Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Ghanyah Al-Qadami and Version 2 by Conner Chen.
Through fermentation, the gut microbiota can produce several types of metabolites, including short-chain fatty acids (SCFAs). SCFAs play an important role in maintaining epithelial barrier functions and intestinal homeostasis.
- microbiota
- short-chain fatty acids
- cancer
- chemotherapy
- radiotherapy
- immunotherapy
1. Introduction
The collection of bacteria and other microorganisms residing in the gastrointestinal tract, termed the gut microbiota, has emerged as an important target in improving and personalizing oncology treatment. A growing body of research has shown potential roles for specific microbial taxa in the efficacy of cancer treatment, as well as in the development of associated toxicities [1][2][3][1,2,3]. The richness of the microbiome has also been investigated, with higher microbial diversity shown to be a key predictor of survival in people having chemoradiation for cervical cancer [4]. A seminal study by Gopalakrishnan et al. [1] clearly showed significant differences in both the diversity and composition of the gut microbiota of people who responded to PD-1 inhibitor immunotherapy for melanoma compared to non-responders. These compositional differences may lead to altered treatment response in a variety of ways, including via changes in direct drug metabolism (for example the action of beta-glucuronidase in the intestinal toxicity of irinotecan [5]), or modulation of the host immune responses [6][7][6,7].
With the advent of more sophisticated analytical methods, there is now more appreciation for the functional aspects of the microbiota, including the production of metabolites. Short-chain fatty acids (SCFAs) are one important class of metabolites produced by the gut microbiota. SCFAs, which include butyrate, acetate, propionate, and others (discussed below), are produced in the gut by bacterial fermentation of indigestible fibers and have a variety of functions both in the gut and distal sites including the brain and kidneys [8]. SCFAs have previously been linked to colorectal cancer development, with multiple studies showing that butyrate, propionate, and acetate induce apoptosis in colorectal cancer cells but not in healthy cells. In addition, some studies have suggested that butyrate and propionate have potent anti-neoplastic effects [9]. This has led to suggestions that SCFA manipulation may be a useful preventative or therapeutic strategy in a variety of cancers [9][10][11][12][13][9,10,11,12,13].
Results linking the microbiota and cancer treatment outcomes have been promising but have so far failed to provide significant improvements to clinical practice [14]. It is therefore now increasingly important to move beyond simply correlating the abundance of particular bacterial taxa with a disease or phenotype, and to understand how the functional capacity of the microbiota may be critical [15]. Furthermore, in the development of microbial-based therapeutics, there is a strong rationale to investigate metabolites such as SCFAs, rather than bacteria themselves. This is because direct metabolite supplementation would remove the need to ensure microorganisms successfully colonize the host, such is the case with probiotics.
2. Short-Chain Fatty Acids
SCFAs are small organic carboxylic acids with 1 to 6 carbon atoms, and in the intestine, are the main product of anaerobic fermentation of indigestible polysaccharides such as resistant starch, inulin, cellulose, and pectin (Figure 1) [16]. Acetate, propionate, and butyrate are the most commonly produced SCFAs in the human gut, in a roughly 3:1:1 ratio [17]. Other SCFAs include formate, isobutyrate, valerate, isovalerate, and 2-methylbutanoate. SCFAs can move from the gut via the bloodstream in differing amounts. A study using stable isotopes in healthy human subjects found that systemic availability of acetate, propionate, and butyrate was 36%, 9%, and 2%, respectively, [18]. Subsequently, SCFAs have a range of functions both in the gut and elsewhere, with differences in local and systemic effects governed by their systemic availability. These include being a key energy source for colonocytes and playing roles in G-protein coupled receptor (GPCR) binding, histone deacetylation, and immune modulation [16][19][16,19]. SCFA quantification in fecal samples is commonly used to quantify SCFA production. This may however not be an accurate measure, as previous research has suggested that a majority of SCFAs produced in the colon are absorbed by the gut mucosa [16][20][16,20]. In addition, systemic SCFA levels may be affected by gut epithelial integrity [21].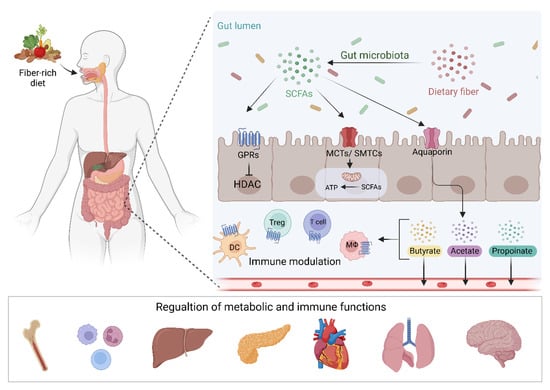
Figure 1. Dietary fiber and other fermentable substrates are fermented by the gut microbiota leading to the production of SCFAs. These metabolites interact with GPCRs or MCTs and SMTCs transporters leading to the regulation of gene transcription and energy productions in IECs. SCFAs can also passively cross the intestinal mucosa and regulate intestinal immunity and pass into the circulation to modulate metabolic and immune functions in different body organs. SCFAs; short-chain fatty acids, GPRs; G protein-coupled receptors, MCTs; Monocarboxylate transporters, SMTCs; Sodium-coupled monocarboxylate transporter; DC, dendritic cell, Treg; T regulatory cell, Mϕ; Macrophage. Figure created with BioRender.com.
