A significant interest was granted lately to enzymes, which are versatile catalysts characterized by natural origin, with high specificity and selectivity for particular substrates. Additionally, some enzymes are involved in the production of high-valuable products, such as antibiotics, while others are known for their ability to transform emerging contaminates, such as dyes and pesticides, to simpler molecules with a lower environmental impact. Nevertheless, the use of enzymes in industrial applications is limited by their reduced stability in extreme conditions and by their difficult recovery and reusability. Rationally, enzyme immobilization on organic or inorganic matrices proved to be one of the most successful innovative approaches to increase the stability of enzymatic catalysts. By the immobilization of enzymes on support materials, composite biocatalysts are obtained that pose an improved stability, preserving the enzymatic activity and some of the support material’s properties. Of high interest are the polymer/enzyme composites, which are obtained by the chemical or physical attachment of enzymes on polymer matrices.
- enzyme immobilization
- biocatalyst
- multilayer
- cross-linking agents
- polymer/enzyme complexes
- enzyme activity
- environmental protection
- biosensors
- biomedical applications
1. Introduction
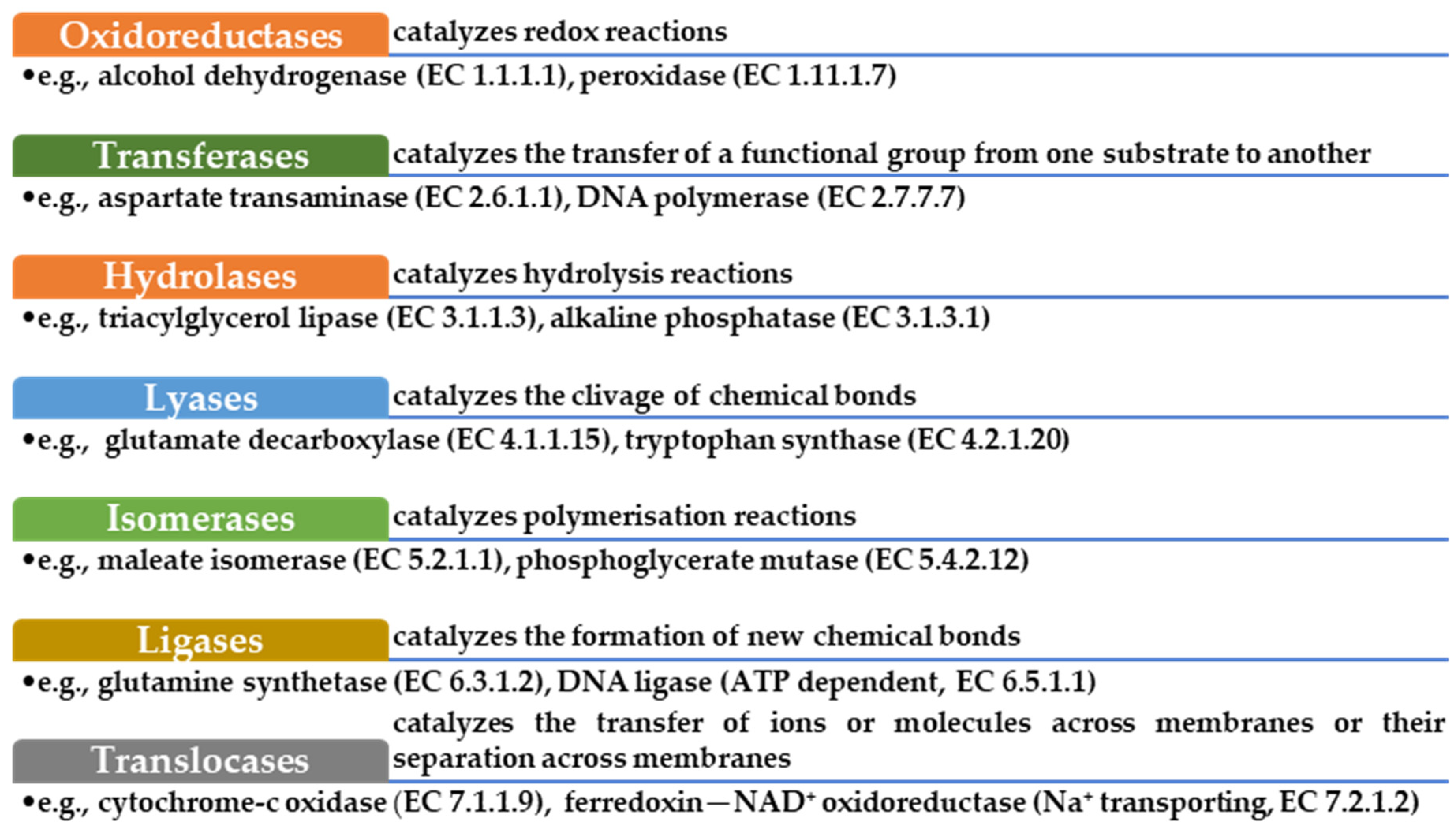
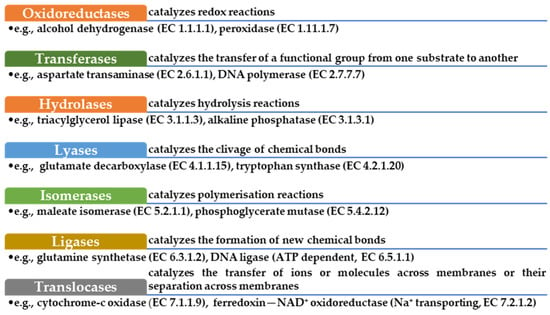
2. Enzymes—Structure, Activity, and Characteristics
3. Applications of Polymer/Enzyme Composite Materials
Polymer/enzyme composite materials are distinguished by high stability, specificity, and selectivity, as well as easier separation and reusability. These are biocatalysts with industrial applicability, ensuring the sustainability and efficiency of industrial processes due to the advantages they offer. Unlike chemical catalysts, biocatalysts operate at mild temperature and pH conditions, have higher selectivity, are nontoxic, and can be used in a greater number of applications (Figure 42).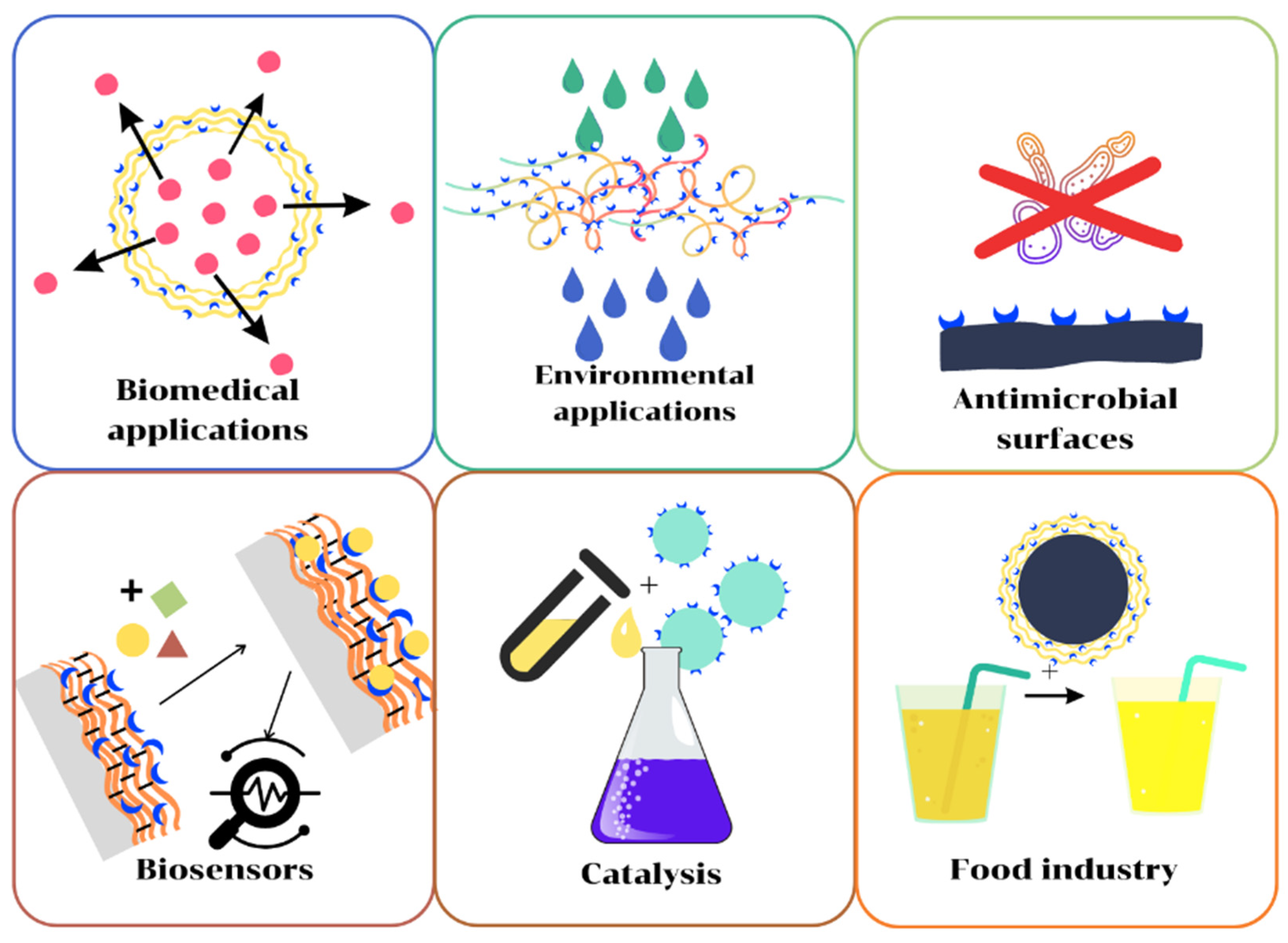
3.1. Applications in Environmental Protection
One of the most important problems our society is facing is the increasing level of water, air, and soil pollution. While pollution is generally hard to mitigate, novel techniques for the efficient removal of pollutants are deeply investigated. A special interest is raised by water pollution since water is a very good solvent for a lot of dangerous chemicals, such as dyes, pesticides, and metal ions, which can pass from water sources to plants, animals, and even humans and raise important health concerns due to their toxicity, bioaccumulation, and carcinogenic effects [116][8]. The use of polymer/enzyme composite materials in environmental applications has attracted increased interest in recent years due to their versatility and stability, satisfactory catalytic activity, and the possibility of reusing the material without an important loss of catalytic activity [117][9]. Several studies have reported the use of composite polymer/enzyme systems for the removal of organic compounds found in wastewater, such as phenols, pesticides, and dyes. Bilal and collaborators immobilized manganese peroxidase in composite microspheres obtained from PVA and ALGNa, which they subsequently used in sorption studies of some dyes from simulated and real polluted water samples [118][10]. Studies in simulated polluted water revealed that composite particles with immobilized peroxidase were able to discolor water samples by 78–92%, depending on the type of dye dissolved in the water, as compared with only 57–74% discoloration when treating water samples with the free enzyme. The materials were later used in the treatment of polluted water samples taken from textile factories and printing houses, containing mixtures of dyes, obtaining satisfactory results. In another study, Aricov and collaborators used laccase/CHI composite microspheres for the removal of indigo carmine, attaining the full discoloration of the sample after 14 min [119][11]. Enzymes immobilized in polymer fibers can also be successfully used in the retention and degradation of some pollutants in wastewater. An example in this regard is proposed by Maryšková and collaborators, who obtained composite fibers based on polyamide and PEI in which they immobilized laccase and subsequently used it to treat polluted water samples with different emerging contaminants [120][12]. The authors used three types of water samples: deionized water, polluted river water treated by ultrafiltration, and polluted water to which citrate buffer solution was added. A mixture of pollutants consisting of bisphenol A, 17 α-ethynyl estradiol, triclosan, and diclofenac was added to these samples at an initial concentration of 10 mg/L. Following the treatment with the composite fibers with laccase, the retention of all four contaminants was noticed in a proportion of at least 10–20%. The best results were recorded when using deionized water, with the percentage of destroyed pollutants varying between 73.6% for triclosan and 17.5% for diclofenac. Leontieș and collaborators studied the discoloration of water samples employing laccase/CHI/PAA composite microparticles [121][13]. The degradation efficiency of these composites was 90% for both naphthol green and indigo carmine, achieved in about 9 and 10 min, respectively. Vera and collaborators used composite materials based on laccase and poly(glycidyl methacrylate) for the enzymatic degradation of diazinon, an insecticidal compound from the class of organophosphorus compounds [122][14]. The use of the biocatalyst in the presence of 2,2-azino-bis-(3-ethylbenzothiazoline-6-sulfonic) acid allowed the total degradation of the pollutant after 48 h of contact and about 88% of the amount of the pollutant, in the absence of the mediator. A study by Lassouane and collaborators used composite materials based on CHI and laccase for the degradation of bisphenol A [123][15]. After optimizing the degradation conditions, the authors achieved a pollutant degradation efficiency of over 99%, demonstrating at the same time that the biocatalyst can be successfully used in at least 10 reaction cycles, with about 70% of the activity maintained.3.2. Catalysts for Chemical Industry
Enzymes are biological catalysts distinguished by increased specificity. Due to their versatility, they can catalyze a large number of chemical transformations of interest, such as the digestion of proteins or carbohydrates, the degradation of organic pollutants to nontoxic small molecular compounds, or the transformation of substrates into products of interest, such as drug intermediates, antibiotics, or organic acids. The increased use of polymer/enzyme composites as catalysts is the result of their stability, easy separation, and reusability, making them suitable candidates for industrial processes. In a recent study, Pereira Cipolatti and collaborators used composite microparticles based on polyurethane and lipase, coated with trehalose to obtain two organic acids (eicosapentaenoic acid and docosahexaenoic acid) that are used in the food industry and some mandelic acid derivatives with pharmaceutical applications [124][16]. The authors demonstrated that lipase-based composite microparticles can be successfully used to obtain compounds of interest, showing increased catalytic activity, as well as stability at temperature, in a wide pH range, and storage time. In another study, Pan and collaborators used composite biocatalysts obtained from CHI-coated magnetic microparticles covalently decorated with lipase [125][17]. The biocatalysts thus obtained were used for the enantioselective acylation of a racemic mixture of L-phenylethylamine. The authors observed that the use of the composite biocatalyst allows the acylation reaction to take place at an elevated temperature without denaturation of the enzyme. Additionally, the biocatalyst could be reused in up to 7 reaction cycles, maintaining about 60% of the activity. Polymer/enzyme composites also find applications in the cosmetic and perfume industry, especially as catalysts for obtaining chemical compounds of interest. An example in this sense was proposed by Melo and collaborators, who tested the catalytic activity of some composite materials obtained by encapsulating lipase B isolated from Candida antarctica in a composite matrix consisting of CHI and sodium polyphosphate [126][18]. The biocatalyst was used to obtain benzyl acetate (synthetic jasmine flavor) under different reaction conditions. The authors demonstrated that the obtained composite biocatalyst shows increased stability under the tested process conditions, being stable in a wide pH range (pH = 4–10), at high temperatures (maximum activity at around 55–60 °C and total inactivation at temperatures higher than 80 °C), but also in the presence of different organic solvents, the best catalytic activity being recorded when using a mixture of dimethyl sulfoxide and phosphate buffer solution (pH = 7). Additionally, this biocatalyst was successfully reused in 5 reaction cycles with the maintenance of about 86% of the enzyme activity. Tudorache and collaborators reported the fabrication of lipase/ALGCa and lipase/κ-carrageenan composite beads that can be used as catalysts for the conversion of α-pinene to oxy-derivatives, followed by isomerization to campholenic aldehyde and trans-carenol, compounds of interest for the cosmetic and fragrance industry [127][19].3.3. Biosensors
Biosensors find applications in the detection of molecules of interest in various fields, including the medicine, cosmetic, and food industries, but also pollution control and environmental protection. Biosensors based on immobilized enzymes are characterized by a high specificity for the molecule of interest, have the ability to mediate a chemical reaction between the receptor and the analyte, and allow the conversion of the chemical response into an electrical response [128][20]. Additionally, biosensors have increased stability and low environmental impact, in recent years, the use of polymer/enzyme composites being established as one of the most versatile and economical detection methods [128][20]. In a recent study, Tutunaru and collaborators developed a composite biosensor based on acetylcholinesterase grafted on carboxyl-modified single-walled carbon nanotubes and poly(3,4-ethylenedioxythiophene), which was subsequently used for the detection of organophosphate insecticides in apple juice [129][21]. The biosensor thus obtained was able to successfully detect and recover dichlorvos, with detection limits of 0.447 and 5.54 ppb, depending on the detection method. Recently, Cano-Raya and collaborators reported the fabrication of a biosensor for pyrocatechol by immobilizing laccase in polyamide 6 microparticles subsequently deposited on a semipermeable support material allowing substrate diffusion [130][22]. To determine the selectivity of the biosensor, the authors tested three substrates: pyrocatechol and two of its isomers, resorcinol and hydroquinone, observing an extremely good selectivity of the biosensor for the target analyte. In addition to high selectivity and satisfactory analytical response under controlled conditions, the authors also observed that the obtained composite biosensor can be successfully used in the detection of pyrocatechol from natural water samples. Thus, the authors tested river water samples collected from various areas and in different seasons, to which they added well-determined amounts of pyrocatechol. The authors obtained pyrocatechol recovery rates of over 97% for all samples tested. A recent example was proposed by Aldea and collaborators, who fabricated a biosensor based on glucose oxidase and PMMA electrospun fibers, coated with a gold layer and deposited on a poly(ethylene terephthalate) film [131][23]. The sensor exhibited a sensitivity of 3.1 µA·cm−2·mM−1 and a detection limit of 0.22 mM.3.4. Antimicrobial Applications
Based on the antimicrobial activity of some natural polymers, such as CHI, but also of some enzymes, polymer/enzyme composites can be used to inhibit the development of some microorganism strains. Among the most significant enzymes with antimicrobial activity, lysozyme exhibits a growing interest based on its ability to lyse a cell wall peptidoglycan layer of Gram-positive bacteria. In a recent study, Bayazidi and collaborators showed that cellulose fibers with immobilized lysozyme show antimicrobial activity against Staphylococcus aureus, Escherichia coli, Listeria monocytogenes, Yersinia enterocolitica, Aspergillus niger, and Saccharomyces cerevisiae [37][24]. Li and collaborators obtained composite nanoparticles based on CHI and alginate lyase and tested their antimicrobial activity on a Pseudomonas aeruginosa strain, observing that the immobilized enzyme showed higher antimicrobial activity than the free enzyme [58][25]. Thus, the biofilm formed by Pseudomonas aeruginosa in the presence of the CHI/alginate-lyase composite was only 21 µm thick, as compared with ~49 µm observed in the presence of the free enzyme and ~87 µm in the absence of any inhibitory agent. In a recent study, Yuan and collaborators demonstrated the potential biomedical applications of composite membranes obtained by layer-by-layer deposition of lysozyme and collagen on a fibroin and nylon 6 support material [132][26]. The antimicrobial activity of the composite membranes was demonstrated on a Staphylococcus aureus strain. The authors observed a decrease of about 98% in the proliferation capacity of the bacterial culture compared with the unmodified membrane. Moreover, these composite membranes with embedded lysozyme showed increased biocompatibility, allowing the development of fibroblast cultures. Baidamshina and collaborators recently reported the fabrication of composite materials with antimicrobial properties by immobilizing papain in a CHI matrix [133][27]. About 90% of papain’s enzymatic activity was maintained in the microparticles, with an extended stability at higher temperature and pH range. The authors demonstrated that the obtained materials can act efficiently on a bacterial biofilm formed by Staphylococcus aureus and Staphylococcus epidermidis species.3.5. Biomedical Applications
Another area of interest for the use of polymer/enzyme composites is represented by the fabrication of materials with biomedical applications. This field can include materials for tissue engineering, implants, systems with controlled release of active substances, and biosensors of medical interest, but also catalysts to obtain biologically active substances. In a 2020 research study, Filho Moreira and collaborators reported the fabrication of a composite material with applications in tissue engineering by immobilizing papain in alginate microparticles [134][28]. The use of this composite is based on the biomedical properties of the two-component materials: alginate, recognized for its properties of stimulating cell regeneration, and papain, involved in the depletion of necrotic tissues. In brief, the enzyme is released through a controlled diffusion, about 64% released for 24 h. Another potential biomedical application of polymer/enzyme composites is related to the prevention of microbial contamination of some implants. Recently, Teske and collaborators reported novel composite films based on papain and poly(L-lactic acid) with antimicrobial properties [135][29]. The composite material was customized by cross-linking papain, under the action of 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride, on the polymer surface. The antimicrobial activity was tested against a Clostridioides difficile strain, one of the common pathogens responsible for the occurrence of nosocomial infections, observing a reduction of about 20% of the biofilm formed on the surface of a cardiovascular implant. Another example was proposed by Visan and collaborators, who used a composite coating based on lysozyme, polycaprolactone, and PEG to prevent the microbial contamination of some Ti implants [102][30]. The composite film exhibited increased antibacterial activity against both Gram-positive and Gram-negative microbial strains. The results highlighted that the composite film inhibited the growth of Escherichia coli, Bacillus subtilis, Enterococcus faecalis, and Staphylococcus aureus strains. Numerous enzymes successfully catalyzed the transformation of some compounds into pharmacological species, this category including antibiotics [136][31], analgesics [137][32], and some anti-inflammatory drugs [138][33]. An example is presented by Gilani and collaborators, who studied the production of (S)-naproxen under the action of three biocatalysts: CHI/lipase composite microparticles, CHI/lipase microparticles cross-linked with GA, and microparticles obtained by lipase immobilization in a commercial resin of Amberlite XAD7 type [138][33]. The authors studied the influence of various parameters on the hydrolysis process of the naproxen methyl ester racemic mixture, determining the optimal conditions for obtaining (S)-naproxen. The optimal parameters were a reaction time of 24 h, in the presence of isooctane and 2-ethoxyethanol as solvent/cosolvent, at 35 °C and pH = 7, successfully reused in about 10 reaction cycles.3.6. Applications for Food Industry
Composite materials with immobilized enzymes are finding increasing use in the food industry. One of their most important applications is in the clarification and discoloration of fruit juices, in order to increase their appearance, stability, and shelf life. The most used types of enzymes for juice clarification are peroxidases and laccases that are able to oxidize phenolic compounds in juices, responsible for sediment formation and changes in their appearance, taste, and smell [139][34]. Irshad and collaborators used composite materials obtained by immobilizing pectinase in CHI microspheres for the clarification of some fruit juices (apple, mango, peach, and apricot), the use of the biocatalyst leading to a considerable reduction of turbidity, viscosity, and discoloration of fruit juice samples [140][35]. Bilal and collaborators used gelatin spheres with manganese peroxidase for the clarification of some fruit juices [139][34]. Following the experiments, a 36.6% decrease in juice turbidity was observed, as well as a 42.7% reduction in juice color intensity. The proposed method has a number of important economic advantages, such as the possibility of reusing the composite material, easy separation, and increased stability over time and with environmental factors. Benucci and coworkers reported papain immobilization on CHI-clay nanocomposite films. One of these films was efficiently used to reduce haze potential and the protein content of white wines [141][36]. Fernandez-Pacheco and collaborators immobilized a commercially available β-glucosidase on ALGCa beads in order to improve white wine aroma by the depletion of flavorless glycosides. The immobilized enzyme displays a superior hydrolytic activity, their activity maintained at 96.5% after reutilization for seven times [142][37]. Another area of interest for the food industry is represented by the prevention of microbiological contamination of some food products. In this regard, Wang and collaborators tested the antimicrobial activity of some composites obtained by immobilizing lysozyme and ALGNa on cellulose acetate nanofibers [143][38]. The composite materials showed increased stability and reusability, with the maintenance of about 70% of the enzyme activity after 4 cycles of use. At the same time, the authors showed the antibacterial activity of these composites against a Staphylococcus aureus strain from UTH milk, at two temperature values, 4 and 25 °C, respectively. Additionally, it was observed that the inhibitory action of composite fibers depends on the number of layers of lysozyme and ALGNa deposited on the support material, the best results being recorded for composites with nine composite layers. Another application for the food industry is represented by the decontamination of some food products. In this sense, Bedade and collaborators studied the degradation of acrylamide from coffee samples, under the action of a composite biocatalyst obtained by immobilizing acrylamidase on ALGNa microparticles coated with CHI [73][39]. The authors studied the retention of the contaminant under the action of the enzyme under static and dynamic conditions, demonstrating the retention of 100% of acrylamide in the studies performed using a batch column under optimal conditions. At the same time, the biocatalyst could be used in 4 reaction cycles with the maintenance of about 80% of the activity.References
- Li, Y.; Wang, X.; Sun, J. Layer-by-layer assembly for rapid fabrication of thick polymeric films. Chem. Soc. Rev. 2012, 41, 5998–6009.
- Chapman, J.; Ismail, A.E.; Zoica Dinu, C. Industrial applications of enzymes: Recent advances, techniques, and outlooks. Catalysts 2018, 8, 238.
- Bilal, M.; Dourado, C.; Mehmood, T.; Nadeem, F.; Tabassam, Q.; Fernando, L.; Ferreira, R. Immobilized lipases-based nano-biocatalytic systems—A versatile platform with incredible biotechnological potential. Int. J. Biol. Macromol. 2021, 175, 108–122.
- Aggarwal, S.; Chakravarty, A.; Ikram, S. A comprehensive review on incredible renewable carriers as promising platforms for enzyme immobilization & thereof strategies. Int. J. Biol. Macromol. 2021, 167, 962–986.
- Jankowska, K.; Su, Z.; Bj, S. Tailor-made novel electrospun polystyrene/poly (D,L-lactide-co-glycolide) for oxidoreductases immobilization: Improvement of catalytic properties under extreme reaction conditions. Bioorg. Chem. 2021, 114, 105036.
- BRENDA Enzyme Database. Available online: www.brenda-enzymes.org (accessed on 4 June 2022).
- Copeland, R.A. Enzymes: A Practical Introduction to Structure, Mechanism, and Data Analysis, 2nd ed.; Wiley-VCH: New York, NY, USA, 2000.
- Bucatariu, F.; Teodosiu, C.; Morosanu, I.; Fighir, D.; Ciobanu, R.; Petrila, L.-M.; Mihai, M. An overview on composite sorbents based on polyelectrolytes used in advanced wastewater treatment. Polymers 2021, 13, 3963.
- Zdarta, J.; Meyer, A.S.; Pinelo, M. Developments in support materials for immobilization of oxidoreductases: A comprehensive review. Adv. Colloid Interface Sci. 2018, 258, 1–20.
- Bilal, M.; Asgher, M. Sandal reactive dyes decolorization and cytotoxicity reduction using manganese peroxidase immobilized onto polyvinyl alcohol–alginate beads. Chem. Cent. J. 2015, 9, 47.
- Aricov, L.; Leonties, A.R.; Gîfu, I.C.; Preda, D.; Raducan, A.; Anghel, D.-F. Enhancement of laccase immobilization onto wet chitosan microspheres using an iterative protocol and its potential to remove micropollutants. J. Environ. Manag. 2020, 276, 111326.
- Maryskova, M.; Schaabova, M.; Tomankova, H.; Novotny, V.; Rysova, M. Wastewater treatment by novel polyamide/polyethylenimine nanofibers with immobilized laccase. Water 2020, 12, 588.
- Leontieș, A.R.; Răducan, A.; Culiță, D.C.; Alexandrescu, E.; Moroșan, A.; Mihaiescu, D.E.; Aricov, L. Laccase immobilized on chitosan-polyacrylic acid microspheres as highly efficient biocatalyst for naphthol green B and indigo carmine degradation. Chem. Eng. J. 2022, 439, 135654.
- Vera, M.; Nyanhongo, G.S.; Guebitz, G.M. Polymeric microspheres as support to co-immobilized Agaricus bisporus and Trametes versicolor laccases and their application in diazinon degradation. Arab. J. Chem. 2019, 13, 4218–4227.
- Lassouane, F.; Aït-Amar, H.; Amrani, S.; Rodriguez-Couto, S. A promising laccase immobilization approach for Bisphenol A removal from aqueous solutions. Bioresour. Technol. 2018, 271, 360–367.
- Pereira Cipolatti, E.; Valério, A.; Henriques, R.O.; Pinto, M.C.C.; Fernandez Lorente, G.; Manoel, E.A.; Guisan, J.M.; Ninow, J.L.; de Oliveira, D.; Costa Pessela, B. Production of new nanobiocatalysts via immobilization of lipase B from C. antarctica on polyurethane nanosupports for application on food and pharmaceutical industries. Int. J. Biol. Macromol. 2020, 165, 2957–2963.
- Pan, J.; Ou, Z.; Tang, L.; Shi, H. Enhancement of catalytic activity of lipase-immobilized Fe3O4 -chitosan microsphere for enantioselective acetylation of racemic 1-phenylethylamine. Korean J. Chem. Eng. 2019, 36, 729–739.
- Melo, A.D.Q.; Silva, F.F.M.; dos Santos, J.C.S.; Fernandez-Lafuente, R.; Lemos, T.L.G.; Dias Filho, F.A. Synthesis of benzyl acetate catalyzed by lipase immobilized in nontoxic chitosan-polyphosphate beads. Molecules 2017, 22, 2165.
- Tudorache, M.; Gheorghe, A.; Negoi, A.; Enache, M.; Maria, G.-M.; Parvulescu, V. Bifunctional carbohydrate biopolymers entrapped lipase as catalyst for the two consecutive conversions of α-pinene to oxy-derivative. Carbohydr. Polym. 2016, 152, 726–733.
- Nemiwal, M.; Zhang, T.C.; Kumar, D. Enzyme immobilized nanomaterials as electrochemical biosensors for detection of biomolecules. Enzyme Microb. Technol. 2022, 156, 110006.
- Tutunaru, O.; Mihailescu, C.M.; Savin, M.; Tincu, B.C.; Stoian, M.C.; Muscalu, G.S.; Firtat, B.; Dinulescu, S.; Craciun, G.; Moldovan, C.A.; et al. Acetylcholinesterase entrapment onto carboxyl-modified single-walled carbon nanotubes and poly(3,4-ethylenedioxythiophene) nanocomposite film, electrosynthesis characterization, and sensor application for dichlorvos detection in apple juice. Microchem. J. 2021, 169, 106573.
- Cano-Raya, C.; Dencheva, N.V.; Braz, J.F.; Malfois, M.; Denchev, Z.Z. Optical biosensor for catechol determination based on laccase-immobilized anionic polyamide 6 microparticles. J. Appl. Polym. Sci. 2020, 137, e49131.
- Aldea, A.; Leote, R.J.B.; Matei, E.; Evanghelidis, A.; Enculescu, I.; Diculescu, V.C. Gold coated electrospun polymeric fibers as new electrode platform for glucose oxidase immobilization. Microchem. J. 2021, 165, 106108.
- Bayazidi, P.; Almasi, H.; Asl, A.K. Immobilization of lysozyme on bacterial cellulose nanofibers: Characteristics, antimicrobial activity and morphological properties. Int. J. Biol. Macromol. 2017, 107, 2544–2551.
- Li, S.; Wang, Y.; Li, X.; Lee, B.S.; Jung, S.; Lee, M. Enhancing the thermo-stability and anti-biofilm activity of alginate lyase by immobilization on low molecular weight chitosan nanoparticles. Int. J. Mol. Sci. 2019, 20, 4565.
- Yuan, M.; Dai, F.; Li, D.; Fan, Y.; Xiang, W.; Tao, F.; Cheng, Y.; Deng, H. Lysozyme/collagen multilayers layer-by-layer deposited nanofibers with enhanced biocompatibility and antibacterial activity. Mater. Sci. Eng. C 2020, 112, 110868.
- Baidamshina, D.R.; Koroleva, V.A.; Olshannikova, S.S.; Trizna, E.Y.; Bogachev, M.I.; Artyukhov, V.G.; Holyavka, M.G.; Kayumov, A.R. Biochemical properties and anti-biofilm activity of chitosan-immobilized papain. Mar. Drugs 2021, 19, 197.
- Filho Moreira, R.N.F.; Vasconcelos, N.F.; Andrade, F.K.; de Freitas Rosa, M.; Silveira Vieira, R. Papain immobilized on alginate membrane for wound dressing application. Colloids Surf. B 2020, 194, 111222.
- Teske, M.; Kießlich, T.; Fischer, J.; Bahl, H.; Wulf, K. Immobilizing hydrolytic active papain on biodegradable PLLA for biofilm inhibition in cardiovascular applications. Curr. Dir. Biomed. Eng. 2020, 6, 6–9.
- Visan, A.; Cristescu, R.; Stefan, N.; Miroiu, M.; Nita, C.; Socol, M.; Florica, C.; Rasoga, O.; Zgura, I.; Sima, L.E.; et al. Antimicrobial polycaprolactone/polyethylene glycol embedded lysozyme coatings of Ti implants for osteoblast functional properties in tissue engineering. Appl. Surf. Sci. 2017, 417, 234–243.
- Sklyarenko, A.V.; El, M.A.; Kurochkina, V.B.; Yarotsky, S.V. Enzymatic synthesis of β-lactam acids (Review). Appl. Biochem. Microbiol. 2015, 51, 627–640.
- Mohammadi, M.; Habibi, Z.; Gandomkar, S.; Yousefi, M. A novel approach for bioconjugation of Rhizomucor miehei lipase (RML) onto amine-functionalized supports; Application for enantioselective resolution of rac-ibuprofen. Int. J. Biol. Macromol. 2018, 117, 523–531.
- Gilani, S.L.; Najafpour, G.D.; Heydarzadeh, H.D.; Moghadamnia, A. Enantioselective synthesis of (S)-naproxen using immobilized lipase on chitosan beads. Chirality 2017, 29, 304–314.
- Bilal, M.; Asgher, M.; Iqbal, H.M.N.; Hu, H. Gelatin-immobilized manganese peroxidase with novel catalytic characteristics and its industrial exploitation for fruit juice clarification purposes. Catal. Lett. 2016, 146, 2221–2228.
- Irshad, M.; Murtza, A.; Zafar, M.; Bhatti, K.H.; Rehman, A.; Anwar, Z. Chitosan-immobilized pectinolytics with novel catalytic features and fruit juice clarification potentialities. Int. J. Biol. Macromol. 2017, 104, 242–250.
- Benucci, I.; Lombardelli, C.; Cacciotti, I.; Esti, M. Papain covalently immobilized on chitosan–clay nanocomposite films: Application in synthetic and real white wine. Nanomaterials 2020, 10, 1622.
- Fernández-Pacheco, P.; García-Béjar, B.; Briones Pérez, A.; Arévalo-Villena, M. Free and immobilised β-glucosidases in oenology: Biotechnological characterisation and its effect on enhancement of wine aroma. Front. Microbiol. 2021, 12, 723815.
- Wang, P.; Zhang, C.; Zou, Y.; Li, Y.; Zhang, H. Immobilization of lysozyme on layer-by-layer self-assembled electrospun films: Characterization and antibacterial activity in milk. Food Hydrocoll. 2021, 113, 106468.
- Bedade, D.K.; Sutar, Y.B.; Singhal, R.S. Chitosan coated calcium alginate beads for covalent immobilization of acrylamidase: Process parameters and removal of acrylamide from coffee. Food Chem. 2019, 275, 95–104.
