Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Robert Ancuceanu and Version 2 by Camila Xu.
Aspergillus species, especially A. fumigatus, and to a lesser extent others (A. flavus, A. niger, A. terreus), although rarely pathogenic to healthy humans, can be very aggressive to immunocompromised patients (they are opportunistic pathogens). Calcineurin is a serine/threonine phosphatase activated by elevated concentrations of calcium, which connects upstream calcium signaling pathways to downstream protein signaling through changes in phosphorylation states.
- Aspergillus
- antifungal
- calcineurin
- heat shock proteins
1. Introduction
Species belonging to the genus Aspergillus are filamentous fungi, widespread in various environments, where they live as saprophyte organisms [1]. Their spores are ubiquitous in the atmosphere and have tiny sizes (less than four μm) that allow them to be easily inhaled into the lower respiratory tract [2]. Although the known number of Aspergillus species exceeds 250, only a handful are known to be associated with human infections, mostly A. fumigatus Fresenius (50–60% of all aspergillosis cases) and, to a lesser extent, A. flavus Link, A. niger van Tieghem, and A. terreus Thom (each responsible for 10–15% of all aspergillosis cases) [2]. Although usually less frequently encountered, other Aspergillus species can also be problematic for clinical practice: A. ustus (Bainier) Thom & Church and A. lentulus Balajee & K.A. Marr are increasingly identified as agents responsible for invasive aspergillosis and are resistant to amphotericin B and at least some of the azoles. A. terreus also manifests intrinsic resistance to amphotericin B [3][4][3,4].
2. Aspergillosis
As for the large majority of fungi, Aspergillus species are rarely pathogenic for humans with a healthy immune system, but they may be very nosogenic for immunocompromised patients, i.e., they are opportunistic pathogens [5]. Such immunocompromised patients are those undergoing an organ transplant [5], those with neutropenia induced by antitumor chemotherapy, or under corticosteroid treatment [6]. Patients with impaired NADPH oxidase complexes, STAT3 or CARD9 signaling pathways, and those with leukocyte adhesion deficiencies or severe congenital neutropenia, are also susceptible to fungal infections, particularly aspergillosis [7]. Lung transplant patients, especially, are vulnerable to infections caused by Aspergillus species, and such conditions are associated with a high rate mortality rate [8]. In tropical and subtropical zones, Aspergillus and other filamentous fungi are the most frequent cause of fungal keratitis (unlike the temperate climates, where Candida sp. represents the leading cause) [9].
Until lately, finding Aspergillus in respiratory biological specimens was typically disregarded as a contaminant, except for cases where the patient was considered immunocompromised [10]. Because it is now accepted that severe disease states (e.g., sepsis) can have a strong negative effect on immunity, and assessing the extent of immunosuppression is challenging in ICU patients, evidence of aspergillosis is currently to be taken seriously in such patients [10].
Chronic pulmonary aspergillosis is a disease that in Europe only affects almost a quarter million persons; unlike invasive aspergillosis, these patients are not immunocompromised [11]. A. fumigatus is often found in respiratory secretions of patients with cystic fibrosis (both children and adults); this does not indicate harmless colonization, although the pathophysiology and clinical management have not been clarified [12]. It tends to be acknowledged, however, that Aspergillus colonization is likely to worsen the evolution of cystic fibrosis even in the absence of allergic bronchopulmonary aspergillosis [13]. A retrospective cohort study in cystic fibrosis patients found that for over a decade (from 1997 to 2007), the frequency of filamentous fungi isolates (mostly Aspergillus species) increased from only 2% to about 28.7% [14]. Moreover, the presence of A. fumigatus in biological samples of patients with chronic respiratory diseases is associated with more frequent manifestations of symptoms, lung fibrosis, and diabetes mellitus [15]. It has been estimated that Aspergillus species cause over 200,000 cases of invasive aspergillosis each year. Over 1.2 million patients are affected by chronic pulmonary aspergillosis (CPA), and between 5 and 10 million patients suffer from allergic bronchopulmonary aspergillosis (ABPA) and severe asthma with fungal sensitization (SAFS) [16]. These are only imperfect estimates because “epidemiological data for fungal infections are notoriously poor” due to frequent misdiagnosis and lack of active data collection on their impact (in the United States, except for coccidioidomycosis, no other fungal disease must be reported to the CDC) [17].
Costs associated with Aspergillus infections are relatively high; the estimates (per case) vary depending on costs considered and assumptions, from EUR 8351–11,821 (when only incremental hospitalization and antifungal product costs are considered) to EUR 26,596–83,300 (when all direct costs are included) [18]. In the USA, it has been estimated that Aspergillus infections are responsible for about USD 1.2 billion (14,820 cases) per year [19].
Both reliable diagnosis and successful treatment of aspergillosis remain a challenge; the presence of the species in a sample is not sufficient to confirm the infection [20], and the therapy is “limited to only a handful of antifungal agents” [21]. Invasive aspergillosis is associated with a gloomy prognosis: its all-cause mortality is estimated to be about 40% at 12 weeks [22]. A review paper from 2018 reported a case fatality rate of 29% in patients with hematological malignancies (almost one in every three patients), which was considered relatively low in comparison with an 88% case fatality rate estimated in 2001 [23]. The survival rates used to be much worse, but the clinical availability of azoles (itraconazole, voriconazole, posaconazole, and, more recently, isavuconazole) resulted in improvements that have been described as “dramatical” [24]. However, antifungal resistance to azoles has emerged and is likely to grow and evolve; the available data suggest that its impact on the clinical outcome is gloomy [25]. The clinical use of polyenes (the primary representative being amphotericin B) is limited by their toxicity [26]. The efficacy of the current antifungals is also restrained by the fact that they act as fungistatic rather than as fungicidal agents, arresting fungal growth but not eradicating the fungal cells [27].
About 16 years ago, the Antimicrobial Availability Task Force of the Infectious Diseases Society of America, discussing aspergillosis, concluded that “more-efficacious and better-tolerated therapies are needed. Orally available compounds would be highly useful” [28]. These have remained mostly desiderata because little progress has been made in this respect, and these needs are as relevant today as they were when first expressed.
Because human and fungal cells are both eukaryotes, the latter share more common metabolic and signaling pathways with the former than microbial cells. Therefore, identifying appropriate targets for antifungal medicines remains a challenge [29]. Since human cells are devoid of a cell wall, whereas fungal cells are endowed with such a wall consisting of polysaccharides (glucan, chitin) and glycoproteins, it has been regarded as “an ideal target” [29]. Although much research has been dedicated to the fungal cell wall as an antifungal target (with good reason), and most currently used antifungals act at the cell wall or plasma membrane levels, other targets have been, until recently, less explored. The flurry of innovation in molecular biology and “omics” sciences in the last two decades has opened the door wide for exploring other potential targets, which could act synergistically with the conventional antifungals and overcome resistant strains. In this respapearch, researchersr, we focus on two related signaling pathways that have attracted much attention in the last years: the calcineurin and heat shock proteins pathways.
3. The Calcineurin Pathway
Calcineurin is a serine/threonine phosphatase activated by elevated concentrations of calcium, which connects upstream calcium signaling pathways to downstream protein signaling through changes in phosphorylation states [30]. It has a large number of substrates and binding partners and a wide range of cell functions, orchestrating a diverse array of cell events and processes [31]. Calcineurin is activated by Ca2+-calmodulin (Figure 1), apparently the only phosphatase that needs both calcium and calmodulin to exert its enzymatic function [32]. It is interesting in this context to mention that known antifungal azoles inhibit calmodulin in a concentration-dependent fashion, and it has been speculated that this inhibition could contribute to their antifungal effects (besides their assumed primary mechanism of ergosterol biosynthesis inhibition) [33].
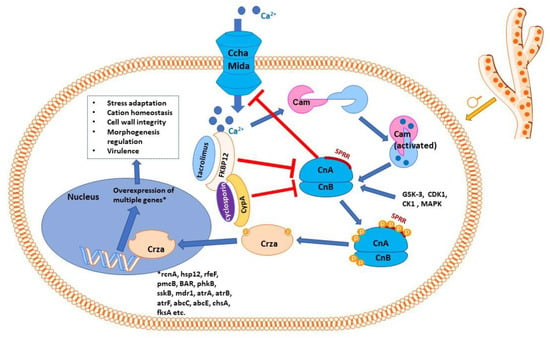
Figure 1. Schematic representation of calcineurin signaling pathway in Aspergillus sp. Calmodulin is activated by calcium ions, which penetrate inside the cell through the high-affinity calcium influx system, consisting mainly of the Ccha and MidA calcium channels, the key access route for calcium in the cell when calcium levels are low. CchA, at least, is negatively regulated by calcineurin. The Ca2+–calmodulin complex activates calcineurin with its two subunits, catalytic (CnA) and regulatory (CnB). Calcineurin presents a serine–proline-rich region (SPRR); phosphorylating the four serine residues of SPRR, as well as phosphorylation of CnA C-terminus and CnB N-terminus, is necessary for calcineurin to exert its effects. Complexes of immunophilins with immunosuppressant molecules (FK506-binding protein with tacrolimus or cyclophilin A with cyclosporin) inhibit calcineurin. Activated (phosphorylated) calcineurin acts on its effector protein, CrzA (the zinc finger transcription factor Crz1 homolog), dephosphorylating it. Dephosphorylated Crza is translocated into the nucleus, where it activates the transcription of a number of genes, which encode proteins involved in cation homeostasis (rcnA, pmcA-pmcB), other proteins involved in stress response (sskB, hsp12), efflux proteins (mdr1, atrA, atrB, atrF, abcC, abcE), proteins responsible for cell wall integrity (chsA, fksA), proteins involved in regulating morphogenesis and in virulence (BAR, but also pmcA, sskB, etc.). As for many such diagrams, only the most relevant molecules and interactions are shown; in reality, the signaling network is more complex and currently only partially known.
Calcineurin is a heterodimer protein consisting of two chains: a catalytic subunit (calcineurin A) and a regulatory subunit (calcineurin B) [34]. Alterations in human calcineurin signaling have been implicated in numerous pathologies, from heart diseases [35] to autoimmune pathologies [36], from nociception [37] to new-onset diabetes mellitus following transplantation [38]. In various fungi species, calcineurin has been found to play multiple regulatory roles, e.g., regulating adaptation to stress, growth at higher values of pH and temperature, cation homeostasis, membrane trafficking and reacting to membrane stress, cell wall integrity, regulating morphogenesis, hyphal branching, the appressorium, sclerotial formation, and virulence [39][40][41][39,40,41]. Although calcineurin pathways seem conserved among different fungal taxons, considerable differences have been identified between various genera; therefore, extrapolating from one taxon to another may not be appropriate [39].
Lung surfactant protein D (SP-D) has a protective role against various microorganisms, its deficiency being associated with increased susceptibility to bacterial or viral infections [42]. Its binding to A. fumigatus hyphae is influenced by the activity of calcineurin. The deletion of the catalytic subunit of the latter (calcineurin A) results in canceling the binding of SP-D to the fungal hyphae [42]. However, the potential clinical application of this finding is still unclear, and more research is necessary to understand its implications.
Data from different fungal genera, including Aspergillus sp., have indicated that calcineurin plays critical roles in the development and virulence of fungi and has an essential effect on the activity of antifungal medicinal products [43]. Mutants of A. fumigatus devoid of the catalytic subunit of calcineurin (CnaA) manifested multiple defective phenotypic features, resulting in reduced filamentation and sporulation and markedly attenuated pathogenicity in several animal models [43][44][43,44]. Deleting the regulatory unit (CnaB) had a similar impact, whereas deletion of both subunits had an additive effect, the double mutants growing slower than each single mutant [39][40][39,40]. Furthermore, the hyphal septa were curved, wavy, or otherwise impaired, indicating disorganization of the cell wall related to a marked decrease in its beta-glucan content and a compensatory increase in its chitin content [39][40][39,40]. However, this compensatory response of increasing chitin seems diminished in mutants devoid of calcineurin or Crza genes (for Crza, see below) [45][46][45,46]. Calcineurin gene has also been proven to be essential for other Aspergillus species, such as A. nidulans [47] and A. oryzae [48], and calcineurin A from the latter species, which has a functional homology with that from yeast [49]. Conidia of A. nidulans with disrupted calcineurin A germinate in a proportion of less than 50%, and conidia with disrupted calmodulin germinate only in a percentage of about 60% [47][50][51][47,50,51]. Growth defects at the hyphae levels observed under calcineurin deletion/suppression are canceled with the deletion of the high-affinity calcium channel CchA, the latter having, as a consequence, a decrease in cytoplasmatic Ca2+ concentrations [52].
In A. niger, deleting calcineurin catalytic subunit or other proteins from the calcineurin signaling pathway resulted in a reduced biofilm formation, lower hydrophobicity and adhesive abilities of conidia (which are necessary for biofilm formation), perturbation of hyphae cell wall structure and hyphae flocculation (and thus also affecting biofilm formation) [53].
Aspergillosis results from inhaling Aspergillus conidia (asexual spores) by immunocompromised patients. Once inhaled, the spores will germinate and form filamentous hyphae, whose growth is essential for the occurrence and then maintenance of invasive aspergillosis [51]. Henceforth, arresting or hindering hyphal growth is critical in controlling and preventing the disease, but hyphal growth physiology is currently little understood. The discovery of calcineurin roles in hyphal development has suggested that this signaling pathway is pivotal for controlling invasive aspergillosis, hence the particular interest in its chain links as potential targets for antifungal products [51].
In vitro, cyclosporin (a calcineurin inhibitor employed as an immunosuppressant) was shown to significantly decrease colonies of A. fumigatus in a dose-dependent manner, tending to confirm the hypothesis that calcineurin inhibition results in antifungal effects [54]. Chromofungin is a short peptide fragment derived from chromogranin A (a peptide secreted by chromaffin cells from the adrenal medulla). It has been claimed that the antifungal effect of chromofungin is related to its inhibition of calcineurin, but this seems a somewhat speculative statement, considering that at the relatively high concentration of 5 μM, calcineurin is only inhibited in a proportion of about 32%. In contrast, complete inhibition could be obtained at 250 μM [55].
Structurally diverse inhibitors of calcineurin, such as tacrolimus (FK506), cyclosporin, FK520, and L685,818, have all demonstrated a synergistic effect when associated with caspofungin, as well as with other antifungals, such as itraconazole or cycloheximide [56][57][58][56,57,58]. Such an effect may be attained with concentrations of calcineurin inhibitors usually found in the blood of patients treated for immunosuppression purposes [59]. In vitro, caspofungin and tacrolimus consistently demonstrated antifungal activity against 12 clinical isolates of A. fumigatus, but associated with caspofungin, they showed slightly additive effects at best and more likely no interaction (FICI for tacrolimus + caspofungin was 0.85 for isolates from transplant recipients and 1.05 for isolates from non-transplant patients; FICI between 0.5 and 4 are interpreted as indicating no interactions [60]) [61]. Three motifs involved in the interaction between calcineurin and its substrates have been identified and characterized up to now [62].
Moreover, it was found that the so-called paradoxical effect of caspofungin seems to be mediated by calcineurin (activated through increased Ca2+ concentrations); therefore, its pharmacological inhibition is able to cancel it [46][63][46,63]. Tacrolimus demonstrated additive (but not synergistic) effects in vitro on A. fumigatus when associated with polyhexamethylene biguanide, amphotericin B, or voriconazole [9]. The association of cyclosporin with voriconazole in vivo resulted in worsened effects of the azole antifungal compared with the monotherapy. Although tacrolimus and cyclosporin act through the same mechanism (inhibiting the calcineurin pathway), they bind to different proteins of the immunophilin family, tacrolimus to FKBP12, whereas cyclosporin to cyclophilin, and it was speculated that this could explain the difference between the effects of the two calcineurin inhibitors [9].
Early on, with the discovery of the importance of the calcineurin signaling pathway in fungi, researchers have been asking the question of how feasible it is to target calcineurin in vivo for antifungal effect, given its role in the mammalian immune system and the fact that there was an extensive clinical experience with calcineurin inhibitors in organ transplantation patients [64]. This was a logical and obvious concern because host immunosuppression brought by calcineurin inhibition could be clinically more relevant than the inhibition of fungal growth by the same mechanism, with the consequence of a lack of effectiveness or even exacerbation of the fungal infection [64]. In humans, the calcineurin–NFAT signaling pathway is strongly involved in myeloid progenitor maintenance, and inhibiting this signaling pathway could hinder the activity of the myeloid cells and increase the sensitivity of immunosuppressed patients to invasive aspergillosis [65]. Moreover, it is known that patients treated with calcineurin inhibitors are also much more susceptible to invasive aspergillosis. More recent research has suggested that activation of calcineurin–NFAT by TLR9 results in impaired recruitment of neutrophils and fungal killing, explaining this increased patient susceptibility to fungal infections [66][67][66,67] and cautioning one to the use of calcineurin inhibitors as potential antifungal agents. Calcineurin also orchestrates a lateral transfer of A. fumigatus between macrophages in a necrosis-dependent process; inhibiting calcineurin results in escaping the immune system by the fungus [68].
Furthermore, in heart transplant recipients, unlike patients treated with other immunosuppressive agents (azathioprine, rabbit antithymocyte globulin), patients receiving cyclosporine had a lower rate of invasive aspergillosis (11% vs. 24%), as well as of viral or bacterial infections and lower mortality [69]. Another study compared the infection and mortality risks in two mini-cohorts of liver transplant recipients, one older and one newer. The former used less tacrolimus and more cyclosporin, the latter less cyclosporin and more tacrolimus. The researcheuthors found that the new cohort (with more tacrolimus) had less disseminated aspergillosis than the former [70]. Both of these studies were observational in nature and used historical controls; therefore, their methodological limitations should be taken into account. Some researchers have seen in these studies evidence for a potential clinical benefit of calcineurin inhibitors in preventing or controlling aspergillosis [61]. Others could look at the empty half and remark that a sizeable proportion (11–24%) of patients treated with calcineurin inhibitors still falls victim to invasive aspergillosis; thus, if beneficial as antifungal, they are definitely imperfect. Experimental data from rabbits showed that challenging the animals with the same inoculum administered intratracheally, rabbits with granulocytopenia induced by araC had a 100% mortality, and rabbits receiving cyclosporin A and methylprednisolone had a 100% survival, whereas in the later conidia germinated abundantly, mature hyphae were rare unlike the granulocytopenic animals [71]. Furthermore, in a murine invasive aspergillosis model, animals treated with cyclosporin survived significantly less than animals treated with vehicle only, as well as less than animals treated with other immunosuppressant agents, also inhibitors of calcineurin. Whereas the median survival for mice treated with cyclosporin was three days, for the vehicle it was 6.5 days (p = 0.001), for tacrolimus 6.5 days (p = 0.03), and for sirolimus 1 and 10 mg/kg 7.5 and 9.5 days (p = 0.002 and p = 0.001) [72].
