Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Miguel Alcoceba and Version 2 by Conner Chen.
The gold standard for determining follicular lymphoma (FL) transformation is based on the histologically confirmed progression of grade 1, 2, or 3A FL to a high-grade lymphoma, consisting of a predominance of large cells and the loss of the follicular architecture. Histological transformation (HT) to a more aggressive disease–mostly diffuse large B-cell lymphoma–is considered one of the most dismal events in the clinical course of FLfollicular lymphoma (FL).
- transformed follicular lymphoma
- genetics
- histological transformation
1. Definition of FL Transformation
The gold standard for determining follicular lymphoma (FL) transformation is based on the histologically confirmed progression of grade 1, 2, or 3A FL to a high-grade lymphoma, consisting of a predominance of large cells and the loss of the follicular architecture [1][2][23,25]. Most of the transformed cases have a diffuse large B-cell lymphoma (DLBCL) histology (>80% of the cases) according to the current WHO classification, although other histologies have been described, such as high-grade B-cell lymphoma, FL grade 3B, Burkitt lymphoma, B lymphoblastic leukemia/lymphoma, and plasmablastic lymphoma [2][3][4][5][25,26,27,28]. There are other atypical forms suggestive of histological transformation, such as the presence at diagnosis of both FL and DLBCL cells, at the same site, referred to as composite lymphoma, or at different sites such as DLBCL in the lymph node and FL in bone marrow, as well as DLBCL cases that undergo a process of reverse transformation, relapsing as a lower grade lymphoma. These forms are not addressed in the prese contentsnt review.
Since lymphoma lesions are not isolated, other tumour areas might have a FL component at the same time in addition to the transformation area [1][6][7][23,29,30]. Positron emission tomography and computerized tomography (PET/CT) could help by selecting the biopsy site according to the highest standardized uptake value (SUV) of 18[F] fluorodeoxyglucose, since a high value (generally > 14) is correlated with more aggressive histology [8][31]. However, only ~50% of patients are biopsied, with inaccessibility of the tumour, the patient’s clinical situation or refusal among the main reasons [9][32]. Based on the clinical behaviour of transformed patients, several clinical criteria of transformation suspicion could be of utility in these cases, including an increase in lactate dehydrogenase (LDH) levels or hypercalcemia, rapid lymphadenopathy growth or the appearance of lymphoma masses or conglomerates, and the novel involvement of extranodal sites and new B symptoms. However, these criteria vary between studies and are not standardised [10][11][12][16,18,19]. Moreover, these clinical criteria are also present in patients who progress without transformation [9][32].
2. Cell of Origin and Pathogenesis of FL Transformation
Transformed cases may have changes in their immunophenotype, with an antigenic drift including CD10 loss or positivity of MUM1/IRF4. Although most transformed FLs are of germinal B-cell DLBCL subtype (GCB), up to 15–20% of the cases change to an activated B-cell (ABC) without differences in survival between both subtypes [4][13][14][27,48,49]. This contrasts with transformation in other B-cell lymphoproliferative disorders, such as chronic lymphocytic leukaemia (CLL), Waldenström macroglobulinemia or marginal zone lymphoma, in which transformed cases are mostly ABC/non-GCB [15][16][17][50,51,52]. Recurrent rearranged genes in DLBCL include BCL2, BCL6 and MYC. There are no major changes in the frequency of BCL2 or BCL6 translocations in transformed samples compared to FL diagnosis, however, MYC translocations are commonly acquired and are present in 25% of transformed cases [4][18][27,42]. The acquisition of MYC translocations implies an increase in the proportion of double-hit lymphomas (presence of both BCL2 and MYC translocations) in transformed patients, which is associated with a shorter survival from transformation (SFT)SFT [4][27], although differences were not statistically significant likely due to the low number of cases analysed. High-resolution genome wide analysis using SNP-array, whole-genome (WGS) or whole-exome sequencing (WES) or WES, and targeted next-generation sequencing studies in transformed FL identify increased genomic complexity and mutational burden at transformation in comparison to FL [5][18][19][20][21][22][23][24][28,39,41,42,53,54,55,56]. The most recurrent genetic lesions acquired in transformed FL cases are summarized in Table 1 and Figure 1 2, and include alterations (mainly mutations and/or deletions) in TP53 in approximately 15–30% transformed cases, CDKN2A/B deletions in 20–30% of cases, and B2M mutations and/or deletions in 20–25% of cases, together with the previously mentioned MYC translocations [5][18][19][20][22][23][25][28,39,41,42,54,55,57]. Of note, although these lesions are commonly acquired in transformation, they are not specific, as they could also be present at diagnosis or acquired during disease recurrence, representing markers of more aggressive disease [2][5][18][19][20][22][23][26][25,28,39,41,42,54,55,58]. In fact, these are also common acquired lesions in refractoriness and/or transformation in other haematological disorders [27][28][43,59].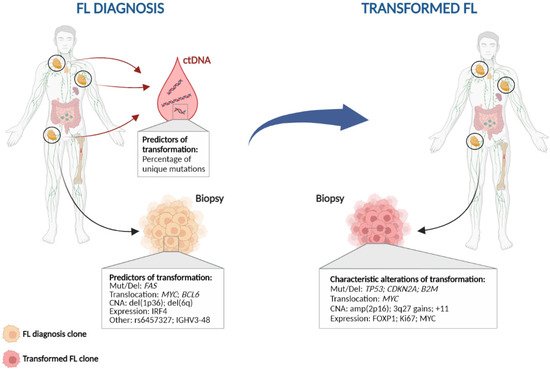
Figure 1 2. Characteristic genetic events in follicular lymphoma histological transformation. Left: Potential predictors of transformation; Right: Genetic alterations commonly found at histological transformation. CNA: copy number alteration; ctDNA: circulating tumour DNA; Mut/Del: mutation and/or deletion.
Table 1.
Biological and genetic factors enriched at follicular lymphoma histological transformation in the literature.
| Category | Variable | Biological Effect | Effect on Transformation |
|---|---|---|---|
| IHQ and microenvironment | IRF4 expression | - | Increased at HT [4][27] |
| MYC expression | - | Increased at HT [34][65] | |
| FOXP1 expression | - | Increased at HT [35][66] | |
| Genomic variants | TP53 mutation and deletion | Cell cycle | Increased at HT [5]28[18][,3919][,4120][23][,42,55] |
| B2microglobulin mutation and deletion | Immune surveillance | Increased at HT [5][18][28,42] | |
| FAS mutation and deletion | Apoptosis | Enriched in transformed cases [18][42] | |
| MYC mutation and translocation | Cell cycle | Increased at HT [4][27[18],42] | |
| CCND3 mutation | Cell cycle, JAK-STAT signalling | Increased at HT [5][28[19],39] | |
| EBF1 mutation | B-cell development | Increased at HT [5][28[20],41] | |
| GNA13 mutation | NF-kB/BCR signalling | Increased at HT [5][28] | |
| P2RY8 mutation | B-cell migration | Increased at HT [5][28] | |
| S1PR2 mutation | Proliferation | Increased at HT [5][28] | |
| CD58 mutation | Immune surveillance | Increased at HT [18][42] | |
| MYD88 mutation | NF-kB/BCR signalling | Increased at HT, ABC-HT related [5][19][20][23][28,39,41,55] | |
| CD79B mutation | NF-kB/BCR signalling | Increased at HT, ABC-HT related [5][19][23][28,39,55] | |
| BCL10 mutation | NF-kB/BCR signalling | Increased at HT, ABC-HT related [5][23][28,55] | |
| CDKN2A/B deletion | Cell cycle | Increased at HT [5][18][20][22][25][31][28,41,42,54,57,61] | |
| BCL6 translocation | B-cell differentiation | Increased at HT [4][36][27,67] | |
| 2p16 (REL) amplification | NF-kB/BCR signalling | Increased at HT, GCB-HT related [18][22][29][30][31][35,42,54,60,61] | |
| 3q27.3-q28 (BCL6) gains | B-cell differentiation | Increased at HT [18][22][29,42][30][35,54,60] | |
| Chromosomes 2, 5 and 11 gains | - | Increased at HT [22][29][35,54] | |
| Genomic complexity -copy-number changes- | - | Increased at HT [5][18][20][21][22][23][24][28,41,42,53,54,55,56] | |
| Genetic complexity -mutations- | - | Increased at HT [5][18][19][20][23][37][28,39,41,42,55,68] |
HT: Histological transformation.
