Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Camila Xu and Version 1 by Ashok Mohanty.
The mammary gland, a unique organ in mammals, is a derivative of ventral skin. The bovine mammary gland is composed of parenchymatous and stromal compartments. The parenchyma is a cellular compartment that contains two main cellular lineages. Mammary gland homeostasis and regeneration are maintained by the controlled
activity of stem cells. These mammary stem cells are multipotent in nature, and give rise to epithelial and myoepithelial cells.
- bovine
- mammary gland development
- mammary hierarchy
1. Introduction
The mammary gland, a unique organ in mammals, is a derivative of ventral skin [1,2][1][2]. The bovine mammary gland is composed of parenchymatous and stromal compartments. The parenchyma is a cellular compartment that contains two main cellular lineages. The major compartment is made up of inner luminal cells that surround a central lumen, while the minor compartment is made up of outer myoepithelial cells that are found at the base of the mammary epithelium, adjacent to the basement membrane (BM), and separate the mammary epithelium from the stroma. The luminal cells can be further divided into ductal (lining the lumen of ducts) and alveolar (milk-synthesizing cells) subtypes. The stromal compartment, unlike the parenchyma, is made up of a variety of cells (fibroblasts, mesenchymal cells, adipocytes, leukocytes, and blood cells) as well as extracellular matrix (ECM) (laminin, fibronectin, collagen, proteoglycan, etc.) [3,4][3][4].
The gross morphology of the mammary gland varies by species, but the microscopic structure is nearly identical. The morphology of the bovine mammary gland is more similar to that of the human breast in terms of the functional unit and stroma composition [5]. The bovine mammary gland, also known as the udder, is divided into two equal and distinct halves by a median suspensory ligament. Each half has two glands, each leading to a teat (Figure 1A). The teat consists of a cistern and canal and an aperture through which milk is discharged. The udder is attached to skeletal muscles, which support its large size [6]. The alveolus is the functional unit of the lactating mammary gland (Figure 1B). A single layer of cuboidal to columnar luminal mammary epithelial cells (MECs), which are primarily engaged in milk synthesis and secretion, line each alveolus. The myoepithelial cells, which have smooth muscle-like properties, surround these cells. These cells form a continuous barrier over epithelial cells in the ducts, preventing them from accessing the BM. In alveoli, the myoepithelial cells form a discontinuous layer so that the MECs are in direct contact with the underlying BM, which plays a crucial role in their differentiation [7]. The capillary network surrounds the alveoli, from which the layers of MECs take milk component precursors for lactose, protein, and milk fat synthesis. Suckling by pups causes the anterior lobe of the pituitary to release oxytocin hormone [8], which binds to oxytocin receptors in the mammary gland [9] and causes myoepithelial cells to contract in lactating mammary glands [10]. This compression aids in the ejection of milk from luminal epithelial cells into the alveolus and subsequently to the milk ducts and teat lumen [6,11][6][11].
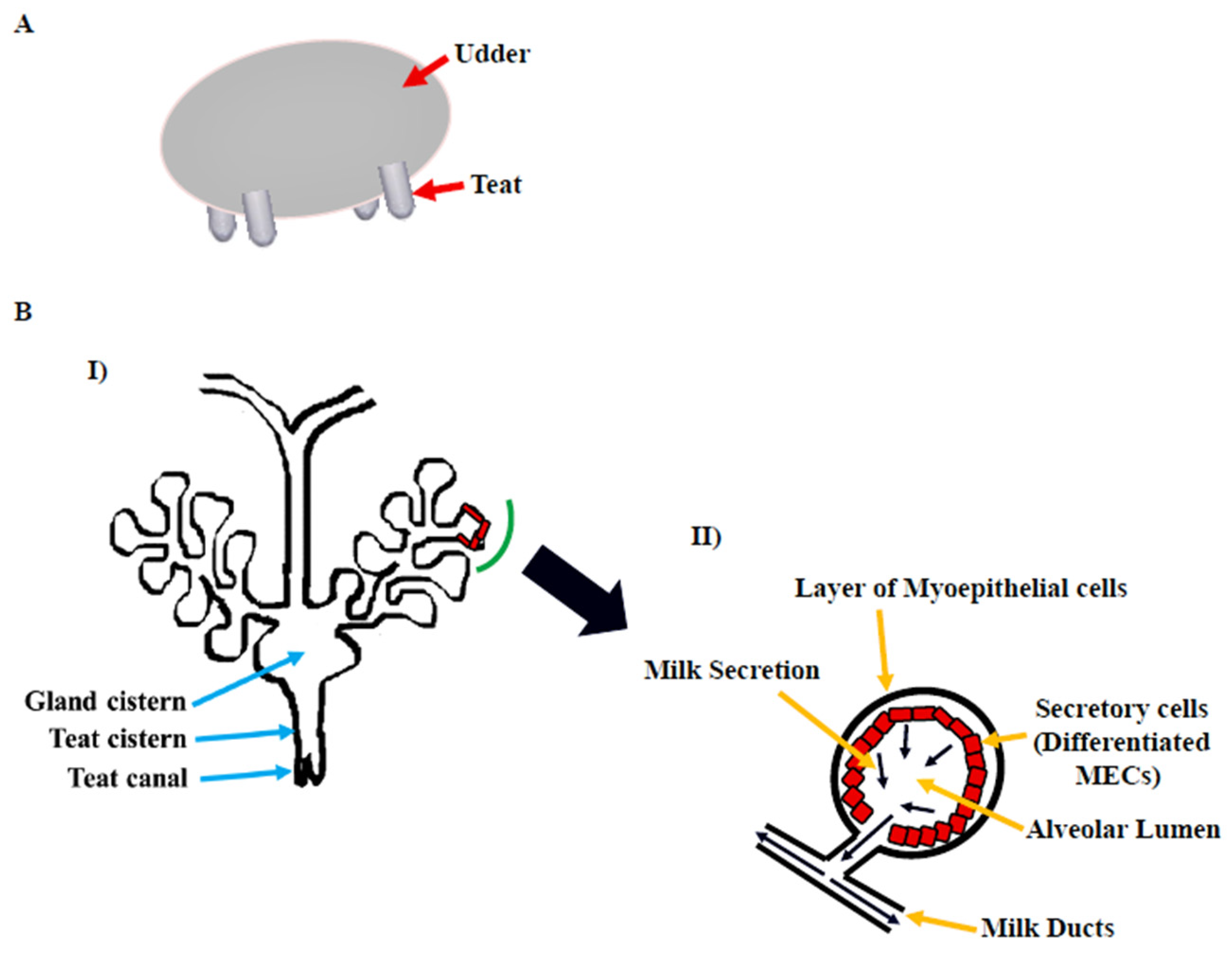
Figure 1. Bovine mammary gland. (A) Diagram illustrating the bovine udder. (B) I: Microscopic anatomy of bovine mammary gland consisting of milk ducts, alveoli, cistern (gland and teat), and teat canal. II: Secretory unit of mammary gland, i.e., alveolus containing milk-secreting mammary epithelial cells (MECs), covered by the myoepithelial cells which are in direct contact with extracellular matrix (ECM) [6].
2. Mammary Hierarchy
2.1. Mammary Stem Cells
Mammary gland homeostasis and regeneration are maintained by the controlled activity of stem cells [12,13][12][13]. Cluster of differentiation 24 (CD24) (heat-stable antigen) and CD49f (α6-integrin) are cell surface markers that distinguish mammary stem cells (MaSCs). These bovine MaSCs (bMaSCs) are multipotent and give rise to bipotent putative stem cells. The putative stem cells differentiate into luminal and basal progenitors. These progenitors differ in the type of cell surface and lineage markers they express (Figure 2). The luminal restricted progenitors are unipotent in nature and differentiate into secretory epithelial cells, whereas the basal progenitors are bipotent and can give rise to ductal epithelial cells and myoepithelial cells. The NOTCH signaling pathway in the putative stem cells and basal progenitors helps in self-renewal [14,15][14][15]. Both bovines and humans have a similar developmental hierarchy [16].
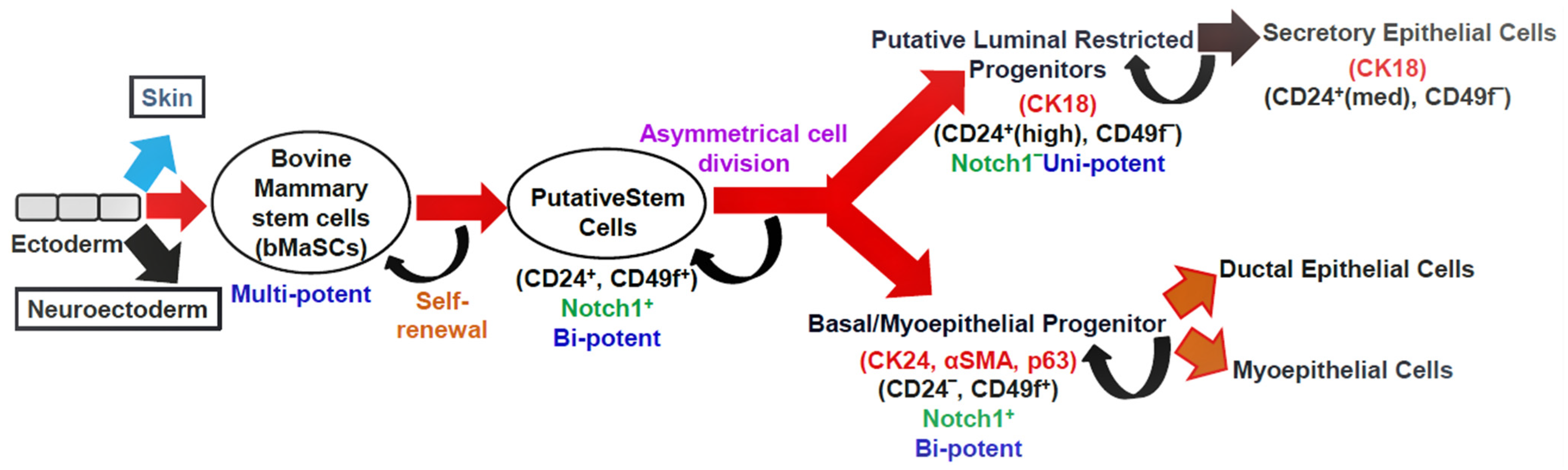
Figure 2. The hypothetical model depicting the mammary epithelial hierarchy in the bovine mammary gland. Cell surface markers: CD24, CD49f; lineage markers: CK18, CK14, αSMA, and p63; CK: cytokeratin, αSMA: smooth muscle alpha-actin, p63: tumor protein p63; med: medium expression.
2.1.1. Estrogen Receptor
In the mammary gland, 30–50% of MECs express estrogen receptor (ER) and progesterone receptor (PR) [17]. Two distinct genes on different chromosomes encode two different isoforms of ER, ERα and ERβ. In bovines, ERα is mostly expressed in luminal MECs, certain fat pad adipocytes, and fibroblast cells. In other animals, including humans, monkeys, and mice, its expression is restricted to luminal epithelial cells. ERβ, on the other hand, is mostly expressed in luminal epithelial, myoepithelial, stromal, and fibroblast cells, and its quantity in the bovine mammary gland is significantly lower than that in humans, monkeys, and mice [18].
17β-estradiol (E2) is an important regulator of mammary gland development that works through the ER [19]. In ERα+ and ERβ+ cells, E2 stimulates proliferation and apoptosis, respectively [20]. The non-pregnant heifer has a higher level of ERα expression, which decreases during lactation and involution [21,22][21][22]. ERβ expression, on the other hand, is stable throughout the stages of mammary gland development, except during the conclusion of udder feeding, when it is expressed considerably [21]. ER+ cells coexist with ER− cells in close proximity. These cells release paracrine chemicals that regulate ER− cell proliferation after being activated by E2 [17].
2.1.2. Progesterone Receptor
Progesterone receptor (PR) is mostly expressed in epithelial, stromal, vascular, and fat cells in bovine [18]. Its expression is restricted to the luminal epithelial cells in other animals such as mice, monkeys, and humans. In the mammary gland, A and B isoforms of PR exist in a specific ratio that varies by species [18], with a 3:1 ratio in bovines [21] and mice [23]. Three different PR isoforms, namely A, B, and C, are expressed in the mammary gland of non-pregnant heifers; however, only the B isoform is expressed during lactation and involution [21].
3. Structural and Functional Development of the Bovine Mammary Gland
In terms of physical anatomy, location, and hormonal requirements, mammary gland development varies among species [24]. In ruminants, the mammary gland is positioned in the inguinal region; in pigs, rats, and mice, it is located in the torso region; and in elephants and primates, it is located in the thoracic region [2]. Unlike other organs that grow during embryogenesis, the mammary gland develops most of its characteristics after birth before fully maturing during pregnancy [25,26][25][26]. Ruminants, humans, and rodents have comparable mammary gland growth patterns [27,28][27][28]. Prepuberty, postpuberty, gestation, and lactation are the four stages of its development [29]. Several factors, including endocrine, autocrine, paracrine, intracellular factors, and extracellular matrix (ECM), regulate each developmental stage [26]. Following cycles of pregnancy, parturition, lactation, and involution, the female mammary gland experiences repeated rounds of apoptosis and growth. As a result, it is a fantastic model for researching stem/progenitor cells that can expand and replenish themselves repeatedly [30].References
- Medina, D. The mammary gland: A unique organ for the study of development and tumorigenesis. J. Mammary Gland Biol. Neoplasia 1996, 1, 5–19.
- Robinson, G.W. Cooperation of signaling pathways in embryonic mammary gland development. Nat. Rev. 2007, 8, 963–973.
- Wang, D.; Cai, C.; Dong, X.; Yu, Q.C.; Zhang, X.; Yang, L.; Zeng, Y.A. Identification of multipotent mammary stemcells by protein C receptor expression. Nature 2014, 517, 81.
- Visvader, J.E.; Stingl, J. Mammary stem cells and the differentiation hierarchy, current status and perspectives. Genes Dev. 2014, 28, 143–1158.
- Rauner, G.; Leviav, A.; Mavor, E.; Barash, I. Development of foreign mammary epithelial morphology in the stroma of immunodeficient mice. PLoS ONE 2013, 8, e68637.
- Nickerson, S.C.; Akers, R.M. Mammary gland anatomy. In Encyclopedia of Dairy Sciences; Fuquay, J.W., Fox, P.F., McSweeney, P.L.H., Eds.; Academic Press: San Diego, CA, USA, 2011; Volume 3, pp. 328–337.
- Gudjonsson, T.; Adriance, M.C.; Sternlicht, M.D.; Petersen, O.W.; Bissell, M.J. Myoepithelial cells: Their origin and function in breast morphogenesis and neoplasia. J. Mammary Gland Biol. Neoplasia 2005, 10, 261–272.
- Benson, G.K.; Folley, S.J. Oxytocin as stimulator for the release of prolactin from the anterior pituitary. Nature 1956, 177, 700.
- Sernia, C.; Thomas, W.G.; Gemmell, R.T. Oxytocin receptors in the mammary gland and reproductive tract of a marsupial, the brushtail possum (Trichosurus vulpecula). Biol. Reprod. 1991, 45, 673–679.
- Moore, D.M.; Vogl, A.W.; Baimbridge, K.E.N.N.E.T.H.; Emerman, J.T. Effect of calcium on oxytocin-induced contraction of mammary gland myoepithelium as visualized by NBD-phallacidin. J. Cell Sci. 1987, 88, 563–569.
- Lincoln, D.W.; Paisley, A.C. Neuroendocrine control of milk ejection. Reproduction 1982, 65, 571–586.
- Kwon, O.J.; Valdez, J.; Zhang, L.; Zhang, B.; Wei, X.; Su, Q.; Ittmann, M.M.; Creighton, C.J.; Xin, L. Increased Notch signaling inhibits anoikis and stimulates proliferation of prostate luminal epithelial cells. Nat. Commun. 2014, 5, 4416.
- Biteau, B.; Hochmuth, C.E.; Jasper, H. Maintaining tissue homeostasis, dynamic control of somatic stem cell activity. Cell Stem Cell 2011, 9, 402–411.
- Rauner, G.; Barash, I. Cell hierarchy and lineage commitment in the bovine mammary gland. PLoS ONE 2012, 7, e30113.
- Rodilla, V.; Dasti, A.; Huyghe, M.; Lafkas, D.; Laurent, C.; Reyal, F.; Fre, S. Luminal Progenitors Restrict Their Lineage Potential during Mammary Gland Development. PLoS Biol. 2015, 13, e1002069.
- Martignani, E.; Eirew, P.; Accornero, P.; Eaves, C.J.; Baratta, M. Human milk protein production in xenografts of genetically engineered bovine mammary epithelial stem cells. PLoS ONE 2010, 5, e13372.
- Clarke, R.B.; Howell, A.; Potten, C.S.; Anderson, E. Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 1997, 57, 4987–4991.
- Connor, E.E.; Meyer, M.J.; Li, R.W.; Van Amburgh, M.E.; Boisclair, Y.R.; Capuco, A.V. Regulation of Gene Expression in the Bovine Mammary Gland by Ovarian Steroids. J. Dairy Sci. 2007, 90, E55–E65.
- Zielniok, K.; Motyl, T.; Gajewska, M. Functional interactions between 17β-estradiol and progesterone regulate autophagy during acini formation by bovine mammary epithelial cells in 3D cultures. BioMed Res. Int. 2014, 2014, 382653.
- Helguero, L.A.; Faulds, M.H.; Gustafsson, J.Å.; Haldosen, L.A. Estrogen receptors alfa (ER ) and beta (ER ) differentially regulate proliferation and apoptosis of the normal murine mammary epithelial cell line HC11. Oncogene 2005, 24, 6605–6616.
- Schams, D.; Kohlenberg, S.; Amselgruber, W.; Berisha, B.; Pfaffl, M.W.; Sinowatz, F. Expression and localisation of oestrogen and progesterone receptors in the bovine mammary gland during development, function and involution. J. Endocrinol. 2003, 177, 305–317.
- Ewan, K.B.; Oketch-Rabah, H.A.; Ravani, S.A.; Shyamala, G.; Moses, H.L.; Barcellos-Hoff, M.H. Proliferation of estrogen receptor-α-positive mammary epithelial cells is restrained by transforming growth factor-β1 in adult mice. Am. J. Pathol. 2005, 167, 409–417.
- Schneider, W.; Ramachandran, C.; Satyaswaroop, P.G.; Shyamala, G. Murine progesterone receptor exists predominantly as the 83-kilodalton ‘A’ form. J. Steroid Biochem. Mol. Biol. 1991, 38, 285–291.
- Nickel, R.; Schummer, A.; Seiferle, E.; Frewein, J.; Wilkens, H.; Wille, K.H.; Siller, W.G.; Stokoe, W.M. The Anatomy of the Domestic Animals. Volume 1. The locomotor system of the domestic mammals. Verlag Paul Parey: Berlin, Germany, 1986.
- Porter, J.C. Hormonal Regulation of breast development and activity. J. Investig. Dermatol. 1974, 63, 85–92.
- Borellini, F.; Oka, T. Growth Control and Differentiation in Mammary Epithelial Cells. Environ. Health Perspect. 1989, 80, 85–99.
- Cadar, M.; Mireşan, V.; Lujerdean, A.; Răducu, C. Mammary gland histological structure in relation with milk production in sheep. Sci. Pap. Sci. Biotechnol. 2012, 45, 146–148.
- Gjorevski, N.; Nelson, C.M. Integrated morphodynamic signaling of the mammary gland. Mol. Cell Biol. 2011, 12, 581–593.
- Yart, L.; Lollivier, V.; Marnet, P.G.; Dessauge, F. Role of ovarian secretions in mammary gland development and function in ruminants. Animal 2014, 8, 72–85.
- Inman, J.L.; Robertson, C.; Mott, J.D.; Bissell, M.J. Mammary gland development, cell fate specification, stem cells and the microenvironment. Development 2015, 142, 1028–1042.
More
