Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Christoph Nienaber and Version 2 by Dean Liu.
The aorta is the largest artery in the body, delivering oxygenated blood from the left ventricle to all organs. Dissection of the aorta is a lethal condition caused by a tear in the intimal layer of the aorta, followed by blood loss within the aortic wall and separation of the layers to full dissection. The aorta can be affected by a wide range of causes including acute conditions such as trauma and mechanical damage; and genetic conditions such as arterial hypertension, dyslipidaemia, and connective tissue disorders; all increasing the risk of dissection. Both rapid diagnostic recognition and advanced multidisciplinary treatment are critical in managing aortic dissection patients.
- aortic dissection
- diagnosis
- TEVAR
- prognosis
1. Introduction
The aorta is the body’s largest blood vessel, and its primary function is to carry oxygen and blood from the left ventricle to other organs of the body. Anatomically, the aorta is divided into four parts: the ascending aorta (which starts from the heart and from which coronary arteries arise), the aortic arch (which bends over the heart and turns towards the posterior thoracic wall), the descending thoracic aorta (which extends through the posterior thoracic cavity in vicinity of the spine) and finally, the abdominal aorta (which runs below the diaphragm) [1][2][1,2]. Clinically, aortic dissection is classified by the location of the dissection and/or origin of the intimal tear and the expanse of the dissection. Stanford type A (or DeBakey type I and II) involves the ascending aorta, while Stanford type B (or DeBakey IIIa and IIIb) involves the descending thoracic and abdominal aorta [3][4][3,4].
The aortic wall comprises three layers: the thin tunica intima which faces the bloodstream, the thick musculo-elastic tunica media, and the outer fibrous tunica adventitia (Figure 1). In acute aortic dissection, a tear in the intima layer results in blood entry to the media layer, developing in an intimal flap and dividing the original vessel into true and false lumen. A broad range of diseases can affect the aorta, including acute (such as trauma and hypertensive emergencies) or chronic conditions (such as chronic arterial hypertension, connective tissue disorders, inflammatory vasculitis and atherosclerosis). Aortic dissection is considered a complex vascular scenario that benefits in terms of prognosis from a rapid diagnosis and advanced teamwork including cardiac surgeons, cardiologists, vascular interventionists, and radiologists.
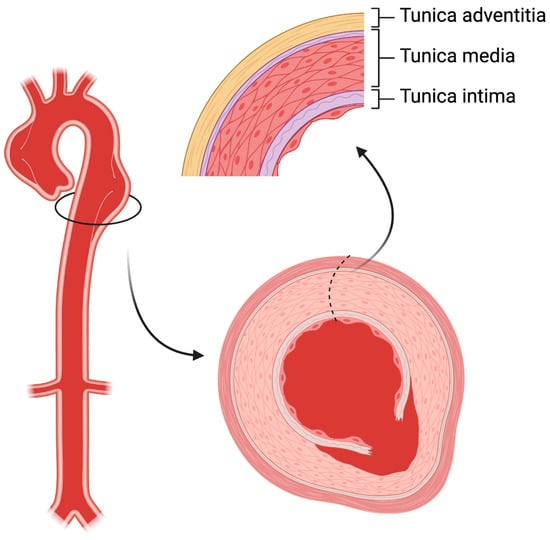
Figure 1. The structure of aortic wall. In aortic dissection, a tear in the intima layer results in blood entry to the media layer, developing in an intimal flap and dividing the original vessel into true and false lumen.
2. Epidemiology
Population-based studies in the US and Europe indicate an incidence of 2.6 to 3.5 cases per 100,000 person-years [5][6][5,6]. Another study from the UK observed 92,728 patients over 10 years, and reported a higher incidence (6 per 100,000 person-years) of acute aortic dissection [7], which is similar to data from Sweden with an incidence of 7.2 per 100,000 person-years [8]. The real-world incidence may be underestimated due to omission of pre-admission deaths in all studies. Interestingly, the demographic forecasts from the UK Office for National Statistics predicts that the incidence of aortic dissection will rise from 3892 in 2010 to 6893 in 2050 in both men and women, with the majority occurring in individuals over 75 [7][9][7,9]. The number of cases of acute aortic dissection seems to be rising in Western countries, possibly related to increased awareness of the disease, as well as access to the use of advanced imaging techniques (particularly CT) in emergency departments [10][11][12][10,11,12]. The frequency of acute aortic dissection is notably higher in males, in particular those who are older [8][13][8,13]. Women are usually older than men at presentation, with a mean age of 72 years vs. 64 years in men [14][15][14,15]. Despite the higher rates of aortic dissection being in men, it is women who have a higher mortality rate. Data from the International Registry of Aortic Dissection (IRAD) indicates that women with aortic dissection typically present at hospital later than men with worse clinical status (coma and tamponade). The data also show they have different types of symptoms and show less typical clinical presentation [16]. These data may partly be explained in a different analysis; after age-adjustment, women have higher rates of mortality then men and more often suffer pre-hospital death [8][16][8,16].3. Classification
Aortic dissection can be categorized in different ways in terms of anatomy and symptom onset. The two most frequent anatomic classification systems are: DeBakey classification, which is based on the site of origin of the intimal tear [4]; and Stanford classification, which specifies the involvement, or lack thereof, of the ascending aorta [3]. DeBakey classification precisely describes the site of the dissected segment or diseased lesion: Type I dissections usually originate from the ascending aorta and have the most extensive involvement including the ascending aorta, aortic arch, descending aorta and further; Type II dissections originate from and are only limited to the ascending aorta; Type III dissections originate from the descending aorta after the left subclavian artery orifice, and affects the descending aorta (Type IIIa) and/or distal abdominal aorta (Type IIIb) [4]. Stanford classification helps indicate different management for dissection cases in clinical practise: Type A dissections involve the ascending aorta (DeBakey type I and II), usually requiring swift surgery; while Type B dissections only involve the descending aorta (DeBakey type III) and can be managed endovascularly or medically [3][17][3,17] (Figure 2). Recently, a unique group, so-called non-A non-B, which does not completely fit DeBakey and Standford classification has been identified. These patients develop a dissection originating from the aortic arch, or the descending aorta and retrogradely involve the arch, without involving the ascending aorta. Several studies suggest that arch dissections could lead to organ malperfusion and aortic rupture, which require prompt intervention and careful management [18][19][18,19]. A new classification system, which includes the type of dissection (adapting Stanford system A, B or non-A non-B), the location of the primary entry tear, and the presence of malperfusion (TEM) has been introduced, but is yet awaiting community acceptance [19].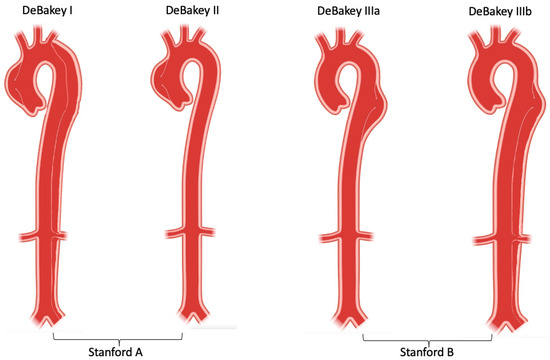
Figure 2. DeBakey and Stanford aortic dissection classification.
