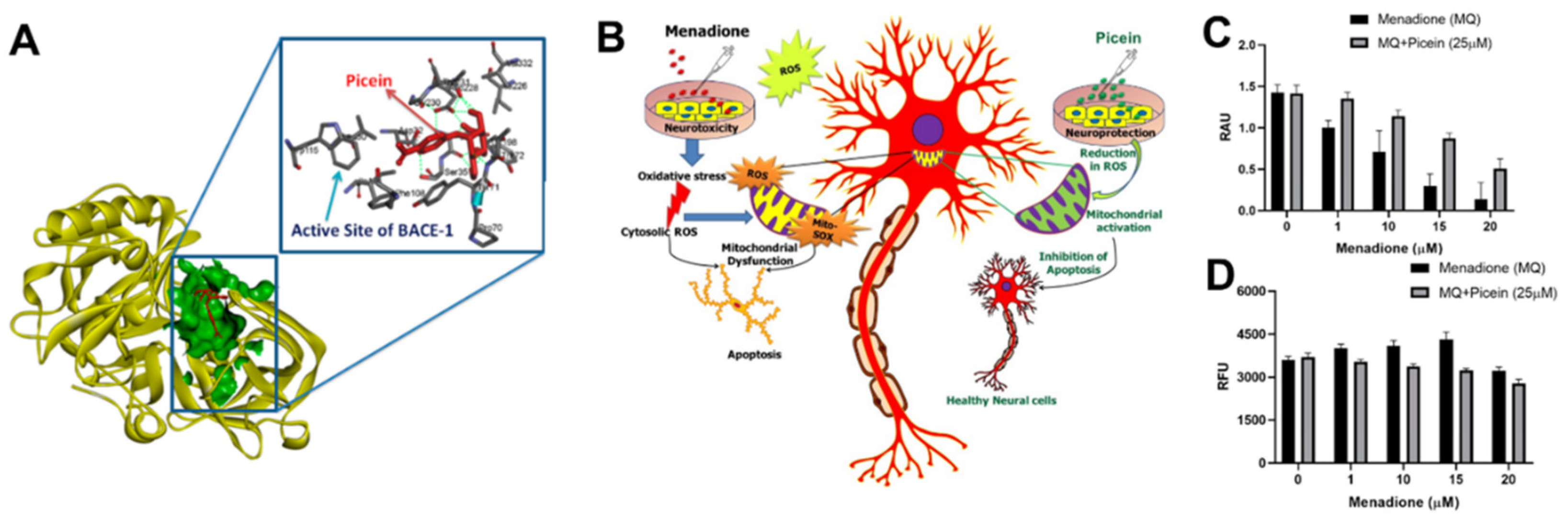
Figure 3. (A) In silico identification of putative biomolecular targets for the willow compound picein. BACE1 was found as the most probable target of picein. The docked pose of picein (red sticks) at the active site (green surface presentation) of BACE1 (yellow cartoon). Inset, the active site residues are shown as grey sticks. (B) An overview of the neuroprotective effects of picein: (C) increase in the level of cell viability, and (D) decrease in the level of ROS [72][11].
References
- Zhang, X.-X.; Tian, Y.; Wang, Z.-T.; Ma, Y.-H.; Tan, L.; Yu, J.-T. The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J. Prev. Alzheimer’s Dis. 2021, 8, 313–321.
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The continuum of aging and age-related diseases: Common mechanisms but different rates. Front. Med. 2018, 5, 61.
- Dorsey, E.R.; George, B.P.; Leff, B.; Willis, A.W. The coming crisis: Obtaining care for the growing burden of neurodegenerative conditions. Neurology 2013, 80, 1989–1996.
- Erkkinen, M.G.; Kim, M.-O.; Geschwind, M.D. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2018, 10, a033118.
- Stanzione, P.; Tropepi, D. Drugs and clinical trials in neurodegenerative diseases. Ann. Dell’istituto Super. Sanità 2011, 47, 49–54.
- Yiannopoulou, K.G.; Papageorgiou, S.G. Current and future treatments for Alzheimer’s disease. Ther. Adv. Neurol. Disord. 2013, 6, 19–33.
- Kalia, L.V.; Kalia, S.K.; Lang, A.E. Disease-modifying strategies for Parkinson’s disease. Mov. Disord. 2015, 30, 1442–1450.
- Castellani, R.J.; Perry, G. Pathogenesis and disease-modifying therapy in Alzheimer’s disease: The flat line of progress. Arch. Med. Res. 2012, 43, 694–698.
- Dunkel, P.; Chai, C.L.; Sperlagh, B.; Huleatt, P.B.; Matyus, P. Clinical utility of neuroprotective agents in neurodegenerative diseases: Current status of drug development for Alzheimer’s, Parkinson’s and Huntington’s diseases, and amyotrophic lateral sclerosis. Expert Opin. Investig. Drugs 2012, 21, 1267–1308.
- Noleto-Dias, C.; Wu, Y.; Bellisai, A.; Macalpine, W.; Beale, M.H.; Ward, J.L. Phenylalkanoid glycosides (non-salicinoids) from wood chips of Salix triandra× dasyclados hybrid willow. Molecules 2019, 24, 1152.
- Kesari, K.K.; Dhasmana, A.; Shandilya, S.; Prabhakar, N.; Shaukat, A.; Dou, J.; Rosenholm, J.M.; Vuorinen, T.; Ruokolainen, J. Plant-derived natural biomolecule picein attenuates menadione induced oxidative stress on neuroblastoma cell mitochondria. Antioxidants 2020, 9, 552.
- Tawfeek, N.; Mahmoud, M.F.; I Hamdan, D.; Sobeh, M.; Farrag, N.; Wink, M.; El-Shazly, A.M. Phytochemistry, pharmacology and medicinal uses of plants of the genus salix: An updated review. Front. Pharmacol. 2021, 12, 50.
- Di Caprio, R.; Monfrecola, G.; Balato, A.; Balato, N.; Gasparri, F.; Micillo, R.; Lembo, S. The anti-inflammatory and antioxidant properties of 1, 2-decanediol and willow bark extract in lipopolysaccharide-stimulated keratinocytes. G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2017, 154, 624–631.
- di Giacomo, V.; Ferrante, C.; Ronci, M.; Cataldi, A.; Di Valerio, V.; Rapino, M.; Recinella, L.; Chiavaroli, A.; Leone, S.; Vladimir-Knežević, S.; et al. Multiple pharmacological and toxicological investigations on Tanacetum parthenium and Salix alba extracts: Focus on potential application as anti-migraine agents. Food Chem. Toxicol. 2019, 133, 110783.
- Mahdi, J.G. Medicinal potential of willow: A chemical perspective of aspirin discovery. J. Saudi Chem. Soc. 2010, 14, 317–322.
- Pobłocka-Olech, L.; van Nederkassel, A.M.; Vander Heyden, Y.; Krauze-Baranowska, M.; Glód, D.; Baczek, T. Chromatographic analysis of salicylic compounds in different species of the genus Salix. J. Sep. Sci. 2007, 30, 2958–2966.
- Maistro, E.L.; Terrazzas, P.M.; Perazzo, F.F.; Gaivão, I.O.N.D.M.; Sawaya, A.C.H.F.; Rosa, P.C.P. Salix alba (white willow) medicinal plant presents genotoxic effects in human cultured leukocytes. J. Toxicol. Environ. Health Part A 2019, 82, 1223–1234.
- Shara, M.; Stohs, S.J. Efficacy and safety of white willow bark (Salix alba) extracts. Phytother. Res. 2015, 29, 1112–1116.
- Durak, A.; Gawlik-Dziki, U.; Sugier, D. Coffee enriched with willow (Salix purpurea and Salix myrsinifolia) bark preparation–Interactions of antioxidative phytochemicals in a model system. J. Funct. Foods 2015, 18, 1106–1116.
- Durak, A.; Gawlik-Dziki, U. The study of interactions between active compounds of coffee and willow (Salix sp.) bark water extract. BioMed Res. Int. 2014, 2014, 386953.
- Pobłocka-Olech, L.; Krauze-Baranowska, M.; Głód, D.; Kawiak, A.; Łojkowska, E. Chromatographic analysis of simple phenols in some species from the genus Salix. Phytochem. Anal. 2010, 21, 463–469.
- Sulima, P.; Krauze-Baranowska, M.; Przyborowski, J.A. Variations in the chemical composition and content of salicylic glycosides in the bark of Salix purpurea from natural locations and their significance for breeding. Fitoterapia 2017, 118, 118–125.
- Heiska, S.; Tikkanen, O.-P.; Rousi, M.; Julkunen-Tiitto, R. Bark salicylates and condensed tannins reduce vole browsing amongst cultivated dark-leaved willows (Salix myrsinifolia). Chemoecology 2007, 17, 245–253.
- Kammerer, B.; Kahlich, R.; Biegert, C.; Gleiter, C.H.; Heide, L. HPLC-MS/MS analysis of willow bark extracts contained in pharmaceutical preparations. Phytochem. Anal. Int. J. Plant Chem. Biochem. Tech. 2005, 16, 470–478.
- Lavola, A.; Maukonen, M.; Julkunen-Tiitto, R. Variability in the composition of phenolic compounds in winter-dormant Salix pyrolifolia in relation to plant part and age. Phytochemistry 2018, 153, 102–110.
- Fischbach, R.J.; Kossmann, B.; Panten, H.; Steinbrecher, R.; Heller, W.; Seidlitz, H.K.; Sandermann, H.; Hertkorn, N.; Schnitzler, J.-P. Seasonal accumulation of ultraviolet-B screening pigments in needles of Norway spruce (Picea abies (L.) Karst.). Plant Cell Environ. 1999, 22, 27–37.
- Tyśkiewicz, K.; Konkol, M.; Kowalski, R.; Rój, E.; Warmiński, K.; Krzyżaniak, M.; Gil, L.; Stolarski, M.J. Characterization of bioactive compounds in the biomass of black locust, poplar and willow. Trees 2019, 33, 1235–1263.
- Heller, W.; Rosemann, D.; Osswald, W.; Benz, B.; Schönwitz, R.; Lohwasser, K.; Kloosa, M.; Sandermann, H., Jr. Biochemical response of Norway spruce (Picea abies (L.) Karst.) towards 14-month exposure to ozone and acid mist: Part I—Effects on polyphenol and monoterpene metabolism. Environ. Pollut. 1990, 64, 353–366.
- Dou, J.; Xu, W.; Koivisto, J.J.; Mobley, J.K.; Padmakshan, D.; Kögler, M.; Xu, C.; Willför, S.M.; Ralph, J.; Vuorinen, T. Characteristics of hot water extracts from the bark of cultivated willow (Salix sp.). ACS Sustain. Chem. Eng. 2018, 6, 5566–5573.
- Dou, J.; Heinonen, J.; Vuorinen, T.; Xu, C.; Sainio, T. Chromatographic recovery and purification of natural phytochemicals from underappreciated willow bark water extracts. Sep. Purif. Technol. 2021, 261, 118247.
- Jeon, S.H.; Chun, W.; Choi, Y.J.; Kwon, Y.S. Cytotoxic constituents from the bark of Salix hulteni. Arch. Pharmacal Res. 2008, 31, 978–982.
- Feng, X.; Wang, W.; Liu, F.; Zhang, P.; Tang, F.; Zeng, L.; Tang, K. Separation of active components tyrosol and salidroside from Rhodiola rosea crude extract by two-step multistage fractionation extraction. Chem. Eng. Process.-Process Intensif. 2022, 172, 108800.
- Corradi, E.; Schmidt, N.; Räber, N.; De Mieri, M.; Hamburger, M.; Butterweck, V.; Potterat, O. Metabolite profile and antiproliferative effects in HaCaT cells of a Salix reticulata extract. Planta Med. 2017, 83, 1149–1158.
- Yang, H.; Lee, S.H.; Sung, S.H.; Kim, J.; Kim, Y.C. Neuroprotective compounds from Salix pseudo-lasiogyne twigs and their anti-amnesic effects on scopolamine-induced memory deficit in mice. Planta Med. 2013, 79, 78–82.
- Masood, M.; Arshad, M.; Qureshi, R.; Sabir, S.; Amjad, M.S.; Qureshi, H.; Tahir, Z. Picrorhiza kurroa: An ethnopharmacologically important plant species of Himalayan region. Pure Appl. Biol. 2015, 4, 407.
- Verma, P.C.; Basu, V.; Gupta, V.; Saxena, G.; Ur Rahman, L. Pharmacology and chemistry of a potent hepatoprotective compound Picroliv isolated from the roots and rhizomes of Picrorhiza kurroa royle ex benth.(kutki). Curr. Pharm. Biotechnol. 2009, 10, 641–649.
- Morikawa, T.; Inoue, N.; Nakanishi, Y.; Manse, Y.; Matsuura, H.; Okino, K.; Hamasaki, S.; Yoshikawa, M.; Muraoka, O.; Ninomiya, K. Collagen synthesis-promoting and collagenase inhibitory activities of constituents isolated from the rhizomes of Picrorhiza kurroa royle ex benth. Fitoterapia 2020, 143, 104584.
- Kant, K.; Walia, M.; Agnihotri, V.; Pathania, V.; Singh, B. Evaluation of antioxidant activity of Picrorhiza kurroa (leaves) extracts. Indian J. Pharm. Sci. 2013, 75, 324.
- Metsämuuronen, S.; Sirén, H. Bioactive phenolic compounds, metabolism and properties: A review on valuable chemical compounds in Scots pine and Norway spruce. Phytochem. Rev. 2019, 18, 623–664.
- Flores-Sanchez, I.J.; Verpoorte, R. Plant polyketide synthases: A fascinating group of enzymes. Plant Physiol. Biochem. 2009, 47, 167–174.
- Turtola, S.; Sallas, L.; Holopainen, J.K.; Julkunen-Tiitto, R.; Kainulainen, P. Long-term exposure to enhanced UV-B radiation has no significant effects on growth or secondary compounds of outdoor-grown Scots pine and Norway spruce seedlings. Environ. Pollut. 2006, 144, 166–171.
- Stolter, C.; Niemelä, P.; Ball, J.P.; Julkunen-Tiitto, R.; Vanhatalo, A.; Danell, K.; Varvikko, T.; Ganzhorn, J.U. Comparison of plant secondary metabolites and digestibility of three different boreal coniferous trees. Basic Appl. Ecol. 2009, 10, 19–26.
- Løkke, H. Picein and piceol concentrations in Norway spruce. Ecotoxicol. Environ. Saf. 1990, 19, 301–309.
- Jensen, J.; Løkke, H. 4-hydroxyacetophenone and its glucoside picein as chemical indicators for stress in Picea abies/4-Hydroxyacetophenon und sein Glucosid Picein als chemische Indikatoren für Stress in Picea abies. Z. Pflanzenkrankh. Pflanzenschutz/J. Plant Dis. Prot. 1990, 97, 328–338.
- Ganthaler, A.; Stöggl, W.; Kranner, I.; Mayr, S. Foliar phenolic compounds in Norway spruce with varying susceptibility to Chrysomyxa rhododendri: Analyses of seasonal and infection-induced accumulation patterns. Front. Plant Sci. 2017, 8, 1173.
- Parent, G.J.; Méndez-Espinoza, C.; Giguère, I.; Mageroy, M.H.; Charest, M.; Bauce, É.; Bohlmann, J.; MacKay, J.J. Hydroxyacetophenone defenses in white spruce against spruce budworm. Evol. Appl. 2020, 13, 62–75.
- Bahnweg, G.; Schubert, R.; Kehr, R.D.; Müller-Starck, G.; Heller, W.; Langebartels, C.; Sandermann, H., Jr. Controlled inoculation of Norway spruce (Picea abies) with Sirococcus conigenus: PCR-based quantification of the pathogen in host tissue and infection-related increase of phenolic metabolites. Trees 2000, 14, 435–441.
- Sarıkahya, N.B.; Pekmez, M.; Arda, N.; Kayce, P.; Yavaşoğlu, N.Ü.K.; Kırmızıgül, S. Isolation and characterization of biologically active glycosides from endemic Cephalaria species in Anatolia. Phytochem. Lett. 2011, 4, 415–420.
- Méndez-Espinoza, C.; Parent, G.J.; Lenz, P.; Rainville, A.; Tremblay, L.; Adams, G.; McCartney, A.; Bauce, E.; Mackay, J. Genetic control and evolutionary potential of a constitutive resistance mechanism against the spruce budworm (Choristoneura fumiferana) in white spruce (Picea glauca). Heredity 2018, 121, 142–154.
- Góngora, L.; Máñez, S.; Giner, R.M.; Recio, M.C.; Gray, A.I.; Ríos, J.-L. Phenolic glycosides from Phagnalon rupestre. Phytochemistry 2002, 59, 857–860.
- Abreu, P.M.; Braham, H.; Jannet, H.B.; Mighri, Z.; Matthew, S. Antioxidant compounds from Ebenus pinnata. Fitoterapia 2007, 78, 32–34.
- Tolonen, A.; Pakonen, M.; Hohtola, A.; Jalonen, J. Phenylpropanoid glycosides from Rhodiola rosea. Chem. Pharm. Bull. 2003, 51, 467–470.
- Chen, D.; Fan, J.; Wang, P.; Zhu, L.; Jin, Y.; Peng, Y.; Du, S. Isolation, identification and antioxidative capacity of water-soluble phenylpropanoid compounds from Rhodiola crenulata. Food Chem. 2012, 134, 2126–2133.
- Morikawa, T.; Imura, K.; Miyake, S.; Ninomiya, K.; Matsuda, H.; Yamashita, C.; Muraoka, O.; Hayakawa, T.; Yoshikawa, M. Promoting the effect of chemical constituents from the flowers of Poacynum hendersonii on adipogenesis in 3T3-L1 cells. J. Nat. Med. 2012, 66, 39–48.
- Flores-Bocanegra, L.; Pérez-Vásquez, A.; Torres-Piedra, M.; Bye, R.; Linares, E.; Mata, R. α-Glucosidase inhibitors from Vauquelinia corymbosa. Molecules 2015, 20, 15330–15342.
- Lai, L.B.; Gopalan, V.; Glew, R.H. Continuous spectrophotometric assays for β-glucosidases acting on the plant glucosides l-picein and prunasin. Anal. Biochem. 1992, 200, 365–369.
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539.
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22.
- Vassar, R. BACE1: The beta-secretase enzyme in Alzheimer’s disease. J. Mol. Neurosci. MN 2004, 23, 105–114.
- Vassar, R.; Kandalepas, P.C. The β-secretase enzyme BACE1 as a therapeutic target for Alzheimer’s disease. Alzheimer’s Res. Ther. 2011, 3, 20.
- Das, B.; Yan, R. A close look at BACE1 inhibitors for Alzheimer’s disease treatment. CNS Drugs 2019, 33, 251–263.
- Zhu, K.; Peters, F.; Filser, S.; Herms, J. Consequences of pharmacological BACE inhibition on synaptic structure and function. Biol. Psychiatry 2018, 84, 478–487.
- Mullard, A. BACE inhibitor bust in Alzheimer trial. Nat. Rev. Drug Discov. 2017, 16, 155–156.
- Huang, L.-K.; Chao, S.-P.; Hu, C.-J. Clinical trials of new drugs for Alzheimer disease. J. Biomed. Sci. 2020, 27, 18.
- Hu, Y.; Li, C.; Shen, W. Gastrodin alleviates memory deficits and reduces neuropathology in a mouse model of Alzheimer’s disease. Neuropathology 2014, 34, 370–377.
- Wang, X.; Shen, Y.; Wang, S.; Li, S.; Zhang, W.; Liu, X.; Lai, L.; Pei, J.; Li, H. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017, 45, W356–W360.
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J. Comput. Chem. 1998, 19, 1639–1662.
More
