Chronic inflammation caused by infections has been suggested to be one of the most important cause of cancers. It has recently been shown that there is correlation between intestinal bacteria and cancer development including metastasis. As over 700 bacterial species exist in an oral cavity, it has been concerning that bacterial infection may cause oral cancer. However, the role of bacteria regarding tumorigenesis of oral cancer remains unclear. Several papers have shown that
Fusobacterium
species deriving the oral cavities, especially, play a crucial role for the development of colorectal and esophageal cancer.
F. nucleatum
is a well-known oral bacterium involved in formation of typical dental plaque on human teeth and causing periodontal diseases. The greatest characteristic of
F. nucleatum
is its ability to adhere to various bacteria and host cells. Interestingly,
F. nucleatum
is frequently detected in oral cancer tissues. Moreover, detection of
F. nucleatum
is correlated with the clinical stage of oral cancer. Although the detailed mechanism is still unclear,
Fusobacterium species have been suggested to be associated with cell adhesion, tumorigenesis, epithelial-to-mesenchymal transition, inflammasomes, cell cycle, etc. in oral cancer. In this entry, we introduce the reports focused on the association of Fusobacterium species with cancer development and progression including oral, esophageal, and colon cancers.
species have been suggested to be associated with cell adhesion, tumorigenesis, epithelial-to-mesenchymal transition, inflammasomes, cell cycle, etc. in oral cancer.
- Fusobacterium nucleatum
- oral cancer
- cancer development and progression
1. Introduction
Oral cancer, predominantly oral squamous cell carcinoma (OSCC), is a significant health problem and is regarded as the main cause of death from oral diseases in many countries. Traditional risk factors of oral cancer include alcohol abuse, tobacco and tobacco-derivate chewing, and oral virus infections. Other factors include infections, exposure to ionizing radiation, and environmental pollutants
[1]
. Thus, various causes of cancer are known to be closely involved in lifestyle choices, such as smoking, drinking, and diet.
Chronic inflammation caused by infections has been suggested to be one of the most important cause of cancers
[2]
. Indeed, infection of various viruses, bacteria, and parasites, such as Hepatitis B virus (HBV), Hepatitis B virus (HCV), Epstein–Barr virus (EBV), human papilloma virus (HPV), human herpes virus 8 (HHV8), human thymus-derived-cell leukemia/lymphoma virus-1 (HTLV-1), human immunodeficiency virus (HIV),
Helicobacter pylori
, Schistosomiasis, and liver flukes are well-known causes of cancer [2]. Recently, commensal bacteria are also involved in carcinogenesis. A huge number of different commensal bacteria naturally colonize in the human body in a relatively stable equilibrium. Microbial-immune network correlates gut bacteria with the whole-body health, and the failure of immune homeostasis manifests significant impact on various diseases, which might result in cancer
. Indeed, some commensal bacteria including
Peptostreptococcus anaerobius
[6]
, enterotoxigenic
Bacteriodes fragiles
[7]
, and
Escherichia coli
[8]
are involved in carcinogenesis of colorectal cancer (CRC).
In the oral cavity, bacteria, fungi, viruses, and archaea naturally colonize in different habitats including the teeth, gingival sulcus, tongue, cheeks, hard and soft palates, and tonsils. The oral microbiota refers to a highly varied and complicated ecosystem of these organisms. Over 700 bacterial species are endemic to the oral cavity, and indigenous oral flora act to prevent the settlement of foreign bacteria. Some bacteria of the oral cavity are harmful and can cause serious disease, while many of the oral bacteria are in fact beneficial in preventing diseases. Thus, the oral cavity is inhabited by complex multispecies bacterial communities that usually exist in a balanced immunoinflammatory state with the host
[9]
. It is now established that many chronic inflammatory conditions are caused by an imbalance between host–microbiota interactions, resulting in a dysbiotic community, deregulated immune responses, and eventually disease outcomes
[10]
. Oral commensal bacteria play a critical role in the development of oral diseases, including periodontal disease and tooth loss, and maintenance of a normal oral physiological environment
. Moreover, oral commensal bacteria are known to be involved in the pathogenesis and development of systematic diseases, such as pneumonia, cardiovascular diseases, diabetes, dementia, etc. It has been of concern that oral commensal bacteria may be involved in the pathogenesis of OSCC
. However, it remains unclear the role of bacteria regarding carcinogenesis of OSCC, even though a lot of bacteria inhabit in the oral cavity. Interestingly, it recently has been shown that one of oral commensal bacteria,
Fusobacterium
species, especially, play a crucial role for the development of CRC
. As
Fusobacterium
species are commensal bacteria in the oral cavity, the cumulative evidences of
Fusobacterium
species in CRC make us hypothesize that
Fusobacterium
species may be involved in the pathogenesis and development of OSCC.
2. Fusobacterium Species and Oral Cancer
There are many reports showing that
Fusobacterium
were detected in OSCC tissues
. The ratio between aerobes and anaerobes within the biofilms on the surfaces of OSCC tissues was approximately 1:2, whereas that in the healthy control was 2:1, indicating that OSCC surfaces provide an important reservoir for anaerobic bacteria
[20]
.
F. nucleatum subsp. polymorphum
was the most significantly overrepresented species in OSCC compared to the control group with deep-epithelium swabs
[21]
. Perera et al. also reported that the
Fusobacterium
was enriched in OSCC biopsy compared to fibroepithelial polyp as a control
[22]
. Moreover, significantly greater bacterial diversity was observed in the swab of OSCC sites than that of normal sites
[23]
. Thus, distribution of bacteria including
Fusobacterium
in OSCC tissues may be distinct from that in healthy oral mucosal tissues. Yang et al. reported that
F. periodonticum
and several bacteria in oral rinse samples were associated with OSCC, and they progressively increased in abundance from stage I to stage IV
[24]
.
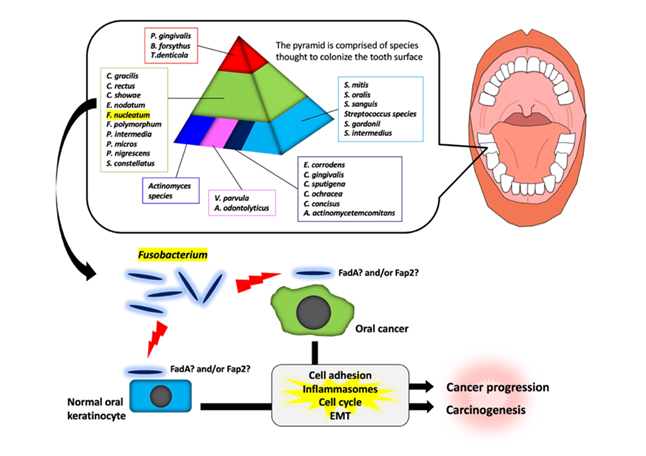
It has been shown that there are specific associations among bacteria within dental plaque by cluster analysis using subgingival plaque samples
. As shown in Figure 1, various bacteria exist as shown in the pyramid.
F. nucleatum
is in the middle of pyramid. Among the species in the middle of pyramid,
F. nucleatum
is dominant in the dental biofilm at a later stage of plaque formation. As described above,
Fusobacterium
infection affects the tumorigenesis and development of OSCC through various responses (Figure 1). However, the target molecules of
Fusobacterium
are still unknown. Further studies will be required for clarifying the evidence of
Fusobacterium
involvement in tumorigenesis and development of OSCC.
Figure 1.
Schematic model for the involvement of
Fusobacterium
species in oral carcinogenesis and cancer progression. In a dental plaque, various bacteria exist as shown in the pyramid. The base of the pyramid is comprised of species thought to colonize the tooth surface and proliferate at an early stage. Then, complex species in the middle of pyramid becomes numerically more dominant later and is thought to bridge the early colonizers. Finally, the complex species in the top of the pyramid numerically more dominant at late stages in plaque development. Among them,
Fusobacterium
species may adhere with oral keratinocyte or oral cancer cells via interaction between FadA/Fap2 and E-cadherin. This interaction may induce carcinogenesis and cancer progression.
3. Conclusions
F. nucleatum is well known oral commensal bacterium that forms typical dental plaque on human teeth is involved periodontal diseases. The involvement of F. nucleatum in carcinogenesis and the development of CRC has attracted attention in the field of CRC research. Importantly, the enrichment of F. nucleatum is frequently observed in OSCC tissues compared to healthy oral mucosal tissues. However, the roles of F. nucleatum in OSCC are still unclear. It is also necessary to investigate whether the mechanism of F. nucleatum in OSCC overlaps with that in CRC. We suggest that oral hygiene managements for reducing the amount of F. nucleatum may contribute to the prevention of OSCC and CRC. Moreover, various commensal bacteria exist in the oral cavity. Therefore, it is interesting to examine the relationship between F. nucleatum and other bacteria in the tumorigenesis and development of OSCC.
References
- Anand, P.; Kunnumakkara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a preventable disease that requires major lifestyle changes. Pharm. Res. 2008, 25, 2097–2116.
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern Med. 2000, 248, 171–183.
- Blumberg, R.; Powrie, F. Microbiota, disease, and back to health: A metastable journey. Sci. Transl. Med. 2012, 4, rv7.
- Erdman, S.E.; Poutahidis, T. Gut bacteria and cancer. Biochim Biophys Acta (BBA)-Rev. Cancer 2015, 1856, 86–90.
- Erdman, S.E.; Rao, V.P.; Olipitz, W.; Taylor, C.L.; Jackson, E.A.; Levkovich, T.; Lee, C.W.; Horwitz, B.H.; Fox, J.G.; Ge, Z.; et al. Unifying roles for regulatory T cells and inflammation in cancer. Int. J. Cancer 2010, 126, 1651–1665.
- Tsoi, H.; Chu, E.S.H.; Zhang, X.; Sheng, J.; Nakatsu, G.; Ng, S.C.; Chan, A.W.H.; Chan, F.K.L.; Sung, J.J.Y.; Yu, J. Peptostreptococcus anaerobius induces intracellular cholesterol biosynthesis in colon cells to induce proliferation and causes dysplasia in mice. Gastroenterology 2017, 152, 1419–1433.e5.
- Thiele Orberg, E.; Fan, H.; Tam, A. J.; Dejea, C.M.; Destefano Shields, C.E.; Wu, S.; Chung, L.; Finard, B.B.; Wu, X.; Fathi, P.; Ganguly, S.; et al. The myeloid immune signature of enterotoxigenic Bacteroides fragilis-induced murine colon tumorigenesis. Mucosal Immunol. 2017, 10, 421–433.
- Zhang, S.; Fu, J.; Dogan, B.; Scherl, E.J.; Simpson, K.W. 5-Aminosalicylic acid downregulates the growth and virulence of Escherichia coli associated with IBD and colorectal cancer, and upregulates host anti-inflammatory activity. J. Antibiot. 2018, 71, 950–961.
- Hajishengallis, G.; Lamont, R.J. Beyond the red complex and into more complexity: The polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol. Oral Microbiol. 2012, 27, 409–419.
- Mohammed, H.; Varoni EM.; Cochis, A.; Cordaro, M.; Gallenzi, P.; Patini, R.; Staderini, E.; Lajolo, C.; Rimondini, L.; Rocchetti, V. Oral Dysbiosis in Pancreatic Cancer and Liver Cirrhosis: A Review of the Literature. Biomedicines 2018, 6, 115.
- Abiko, Y.; Sato, T.; Mayanagi, G.; Takahashi, N. Profiling of subgingival plaque biofilm microflora from periodontally healthy subjects and from subjects with periodontitis using quantitative real-time PCR. J. Periodontal Res. 2010, 45, 389–395.
- Preza, D.; Olsen, I.; Willumsen, T.; Boches, S.K.; Cotton, S.L.; Grinde, B.; Paster, B.J. Microarray analysis of the microflora of root caries in elderly. Eur. J. Clin. Microbiol. Infect Dis. 2009, 28, 509–517.
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front Cell Infect Microbiol. 2020, 9, 476.
- Kudo, Y.; Tada, H.; Fujiwara, N.; Tada, Y.; Tsunematsu, T.; Miyake, Y.; Ishimaru, N. Oral environment and cancer. Genes Environ. 2016, 38, 13.
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/beta-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206.
- Gur, C.; Ibrahim, Y.; Isaacson, B.; Yamin, R.; Abed, J.; Gamliel, M.; Enk, J.; Bar-On, Y.; Stanietsky-Kaynan, N.; Coppenhagen-Glazer, S.; et al. Binding of the Fap2 protein of Fusobacterium nucleatum to human inhibitory receptor TIGIT protects tumors from immune cell attack. Immunity 2015, 42, 344–355.
- Abed, J.; Emgard, J.E.; Zamir, G.; Faroja, M.; Almogy, G.; Grenov, A.; Sol, A.; Naor, R.; Pikarsky, E.; Atlan, K.A.; et al. Fap2 Mediates Fusobacterium nucleatum Colorectal Adenocarcinoma Enrichment by Binding to Tumor-Expressed Gal-GalNAc. Cell Host Microbe 2016, 20, 215–225.
- Nagy, K.N.; Sonkodi, I.; Szoke, I.; Nagy, E.; Newman, H.N. The microflora associated with human oral carcinomas. Oral Oncol. 1998, 34, 304–308.
- Schmidt, B.L.; Kuczynski, J.; Bhattacharya, A.; Huey, B.; Corby, P.M.; Queiroz, E.L.; Nightingale, K.; Kerr, A.R.; DeLacure, M.D.; Veeramachaneni, R.; et al. Changes in abundance of oral microbiota associated with oral cancer. PLoS ONE 2014, 9, e98741.
- Bolz, J.; Dosa, E.; Schubert, J.; Eckert, A.W. Bacterial colonization of microbial biofilms in oral squamous cell carcinoma. Clin. Oral Investig. 2014, 18, 409–414.
- Al-Hebshi, N.N.; Nasher, A.T.; Maryoud, M.Y.; Homeida, H.E.; Chen, T.; Idris, A.M.; Johnson, N.W. Inflammatory bacteriome featuring Fusobacterium nucleatum and Pseudomonas aeruginosa identified in association with oral squamous cell carcinoma. Sci. Rep. 2017, 7, 1834.
- Perera, M.; Al-Hebshi, N.N.; Perera, I.; Ipe, D.; Ulett, G.C.; Speicher, D.J.; Chen, T.; Johnson, N.W. Inflammatory Bacteriome and Oral Squamous Cell Carcinoma. J. Dent. Res. 2018, 97, 725–732.
- Zhao, H.; Chu, M.; Huang, Z.; Yang, X.; Ran, S.; Hu, B.; Zhang, C.; Liang, J. Variations in oral microbiota associated with oral cancer. Sci. Rep. 2017, 7, 11773.
- Yang, C.Y.; Yeh, Y.M.; Yu, H.Y.; Chin, C.Y.; Hsu, C.W.; Liu, H.; Huang, P.J.; Hu, S.N.; Liao, C.T.; Chang, K.P.; et al. Oral Microbiota Community Dynamics Associated With Oral Squamous Cell Carcinoma Staging. Front. Microbiol. 2018, 9, 862.
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J.J. Clin. Periodontol. 1998, 25, 134–144.
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontol. 2000 2002, 28, 12–55.