Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Liang-en Yu.
Neurological and psychiatric patients have increased dramatically in number in the past few decades. Effective treatments for these diseases and disorders are limited due to heterogeneous and unclear pathogenic mechanisms.
- enteroendocrine cells
- enterochromaffin
- GLP1
- GLP2
1. Introduction
The number of patients suffering from neurological and psychiatric disorders has increased dramatically in the past few decades. According to the World Health Organization (WHO) epidemiology statistics, the number of Parkinson’s disease (PD) patients has doubled within the last 25 years [1]. Moreover, recent updates from the WHO indicate that there are nearly a billion people suffering from mental disorders, while approximately 280 million people suffer from depression around the world [2,3][2][3]. However, effective treatments for neurological and psychiatric disorders are currently limited due to the heterogeneous disease pathogenesis and targets of treatments. For example, it has been shown that visceral pain or depression patients sometimes express resistance to treatment [4,5,6,7][4][5][6][7]. Therefore, in order to develop novel therapeutic strategies, the exploration of novel aspects of neurological or psychiatric pathogenic mechanisms is urgently required.
Enteroendocrine cells (EECs) are chemosensory cells residing in the intestinal epithelium, and they function as important sensors monitoring changes in the lumen of the gastrointestinal (GI) tract. The EECs orchestrate not only the communication with luminal microorganisms via microbial metabolites, but also the communication with host body systems via neuroendocrine hormones (Figure 1 and Figure 2). For example, epithelial EECs continuously respond to short-chain fatty acids (SCFAs) generated by luminal microorganisms via free fatty acid receptor 2 and 3 (FFAR2/3). Following this transceptor or receptor activation, EECs secrete pre-made peptide hormones to conduct paracrine and endocrine functions [8,9,10,11][8][9][10][11]. Further, the neuropod structure of EECs allows direct or indirect signal transductions to enteric glia cells and enteric neurons [12,13][12][13]. An increasing body of evidence indicates the unique involvement or pathogenic role of the gut–brain axis and gut microbiota in neurological and psychiatric disorders, in which EECs might participate [14,15,16,17,18,19][14][15][16][17][18][19].
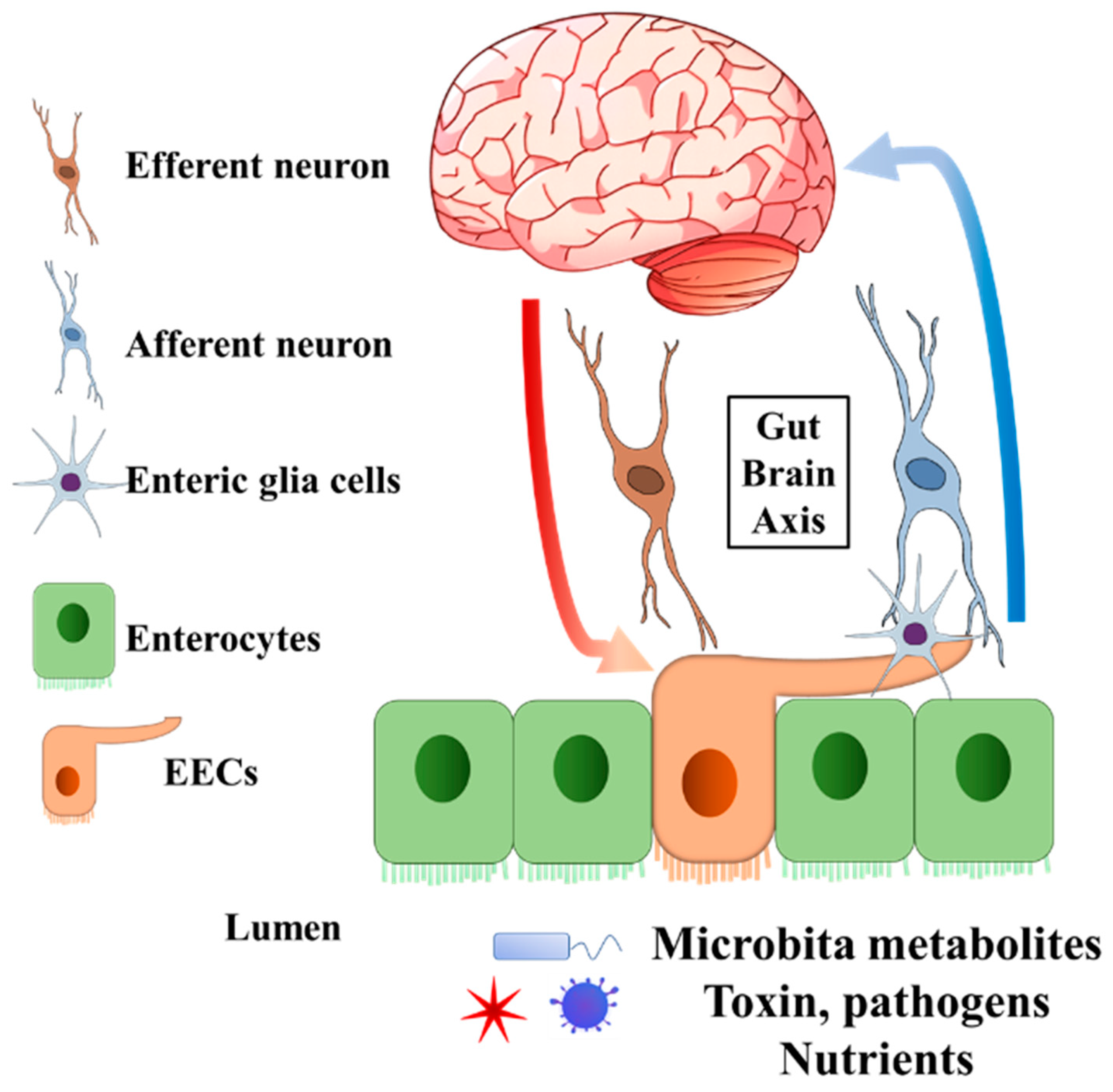
Figure 1. General structure of EECs involved in gut–brain axis. The EECs present receptors on the brush border to sense the microbiota metabolites, toxins, pathogens, and nutrients in the lumen. Enteric glia cells and neurons connect to EECs. The secreted endocrine molecules affect afferent neuron signalling directly and (or) indirectly via EECs enteric glia cells. Efferent neurons bring the signal into the central nervous system. On the other hand, the central nervous system can pass the signal to EECs through efferent neurons. EECs, enteroendocrine cells.
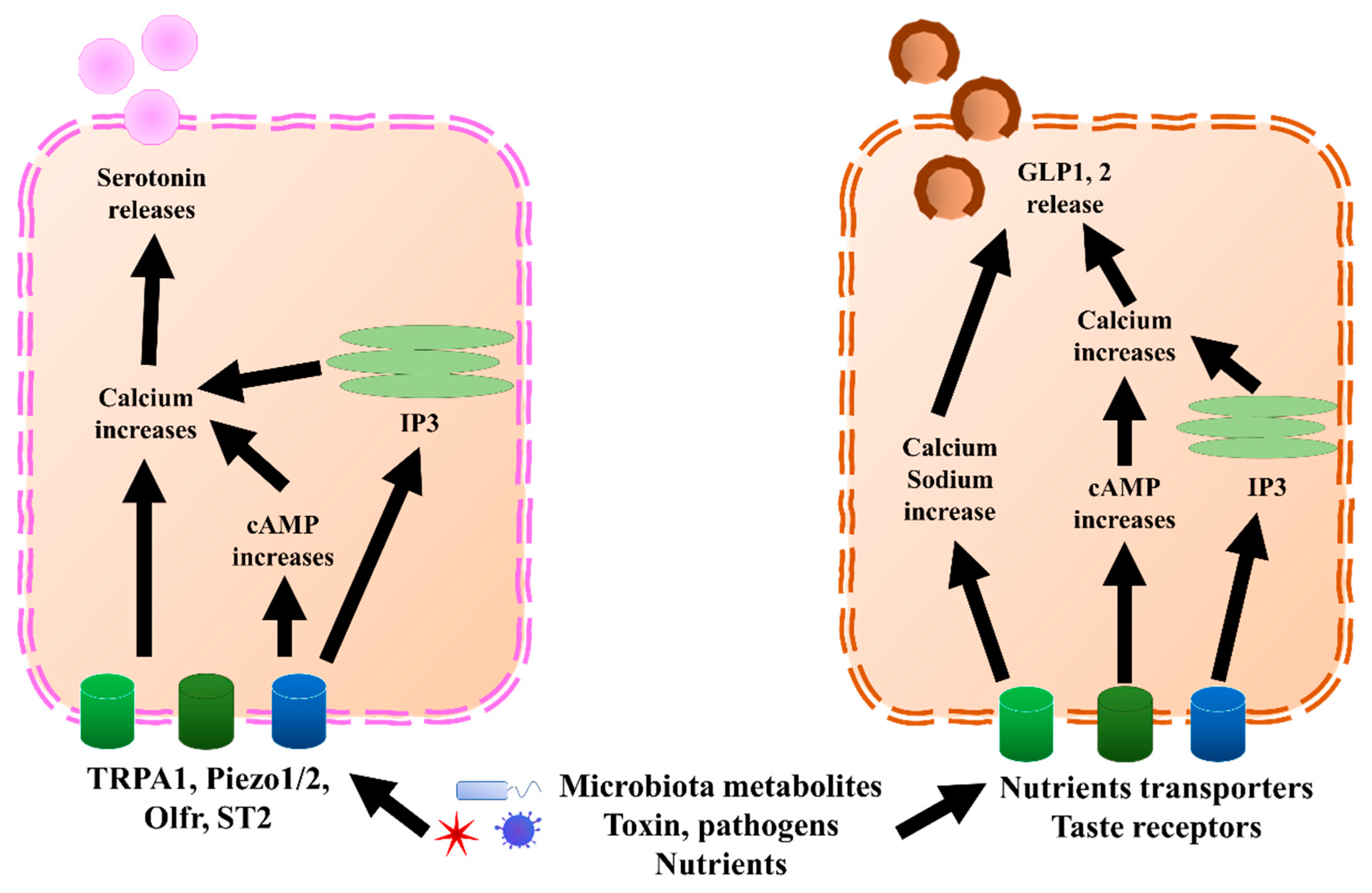
Figure 2. GLP1, 2, and serotonin secretion mechanisms in EECs and ECs, respectively. In ECs, TRPA1, piezol 1/2, Olfr, and ST2 bind to microbiota metabolites, pathogens, or nutrients, which increases calcium levels through directly increasing cAMP or endoplasmic reticulum IP3. Once the calcium level increases, it triggers the release of serotonin. Similar to ECs, EECs sense microbiota metabolites, toxins, pathogens, and nutrients through nutrient transporters and taste receptors. Further, they trigger an increase in calcium and sodium by directly increasing cAMP or endoplasmic reticulum IP3. Once the calcium level increases, it triggers the release of GLP1 and GLP2. cAMP, cyclic adenosine monophosphate; ECs, enterochromaffin cells; EECs, enteroendocrine cells; GLP1, glucagon-like peptide 1; GLP2, glucagon-like peptide 2; IP3, inositol trisphosphate; Olfr, olfactory receptor; TRPA1, transient receptor potential ankyrin 1.
2. Enteroendocrine Cell Functions That Might Be Related to Neurological and Psychiatric Disorders
EECs are located in the epithelium throughout the GI tract. They dynamically produce and store various peptide hormones and bioactive components, depending on the intestinal segments and epithelial homeostasis status. The regulation of EECs’ content profile, as well as their functions in energy metabolism and roles as incretins, has been reviewed elsewhere [20,21][20][21]. EECs can be further categorized into multiple subtypes, depending on their endocrine molecules production and secretion. For example, G cells can be identified by the secretion of gastrin; K cells uniquely secrete gastric inhibitory peptides; L cells produce and secrete GLP1, GLP2, PYY, and oxyntomodulin; I cells produce cholecystokinin (CCK); N cells secrete neurotensin; S cells secrete secretin; enterochromaffin cells (ECs) secrete serotonin; and enterochromaffin-like cells secrete histamine [20,21][20][21]. Here, we only focus on the subtypes of EECs that are potentially involved in neurological and psychiatric disorders.
L cells mostly secrete GLP1, GLP2, PYY, and oxyntomodulin, although PYY might also be co-expressed with gastrin, which is mostly secreted by G cells [22]. GLP1, GLP2, and PYY, which are secreted by L cells, and serotonin, secreted predominantly by ECs (Figure 2), are discussed in this paper. GLP1 and GLP2 have been shown to correlate with multiple neurological disorders. Serotonin also showed a correlation with depression and visceral pain, although this is still under debate. Besides incretin functions, GLP1 has been shown to exert anti-inflammatory effects in both the GI tract and central nervous system (CNS) [23,24,25][23][24][25]. Moreover, GLP1 possesses neuroprotective effects and triggers neurogenesis [26,27,28,29,30,31][26][27][28][29][30][31]. New evidence suggested that GLP1 and glucagon-like peptide 1 receptor (GLP1R), a receptor of GLP1, have protective effects on hypothalamic inflammation and leptin sensitivity in mice [25,32][25][32]. Despite the well-known source and their effects within the CNS, the GLP1 derived from intestinal EECs has also been suggested to play a role in neurological pathology, due to the feature wherein GLP1 is able to pass through the brain–blood barrier [33,34,35][33][34][35]. Similar to GLP1, L-cell-secreted GLP2 also possesses anti-inflammation effects [36,37,38][36][37][38]. In cows, GLP2 administration increased the intestinal villi height, mucosal surface, and proliferating cells, and decreased inflammation [39]. Further, GLP2 has a neuroprotective effect and can trigger neurogenesis in a similar manner as GLP1 [29,37,40,41,42][29][37][40][41][42]. Interestingly, the anti-inflammatory effects of other components of EEC content have recently been revealed, including PYY [43,44][43][44] and serotonin [45[45][46],46], which are likely associated with neuroinflammation. Therefore, accumulating evidence suggests that EECs and ECs could play important pathogenic and regulatory roles in neurological and psychiatric disorders.
3. Enteroendocrine Cells in Parkinson’s Disease
PD is a common movement disorder that was originally characterized as a neurodegenerative disorder due to the loss of dopaminergic neurons and accumulated aggregation of α-synuclein fibrils (called Lewy bodies) (reviewed elsewhere previously [47]). However, studies have shown a new pathogenic aspect of PD, which could be linked to intestinal disorders, as well as to changes in intestinal microbiota and metabolites [15,48,49][15][48][49]. For instance, inflammatory bowel disease (IBD) has increased by 22 to 35% regarding the incidence of PD [50]. In addition, Sampson et al. reported that the GI microbiota was required for motor deficits, microglia activation, and α-synuclein pathology (PD symptoms), in a germ-free mice model overexpressing α-synuclein. Further, their results indicated that the microbial metabolites produced in PD patients enhanced the pathophysiology of PD [15]. Although with a negative correlation, others also found an association among the GI microbiota, the total faecal SCFAs, and PD incidence [48]. Researchers hypothesized that the origin of PD might lie in the enteric nervous system (ENS) [51,52][51][52]. Accordingly, α-synuclein was detected in GI mucosa in early PD patients [53,54][53][54].
Given the important luminal chemo-sensing and neuroendocrine functions of EECs, these recent results point to a hypothesis that EECs contribute to and regulate the pathogenesis of PD. Interestingly, in human intestinal tissue, the α-synuclein that triggers PD was colocalized with EECs, such as L cells and K cells [55,56][55][56]. Although the authors have not confirmed the original secretion location of the α-synuclein, the data in these studies strengthen the possibility of EECs’ involvement in PD progression.
Two potential mechanisms of EECs’ contribution in PD pathogenesis have been proposed. On one hand, the EECs are likely to be a source of α-synuclein, which is generated in response to specific microbial activation. Thereafter, the α-synuclein is transported into the brain via nerves, leading to the accumulation of α-synuclein [57] (Figure 3a). In line with this hypothesis, a very recent research work revealed the potential mechanisms. The authors identified an increased population of microorganism Akkermansia muciniphila in the guts of PD patients. The metabolites of this microorganism initiated α-synuclein aggregation in EECs, via activation of ryanodine receptor (RyR), calcium ion (Ca2+) release, and increased mitochondrial reactive oxygen species (ROS) generation [58] (Figure 3b). Moreover, a newly published paper indicated that another microbial metabolite, sodium butyrate, increased the α-synuclein mRNA expression in EECs through the autophagy-related 5 (Atg5) dependent autophagy pathway [59]. Holmqvist et al. provided evidence that α-synuclein was able to move from the intestine to the brain in rats [60]. Further, the transportation of α-synuclein from EECs to neurons requires GTPase called Ras-related protein Rab-35 (Rab35) and cell-to-cell contact, which is in line with the EECs’ characteristics [61].
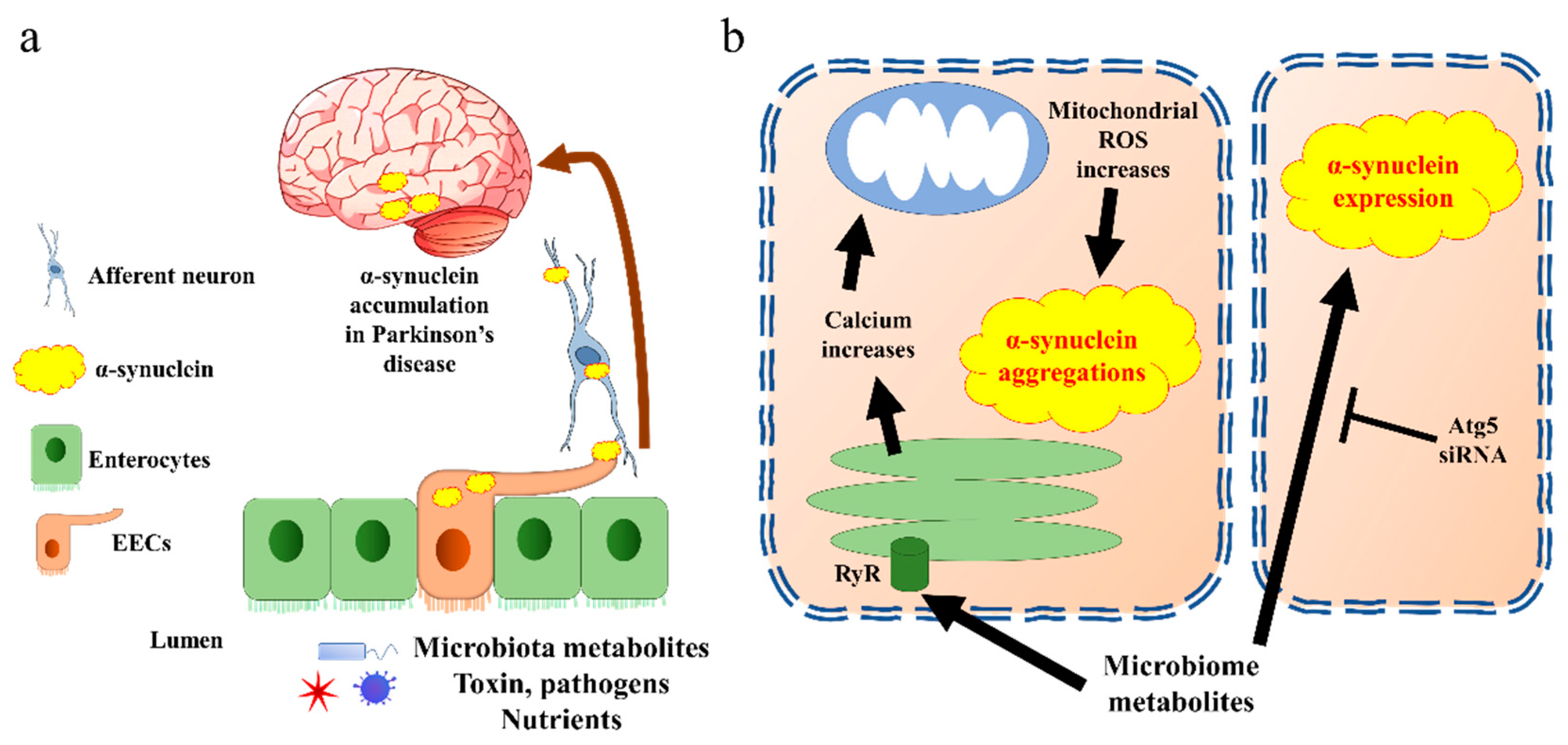
Figure 3. α-synuclein accumulates in Parkinson’s disease through EECs. (a) The general pathway by which EECs trigger α-synuclein transfer into brain. Aggregated α-synuclein produced by EECs is transported into brain through afferent neurons and vagal nerve. (b) Cell signalling of α-synuclein aggregation present in EECs. The EECs present receptors on the brush border to sense the microbiota metabolites. Triggering of endoplasmic reticulum releases calcium through RyR. The increase in calcium induces reactive oxygen species (ROS) synthesis in mitochondria, which further creates α-synuclein aggregates. Microbiota metabolites also increase α-synuclein expression through Atg5 pathway in EECs. Atg5, autophagy-related 5; EECs, enteroendocrine cells; ROS, reactive oxygen species; RyR, ryanodine receptor.
On the other hand, the EECs’ secretion could also be suppressed by alterations in luminal SCFA concentrations and profiles. This could be the consequence of changes in specific microbes, which then increase the systemic inflammation, and this eventually enhances the progression of PD [62,63,64][62][63][64]. It was suggested that sodium butyrate increased the pro-inflammatory cytokines and α-synuclein mRNA expression in an EECs cell line and neuroblast cell line treated with EECs conditional medium [59]. Further, the EECs facilitate α-synuclein transport, which could trigger inflammation responses in microglia [65,66][65][66]. In contrast, a study in a PD mouse model suggested that the oral administration of butyrate could have protective effects on the neurobehavioral impairment via increased EEC activities, such as increased colonic GLP1 expression and brain GLP1R gene expression [67]. A recent animal study also indicated the neuroprotective effect of GLP1, triggered by chlorogenic acid [31]. These conflicting characteristics of EECs might be due to the variations in EECs’ homeostasis status or the hormone composition of EECs. In other words, the EECs that secrete GLP1 could be beneficial in terms of inflammation reduction, while the EECs that cannot secrete GLP1 but produce α-synuclein could be harmful. However, the detailed mechanism for either hypothesis is still unclear, especially regarding the extent to which EECs contribute to inflammation in PD patients. Future study will be needed to investigate the detailed mechanisms of EECs in PD progression.
References
- WHO. Parkinson Disease. Available online: https://www.who.int/news-room/fact-sheets/detail/parkinson-disease (accessed on 16 May 2022).
- WHO. Depression. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 16 May 2022).
- WHO. Nearly One Billion People Have a Mental Disorder: WHO. UN News, 17 June 2022.
- Hanson, K.A.; Loftus, E.V., Jr.; Harmsen, W.S.; Diehl, N.N.; Zinsmeister, A.R.; Sandborn, W.J. Clinical features and outcome of patients with inflammatory bowel disease who use narcotics: A case-control study. Inflamm. Bowel Dis. 2009, 15, 772–777.
- Drossman, D.A.; Morris, C.B.; Edwards, H.; Wrennall, C.E.; Weinland, S.R.; Aderoju, A.O.; Kulkarni-Kelapure, R.R.; Hu, Y.J.; Dalton, C.; Bouma, M.H.; et al. Diagnosis, characterization, and 3-month outcome after detoxification of 39 patients with narcotic bowel syndrome. Am. J. Gastroenterol. 2012, 107, 1426–1440.
- Mueller, T.I.; Leon, A.C.; Keller, M.B.; Solomon, D.A.; Endicott, J.; Coryell, W.; Warshaw, M.; Maser, J.D. Recurrence after recovery from major depressive disorder during 15 years of observational follow-up. Am. J. Psychiatry 1999, 156, 1000–1006.
- Trivedi, M.H.; Fava, M.; Wisniewski, S.R.; Thase, M.E.; Quitkin, F.; Warden, D.; Ritz, L.; Nierenberg, A.A.; Lebowitz, B.D.; Biggs, M.M.; et al. Medication augmentation after the failure of SSRIs for depression. N. Engl. J. Med. 2006, 354, 1243–1252.
- Tolhurst, G.; Heffron, H.; Lam, Y.S.; Parker, H.E.; Habib, A.M.; Diakogiannaki, E.; Cameron, J.; Grosse, J.; Reimann, F.; Gribble, F.M. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 2012, 61, 364–371.
- Psichas, A.; Sleeth, M.L.; Murphy, K.G.; Brooks, L.; Bewick, G.A.; Hanyaloglu, A.C.; Ghatei, M.A.; Bloom, S.R.; Frost, G. The short chain fatty acid propionate stimulates GLP-1 and PYY secretion via free fatty acid receptor 2 in rodents. Int. J. Obes. 2015, 39, 424–429.
- Tazoe, H.; Otomo, Y.; Karaki, S.; Kato, I.; Fukami, Y.; Terasaki, M.; Kuwahara, A. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 2009, 30, 149–156.
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.Y.; Lannoy, V.; Decobecq, M.E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 2003, 278, 25481–25489.
- Bohorquez, D.V.; Samsa, L.A.; Roholt, A.; Medicetty, S.; Chandra, R.; Liddle, R.A. An enteroendocrine cell-enteric glia connection revealed by 3D electron microscopy. PLoS ONE 2014, 9, e89881.
- Bohorquez, D.V.; Shahid, R.A.; Erdmann, A.; Kreger, A.M.; Wang, Y.; Calakos, N.; Wang, F.; Liddle, R.A. Neuroepithelial circuit formed by innervation of sensory enteroendocrine cells. J. Clin. Investig. 2015, 125, 782–786.
- Tian, P.; Wang, G.; Zhao, J.; Zhang, H.; Chen, W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J. Nutr. Biochem. 2019, 66, 43–51.
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e1412.
- Kelly, J.R.; Minuto, C.; Cryan, J.F.; Clarke, G.; Dinan, T.G. The role of the gut microbiome in the development of schizophrenia. Schizophr. Res. 2021, 234, 4–23.
- Socala, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Wlodarczyk, M.; Zielinska, A.; Poleszak, E.; Fichna, J.; Wlaz, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840.
- Maiuolo, J.; Gliozzi, M.; Musolino, V.; Carresi, C.; Scarano, F.; Nucera, S.; Scicchitano, M.; Oppedisano, F.; Bosco, F.; Ruga, S.; et al. The Contribution of Gut Microbiota-Brain Axis in the Development of Brain Disorders. Front. Neurosci. 2021, 15, 616883.
- Suganya, K.; Koo, B.S. Gut-Brain Axis: Role of Gut Microbiota on Neurological Disorders and How Probiotics/Prebiotics Beneficially Modulate Microbial and Immune Pathways to Improve Brain Functions. Int. J. Mol. Sci. 2020, 21, 7551.
- Xie, C.; Jones, K.L.; Rayner, C.K.; Wu, T. Enteroendocrine Hormone Secretion and Metabolic Control: Importance of the Region of the Gut Stimulation. Pharmaceutics 2020, 12, 790.
- Martin, A.M.; Sun, E.W.; Keating, D.J. Mechanisms controlling hormone secretion in human gut and its relevance to metabolism. J. Endocrinol. 2019, 244, R1–R15.
- Upchurch, B.H.; Fung, B.P.; Rindi, G.; Ronco, A.; Leiter, A.B. Peptide YY expression is an early event in colonic endocrine cell differentiation: Evidence from normal and transgenic mice. Development 1996, 122, 1157–1163.
- Barreto-Vianna, A.R.C.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Beneficial effects of liraglutide (GLP1 analog) in the hippocampal inflammation. Metab. Brain Dis. 2017, 32, 1735–1745.
- Solmaz, V.; Cinar, B.P.; Yigitturk, G.; Cavusoglu, T.; Taskiran, D.; Erbas, O. Exenatide reduces TNF-alpha expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. Eur. J. Pharmacol. 2015, 765, 482–487.
- Heiss, C.N.; Manneras-Holm, L.; Lee, Y.S.; Serrano-Lobo, J.; Hakansson Gladh, A.; Seeley, R.J.; Drucker, D.J.; Backhed, F.; Olofsson, L.E. The gut microbiota regulates hypothalamic inflammation and leptin sensitivity in Western diet-fed mice via a GLP-1R-dependent mechanism. Cell Rep. 2021, 35, 109163.
- Perry, T.; Lahiri, D.K.; Chen, D.; Zhou, J.; Shaw, K.T.; Egan, J.M.; Greig, N.H. A novel neurotrophic property of glucagon-like peptide 1: A promoter of nerve growth factor-mediated differentiation in PC12 cells. J. Pharm. Exp. Ther. 2002, 300, 958–966.
- Perry, T.; Haughey, N.J.; Mattson, M.P.; Egan, J.M.; Greig, N.H. Protection and reversal of excitotoxic neuronal damage by glucagon-like peptide-1 and exendin-4. J. Pharm. Exp. Ther. 2002, 302, 881–888.
- During, M.J.; Cao, L.; Zuzga, D.S.; Francis, J.S.; Fitzsimons, H.L.; Jiao, X.; Bland, R.J.; Klugmann, M.; Banks, W.A.; Drucker, D.J.; et al. Glucagon-like peptide-1 receptor is involved in learning and neuroprotection. Nat. Med. 2003, 9, 1173–1179.
- Voss, U.; Sand, E.; Hellstrom, P.M.; Ekblad, E. Glucagon-like peptides 1 and 2 and vasoactive intestinal peptide are neuroprotective on cultured and mast cell co-cultured rat myenteric neurons. BMC Gastroenterol. 2012, 12, 30.
- Zhang, L.; Zhang, L.; Li, Y.; Li, L.; Melchiorsen, J.U.; Rosenkilde, M.; Holscher, C. The Novel Dual GLP-1/GIP Receptor Agonist DA-CH5 Is Superior to Single GLP-1 Receptor Agonists in the MPTP Model of Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 523–542.
- Sharma, N.; Soni, R.; Sharma, M.; Chatterjee, S.; Parihar, N.; Mukarram, M.; Kale, R.; Sayyed, A.A.; Behera, S.K.; Khairnar, A. Chlorogenic Acid: A Polyphenol from Coffee Rendered Neuroprotection Against Rotenone-Induced Parkinson’s Disease by GLP-1 Secretion. Mol. Neurobiol. 2022.
- Erdogan, M.A.; Erdogan, A.; Erbas, O. The Anti-Seizure Effect of Liraglutide on Ptz-Induced Convulsions Through its Anti-Oxidant and Anti-Inflammatory Properties. Neurochem. Res. 2022.
- Kastin, A.J.; Akerstrom, V.; Pan, W. Interactions of Glucagon-Like Peptide-1 (GLP-1) with the Blood-Brain Barrier. J. Mol. Neurosci. 2002, 18, 7–14.
- Kastin, A.J.; Akerstrom, V. Entry of exendin-4 into brain is rapid but may be limited at high doses. Int. J. Obes. Relat. Metab. Disord. 2003, 27, 313–318.
- Hunter, K.; Holscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33.
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103.
- Nuzzo, D.; Baldassano, S.; Amato, A.; Picone, P.; Galizzi, G.; Caldara, G.F.; Di Carlo, M.; Mule, F. Glucagon-like peptide-2 reduces the obesity-associated inflammation in the brain. Neurobiol. Dis. 2019, 121, 296–304.
- Xie, S.; Liu, B.; Fu, S.; Wang, W.; Yin, Y.; Li, N.; Chen, W.; Liu, J.; Liu, D. GLP-2 suppresses LPS-induced inflammation in macrophages by inhibiting ERK phosphorylation and NF-kappaB activation. Cell Physiol. Biochem. 2014, 34, 590–602.
- Kvidera, S.K.; Horst, E.A.; Sanz Fernandez, M.V.; Abuajamieh, M.; Ganesan, S.; Gorden, P.J.; Green, H.B.; Schoenberg, K.M.; Trout, W.E.; Keating, A.F.; et al. Characterizing effects of feed restriction and glucagon-like peptide 2 administration on biomarkers of inflammation and intestinal morphology. J. Dairy Sci. 2017, 100, 9402–9417.
- Li, N.; Liu, B.W.; Ren, W.Z.; Liu, J.X.; Li, S.N.; Fu, S.P.; Zeng, Y.L.; Xu, S.Y.; Yan, X.; Gao, Y.J.; et al. GLP-2 Attenuates LPS-Induced Inflammation in BV-2 Cells by Inhibiting ERK1/2, JNK1/2 and NF-κB Signaling Pathways. Int. J. Mol. Sci. 2016, 17, 190.
- Zhang, Z.; Hao, L.; Shi, M.; Yu, Z.; Shao, S.; Yuan, Y.; Zhang, Z.; Holscher, C. Neuroprotective Effects of a GLP-2 Analogue in the MPTP Parkinson’s Disease Mouse Model. J. Parkinsons Dis. 2021, 11, 529–543.
- Su, Y.; Zhang, Z.; Li, H.; Ma, J.; Sun, L.; Shao, S.; Zhang, Z.; Holscher, C. A GLP-2 Analogue Protects SH-SY5Y and Neuro-2a Cells Against Mitochondrial Damage, Autophagy Impairments and Apoptosis in a Parkinson Model. Drug Res. 2021, 71, 43–50.
- Li, Z.; Kuang, X.; Chen, T.; Shen, T.; Wu, J. Peptide YY 3-36 attenuates trinitrobenzene sulfonic acid-induced colitis in mice by modulating Th1/Th2 differentiation. Bioengineered 2022, 13, 10144–10158.
- Guzzardi, M.A.; La Rosa, F.; Campani, D.; Cacciato Insilla, A.; Nannipieri, M.; Brunetto, M.R.; Bonino, F.; Iozzo, P. Evidence of a Gastro-Duodenal Effect on Adipose Tissue and Brain Metabolism, Potentially Mediated by Gut-Liver Inflammation: A Study with Positron Emission Tomography and Oral (18)FDG in Mice. Int. J. Mol. Sci. 2022, 23, 2695.
- Mota, C.M.D.; Rodrigues-Santos, C.; Fernandez, R.A.R.; Carolino, R.O.G.; Antunes-Rodrigues, J.; Anselmo-Franci, J.A.; Branco, L.G.S. Central serotonin attenuates LPS-induced systemic inflammation. Brain Behav. Immun. 2017, 66, 372–381.
- Arnold, W.R.; Carnevale, L.N.; Xie, Z.; Baylon, J.L.; Tajkhorshid, E.; Hu, H.; Das, A. Anti-inflammatory dopamine- and serotonin-based endocannabinoid epoxides reciprocally regulate cannabinoid receptors and the TRPV1 channel. Nat. Commun. 2021, 12, 926.
- Davie, C.A. A review of Parkinson’s disease. Br. Med. Bull 2008, 86, 109–127.
- Unger, M.M.; Spiegel, J.; Dillmann, K.U.; Grundmann, D.; Philippeit, H.; Burmann, J.; Fassbender, K.; Schwiertz, A.; Schafer, K.H. Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat. Disord. 2016, 32, 66–72.
- Hirayama, M.; Ohno, K. Parkinson’s Disease and Gut Microbiota. Ann. Nutr. Metab. 2021, 77 (Suppl. 2), 28–35.
- Lin, J.C.; Lin, C.S.; Hsu, C.W.; Lin, C.L.; Kao, C.H. Association Between Parkinson’s Disease and Inflammatory Bowel Disease: A Nationwide Taiwanese Retrospective Cohort Study. Inflamm. Bowel Dis. 2016, 22, 1049–1055.
- Braak, H.; Rub, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural Transm. 2003, 110, 517–536.
- Del Tredici, K.; Rub, U.; De Vos, R.A.; Bohl, J.R.; Braak, H. Where does parkinson disease pathology begin in the brain? J. Neuropathol. Exp. Neurol. 2002, 61, 413–426.
- Shannon, K.M.; Keshavarzian, A.; Mutlu, E.; Dodiya, H.B.; Daian, D.; Jaglin, J.A.; Kordower, J.H. Alpha-synuclein in colonic submucosa in early untreated Parkinson’s disease. Mov. Disord. 2012, 27, 709–715.
- Sanchez-Ferro, A.; Rabano, A.; Catalan, M.J.; Rodriguez-Valcarcel, F.C.; Fernandez Diez, S.; Herreros-Rodriguez, J.; Garcia-Cobos, E.; Alvarez-Santullano, M.M.; Lopez-Manzanares, L.; Mosqueira, A.J.; et al. In vivo gastric detection of alpha-synuclein inclusions in Parkinson’s disease. Mov. Disord. 2015, 30, 517–524.
- Chandra, R.; Hiniker, A.; Kuo, Y.M.; Nussbaum, R.L.; Liddle, R.A. alpha-Synuclein in gut endocrine cells and its implications for Parkinson’s disease. JCI Insight 2017, 2, e92295.
- Casini, A.; Mancinelli, R.; Mammola, C.L.; Pannarale, L.; Chirletti, P.; Onori, P.; Vaccaro, R. Distribution of alpha-synuclein in normal human jejunum and its relations with the chemosensory and neuroendocrine system. Eur. J. Histochem. EJH 2021, 65, 3310.
- Liddle, R.A. Parkinson’s disease from the gut. Brain Res. 2018, 1693, 201–206.
- Amorim Neto, D.P.; Bosque, B.P.; Pereira de Godoy, J.V.; Rodrigues, P.V.; Meneses, D.D.; Tostes, K.; Costa Tonoli, C.C.; Faustino de Carvalho, H.; Gonzalez-Billault, C.; de Castro Fonseca, M. Akkermansia muciniphila induces mitochondrial calcium overload and alpha -synuclein aggregation in an enteroendocrine cell line. iScience 2022, 25, 103908.
- Qiao, C.M.; Sun, M.F.; Jia, X.B.; Shi, Y.; Zhang, B.P.; Zhou, Z.L.; Zhao, L.P.; Cui, C.; Shen, Y.Q. Sodium butyrate causes alpha-synuclein degradation by an Atg5-dependent and PI3K/Akt/mTOR-related autophagy pathway. Exp. Cell Res. 2020, 387, 111772.
- Holmqvist, S.; Chutna, O.; Bousset, L.; Aldrin-Kirk, P.; Li, W.; Bjorklund, T.; Wang, Z.Y.; Roybon, L.; Melki, R.; Li, J.Y. Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol. 2014, 128, 805–820.
- Rodrigues, P.V.; de Godoy, J.V.P.; Bosque, B.P.; Amorim Neto, D.P.; Tostes, K.; Palameta, S.; Garcia-Rosa, S.; Tonoli, C.C.C.; de Carvalho, H.F.; de Castro Fonseca, M. Transcellular propagation of fibrillar alpha-synuclein from enteroendocrine to neuronal cells requires cell-to-cell contact and is Rab35-dependent. Sci. Rep. 2022, 12, 4168.
- Tansey, M.G.; Wallings, R.L.; Houser, M.C.; Herrick, M.K.; Keating, C.E.; Joers, V. Inflammation and immune dysfunction in Parkinson disease. Nat. Rev. Immunol. 2022.
- Boyle, J.G.; Livingstone, R.; Petrie, J.R. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: A comparative review. Clin. Sci. 2018, 132, 1699–1709.
- Drobny, A.; Ngo, P.A.; Neurath, M.F.; Zunke, F.; Lopez-Posadas, R. Molecular Communication Between Neuronal Networks and Intestinal Epithelial Cells in Gut Inflammation and Parkinson’s Disease. Front. Med. 2021, 8, 655123.
- Alvarez-Erviti, L.; Couch, Y.; Richardson, J.; Cooper, J.M.; Wood, M.J. Alpha-synuclein release by neurons activates the inflammatory response in a microglial cell line. Neurosci. Res. 2011, 69, 337–342.
- Beraud, D.; Hathaway, H.A.; Trecki, J.; Chasovskikh, S.; Johnson, D.A.; Johnson, J.A.; Federoff, H.J.; Shimoji, M.; Mhyre, T.R.; Maguire-Zeiss, K.A. Microglial activation and antioxidant responses induced by the Parkinson’s disease protein alpha-synuclein. J. Neuroimmune Pharm. 2013, 8, 94–117.
- Liu, J.; Wang, F.; Liu, S.; Du, J.; Hu, X.; Xiong, J.; Fang, R.; Chen, W.; Sun, J. Sodium butyrate exerts protective effect against Parkinson’s disease in mice via stimulation of glucagon like peptide-1. J. Neurol. Sci. 2017, 381, 176–181.
More
