This entry examines the role of reactive species RS (of oxygen ROS, nitrogen RNS and halogen RHS) on innate immunity is examined. The importance of these species in innate immunity was first recognized in phagocytes that underwent a “respiratory burst” after activation. The anion superoxide •O2− and hydrogen peroxide H2O2 are detrimental to the microbial population. NADPH oxidase NOx, as an •O2− producer is essential for microbial destruction, and patients lacking this functional oxidase are more susceptible to microbial infections. Reactive nitrogen species RNS (the most important are nitric oxide radical -•NO, peroxynitrite ONOO— and its derivatives), are also harmful to microorganisms, including bacteria, viruses, and parasites. Hypochlorous acid HOCl and hypothiocyanous acid HOSCN synthesized through the enzyme myeloperoxidase MPO, which catalyzes the reaction between H2O2 and Cl− or SCN−, are important inorganic bactericidal molecules, effective against a wide range of microbes.
- reactive species
- ROS
- RNS and RHS
- innate immunity
- antimicrobial
1. Introduction
2. Function and Features of Immunity and Innate Immune System
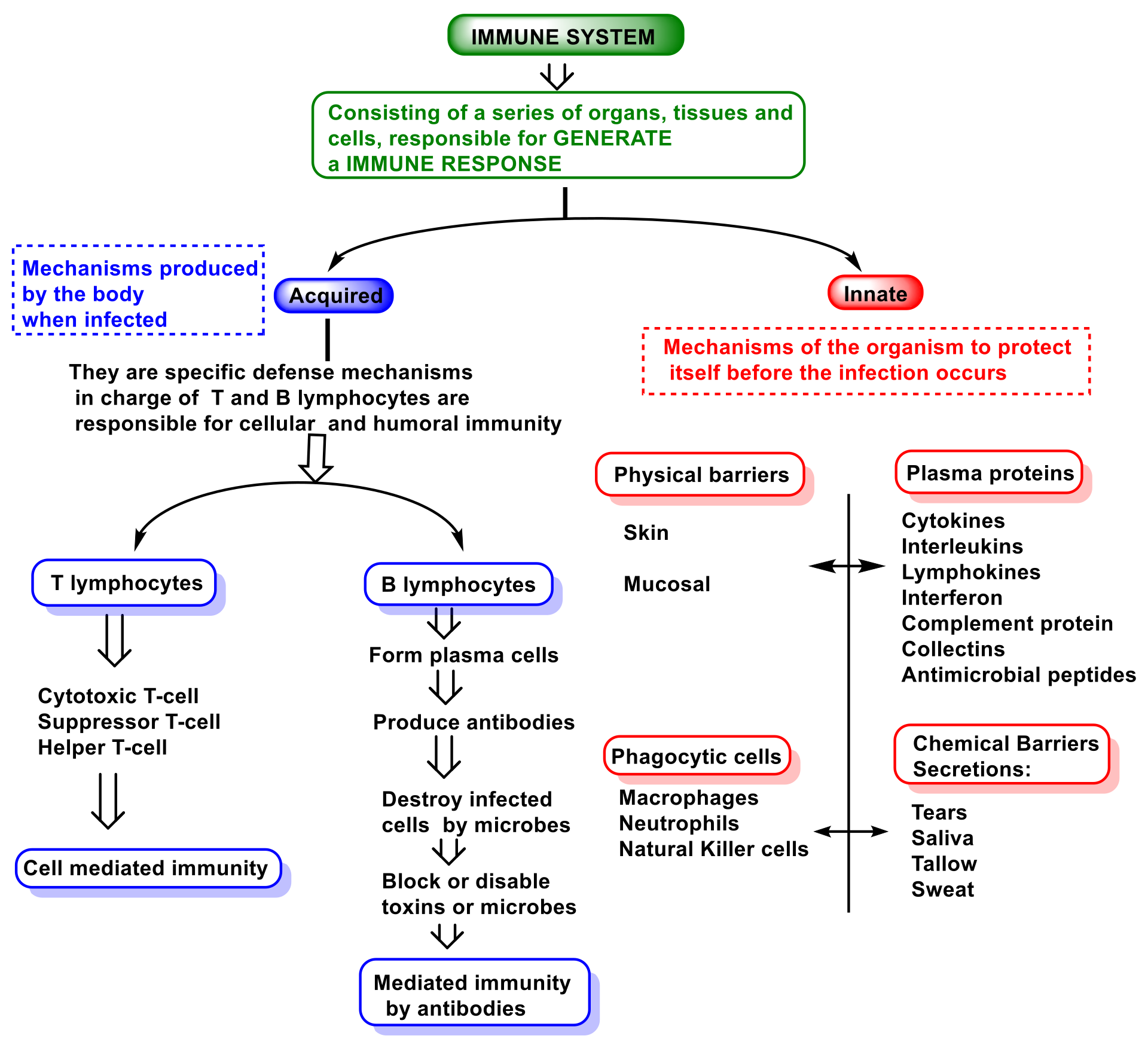
- (i)
-
The so-called alternative pathway is activated by the presence of bacterial surfaces that can bind complement protein C3. C3-coated bacteria are rapidly and efficiently phagocytosed and destroyed. C3 can activate other complement components by inducing a protein called C9 to insert itself into the cell walls of bacteria, causing them to rupture;
- (ii)
-
A second pathway of complement activation is triggered when bacterial surface carbohydrates bind to a mannose-binding lectin (MBL), collectin 11 (CL-K1), and ficolins (Ficolin-1, Ficolin-2, and Ficolin-3). Its activation leads to C4 and C2 activation by their serine-proteases; or
- (iii)
-
The classical complement pathway is initiated by antigen-antibody complexes with the antibody isotypes IgG and IgM. Upon activation, several proteins are recruited to generate C3 convertase, which cleaves the C3 protein. The C3b component of cleaved C3 binds to the C3 convertase to generate the C5 convertase, which cleaves the C5 protein. The cleaved products attract phagocytes to the site of infection and mark target cells for elimination by phagocytosis. C5 convertase initiates the terminal phase of the complement system, resulting in the assembly of the MAC membrane attack complex, creating a pore in the target cell membrane, and inducing its lysis [72].
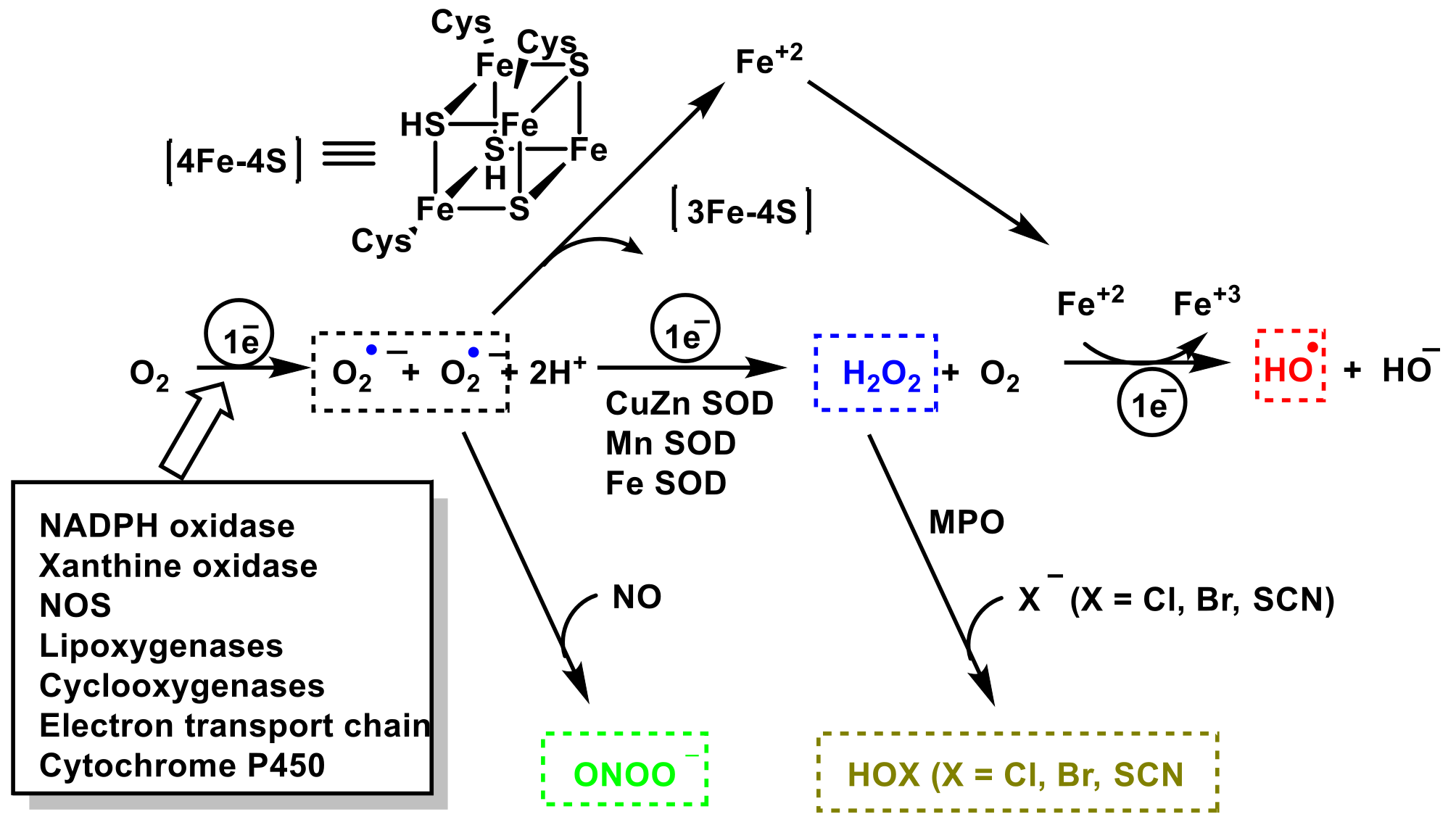
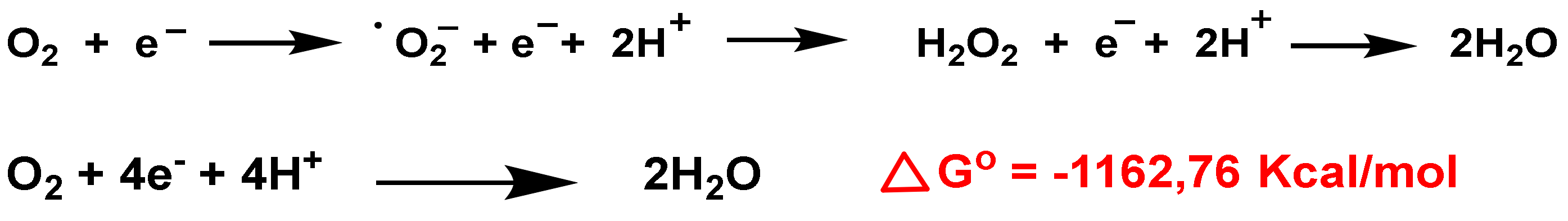
3. Role of Superoxide Anion •O2− and Hydrogen Peroxide H2O2 on Innate Immunity
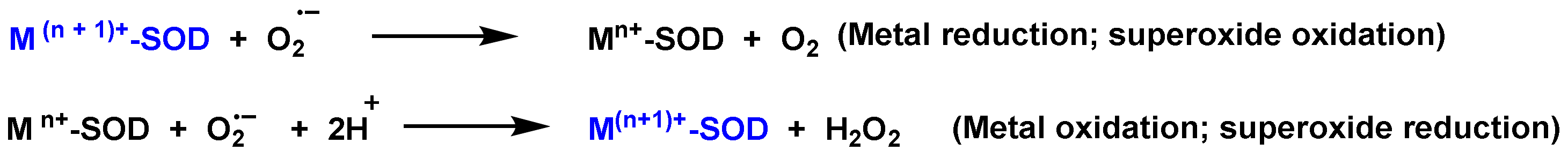
References
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma Clin. Immunol. 2018, 14, 1–10.
- Malech, H.L.; DeLeo, F.R.; Quinn, M.T. The Role of Neutrophils in the Immune System: An Overview. Methods Mol. Biol 2020, 2087, 3–10.
- Hackett, C.J. Innate immune activation as a broad-spectrum biodefense strategy: Prospects and research challenges. J. Allergy Clin. Immunol. 2003, 112, 686–694.
- Vijay, K. Toll-like receptors in immunity and inflammatory diseases: Past, present, and future. Int. Immunopharmacol. 2018, 59, 391–412.
- Li, H.; Zhou, X.; Huang, Y.; Liao, B.; Cheng, L.; Ren, B. Reactive oxygen species in pathogen clearance: The killing mechanisms, the adaption response, and the side effects. Front. Microbiol. 2021, 11, 622534.
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I. Lifestyle, oxidative stress, and antioxidants: Back and forth in the pathophysiology of chronic diseases. Front. Physiol. 2020, 11, 694.
- Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. The Nitration of Proteins, Lipids and DNA by Peroxynitrite Derivatives-Chemistry Involved and Biological Relevance. Stresses 2022, 2, 53–64.
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regenerat. Res. 2013, 8, 2003.
- Fisher, A.B. Redox signaling across cell membranes. Antioxid Redox Signal. 2009, 11, 1349–1356.
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344.
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019.
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274.
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The chemistry of reactive oxygen species (ROS) revisited: Outlining their role in biological macromolecules (DNA, lipids and proteins) and induced pathologies. Int. J. Mol. Sci. 2021, 22, 4642.
- Royer-Pokora, B.; Kunkel, L.M.; Monaco, A.P.; Goff, S.C.; Newburger, P.E.; Baehner, R.L.; Cole, F.S.; Curnutte, J.T.; Orkin, S.H. Cloning the gene for an inherited human disorder—chronic granulomatous disease—On the basis of its chromosomal location. Nature 1986, 322, 32–38.
- Dinauer, M.C.; Orkin, S.H.; Brown, R.; Jesaitis, A.J.; Parkos, C.A. The glycoprotein encoded by the X-linked chronic granulomatous disease locus is a component of the neutrophil cytochrome b complex. Nature 1987, 327, 717–720.
- Parkos, C.A.; Allen, R.A.; Cochrane, C.G.; Jesaitis, A.J. Purified cytochrome b from human granulocyte plasma membrane is comprised of two polypeptides with relative molecular weights of 91,000 and 22,000. J. Clin. Investig. 1987, 80, 732–742.
- Segal, A.W. Absence of both cytochrome b−245 subunits from neutrophils in X-linked chronic granulomatous disease. Nature 1987, 326, 88–91.
- Segal, A.W.; Heyworth, P.G.; Cockcroft, S.; Barrowman, M.M. Stimulated neutrophils from patients with autosomal recessive chronic granulomatous disease fail to phosphorylate a Mr-44,000 protein. Nature 1985, 316, 547–549.
- Volpp, B.D.; Nauseef, W.M.; Clark, R.A. Two cytosolic neutrophil oxidase components absent in autosomal chronic granulomatous disease. Science 1988, 242, 1295–1297.
- Wientjes, F.B.; Hsuan, J.J.; Totty, N.F.; Segal, A.W. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J. 1993, 296 (Pt. 3), 557–561.
- Abo, A.; Pick, E. Purification and characterization of a third cytosolic component of the superoxide-generating NADPH oxidase of macrophages. J. Biol. Chem. 1991, 266, 23577–23585.
- Roberts, A.W.; Kim, C.; Zhen, L.; Lowe, J.B.; Kapur, R.; Petryniak, B.; Spaetti, A.; Pollock, J.D.; Borneo, J.B.; Bradford, G.B.; et al. Deficiency of the Hematopoietic Cell-Specific Rho Family GTPase Rac2 Is Characterized by Abnormalities in Neutrophil Function and Host Defense. Immunity 1999, 10, 183–196.
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric oxide and peroxynitrite in health and disease. Physiol. Rev. 2007, 87, 315–424.
- Förstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735.
- Babu, B.R.; Frey, C.; Griffith, O.W. l-arginine binding to nitric-oxide synthase: The role of H-bonds to the nonreactive guanidinium nitrogens. J. Biol. Chem. 1999, 274, 25218–25226.
- Radi, R.; Peluffo, G.; Alvarez, M.a.N.; Naviliat, M.; Cayota, A. Unraveling peroxynitrite formation in biological systems. Free Radic. Biol. Med. 2001, 30, 463–488.
- Prolo, C.; Álvarez, M.N.; Radi, R. Peroxynitrite, a potent macrophagd-derived oxidizing cytotoxin to combat invading pathogens. Biofactors 2014, 40, 215–225.
- Tobler, A.; Koeffler, H.P. Myeloperoxidase: Localization, structure, and function. In Blood Cell Biochemistry Volume 3; Springer: Berlin/Heidelberg, Germany, 1991; pp. 255–288.
- Hurst, J.K. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012, 53, 508–520.
- Davies, M.J. Myeloperoxidase-derived oxidation: Mechanisms of biological damage and its prevention. J. Clin. Biochem. Nutr. 2010, 48, 8–19.
- Janeway, C.A., Jr.; Travers, P.; Walport, M.; Shlomchik, M.J. Principles of innate and adaptive immunity. In Immunobiology: The Immune System in Health and Disease, 5th ed.; Garland Science: New York, NY, USA, 2001.
- Kiboneka, A. Principals of innate and adaptive immunity. Immunity to microbes & fundamental concepts in immunology. World J. Adv. Res. Rev. 2021, 10, 188–197.
- Zimmerman, L.; Vogel, L.; Bowden, R. Understanding the vertebrate immune system: Insights from the reptilian perspective. J. Exp. Biol. 2010, 213, 661–671.
- Alberts, B. Molecular Biology of the Cell; WW Norton & Company: New York, NY, USA, 2017.
- Riera Romo, M.; Pérez-Martínez, D.; Castillo Ferrer, C. Innate immunity in vertebrates: An overview. Immunology 2016, 148, 125–139.
- LaRosa, D.F.; Rahman, A.H.; Turka, L.A. The innate immune system in allograft rejection and tolerance. J. Immunol. 2007, 178, 7503–7509.
- Tanaka, K.; Heil, M. Damage-associated molecular patterns (DAMPs) in plant innate immunity: Applying the danger model and evolutionary perspectives. Annu. Rev. Phytopathol. 2021, 59, 53–75.
- DeLeo, F.R.; Diep, B.A.; Otto, M. Host defense and pathogenesis in Staphylococcus aureus infections. Infect. Dis. Clin. N. Am. 2009, 23, 17–34.
- Khovidhunkit, W.; Kim, M.-S.; Memon, R.A.; Shigenaga, J.K.; Moser, A.H.; Feingold, K.R.; Grunfeld, C. Effects of infection and inflammation on lipid and lipoprotein metabolism: Mechanisms and consequences to the host. J. Lipid Res. 2004, 45, 1169–1196.
- Kieser, K.J.; Kagan, J.C. Multi-receptor detection of individual bacterial products by the innate immune system. Nat. Rev. Immunol. 2017, 17, 376–390.
- Finberg, R.W.; Wang, J.P.; Kurt-Jones, E.A. Toll like receptors and viruses. Rev. Med. Virol. 2007, 17, 35–43.
- Wu, J.; Niu, P.; Zhao, Y.; Cheng, Y.; Chen, W.; Lin, L.; Lu, J.; Cheng, X.; Xu, Z. Impact of miR-223-3p and miR-2909 on inflammatory factors IL-6, IL-1ß, and TNF-α, and the TLR4/TLR2/NF-κB/STAT3 signaling pathway induced by lipopolysaccharide in human adipose stem cells. PLoS ONE 2019, 14, e0212063.
- Moser, B.; Wolf, M.; Walz, A.; Loetscher, P. Chemokines: Multiple levels of leukocyte migration control. Trends Immunol. 2004, 25, 75–84.
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626.
- Mortaz, E.; Alipoor, S.D.; Adcock, I.M.; Mumby, S.; Koenderman, L. Update on neutrophil function in severe inflammation. Front. Immunol. 2018, 9, 2171.
- Muller, W. Getting leukocytes to the site of inflammation. Vet. Pathol. 2013, 50, 7–22.
- Ebbo, M.; Crinier, A.; Vély, F.; Vivier, E. Innate lymphoid cells: Major players in inflammatory diseases. Nat. Rev. Immunol. 2017, 17, 665–678.
- Shaikh, P.Z. Cytokines & their physiologic and pharmacologic functions in inflammation: A review. Int. J. Pharm. Life Sci. 2011, 2, 212599524.
- Slauch, J.M. How does the oxidative burst of macrophages kill bacteria? Still an open question. Mol. Microbiol. 2011, 80, 580–583.
- Tesmer, L.A.; Lundy, S.K.; Sarkar, S.; Fox, D.A. Th17 cells in human disease. Immunol. Rev. 2008, 223, 87–113.
- Favaro, R.R.; Phillips, K.; Delaunay-Danguy, R.; Ujčič, K.; Markert, U.R. Emerging Concepts in Innate Lymphoid Cells, Memory, and Reproduction. Front. Immunol. 2022, 13, 824263.
- Laskowski, T.J.; Biederstädt, A.; Rezvani, K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat. Rev. Cancer 2022, 22, 1–19.
- Ochel, A.; Tiegs, G.; Neumann, K. Type 2 innate lymphoid cells in liver and gut: From current knowledge to future perspectives. Int. J. Mol. Sci. 2019, 20, 1896.
- Maazi, H.; Akbari, O. Type two innate lymphoid cells: The Janus cells in health and disease. Immunol. Rev. 2017, 278, 192–206.
- Withers, D.R.; Hepworth, M.R. Group 3 innate lymphoid cells: Communications hubs of the intestinal immune system. Front. Immunol. 2017, 8, 1298.
- Withers, D.R. Lymphoid tissue inducer cells. Curr. Biol. 2011, 21, R381–R382.
- Klose, C.S.; Artis, D. Innate lymphoid cells as regulators of immunity, inflammation and tissue homeostasis. Nat. Immunol. 2016, 17, 765–774.
- Ulfig, A.; Leichert, L.I. The effects of neutrophil-generated hypochlorous acid and other hypohalous acids on host and pathogens. Cell. Mol. Life Sci. 2021, 78, 385–414.
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440.
- Atri, C.; Guerfali, F.Z.; Laouini, D. Role of human macrophage polarization in inflammation during infectious diseases. Int. J. Mol. Sci. 2018, 19, 1801.
- Azzouz, D.; Khan, M.A.; Palaniyar, N. ROS induces NETosis by oxidizing DNA and initiating DNA repair. Cell Death Discov. 2021, 7, 1–10.
- Huang, S.U.-S.; O’Sullivan, K.M. The expanding role of extracellular traps in inflammation and autoimmunity: The new players in casting dark webs. Int. J. Mol. Sci. 2022, 23, 3793.
- Vorobjeva, N.; Prikhodko, A.; Galkin, I.; Pletjushkina, O.; Zinovkin, R.; Sud’ina, G.; Chernyak, B.; Pinegin, B. Mitochondrial reactive oxygen species are involved in chemoattractant-induced oxidative burst and degranulation of human neutrophils in vitro. Eur. J. Cell Biol. 2017, 96, 254–265.
- Vorobjeva, N.; Galkin, I.; Pletjushkina, O.; Golyshev, S.; Zinovkin, R.; Prikhodko, A.; Pinegin, V.; Kondratenko, I.; Pinegin, B.; Chernyak, B. Mitochondrial permeability transition pore is involved in oxidative burst and NETosis of human neutrophils. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165664.
- Yipp, B.G.; Kubes, P. NETosis: How vital is it? Blood J. Am. Soc. Hematol. 2013, 122, 2784–2794.
- Vorobjeva, N.V.; Chernyak, B.V. NETosis: Molecular Mechanisms, Role in Physiology and Pathology. Biochemistry (Mosc) 2020, 85, 1178–1190.
- Schramm, M.; Wiegmann, K.; Schramm, S.; Gluschko, A.; Herb, M.; Utermöhlen, O.; Krönke, M. Riboflavin (vitamin B2) deficiency impairs NADPH oxidase 2 (Nox2) priming and defense against Listeria monocytogenes. Eur. J. Immunol. 2014, 44, 728–741.
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491.
- Allan, E.R.; Tailor, P.; Balce, D.R.; Pirzadeh, P.; McKenna, N.T.; Renaux, B.; Warren, A.L.; Jirik, F.R.; Yates, R.M. NADPH oxidase modifies patterns of MHC class II–restricted epitopic repertoires through redox control of antigen processing. J. Immunol. 2014, 192, 4989–5001.
- Metz-Boutigue, M.-H.; Shooshtarizadeh, P.; Prevost, G.; Haikel, Y.; Chich, J.-F. Antimicrobial peptides present in mammalian skin and gut are multifunctional defence molecules. Curr. Pharm. Design 2010, 16, 1024–1039.
- Sarma, J.V.; Ward, P.A. The complement system. Cell Tissue Res. 2011, 343, 227–235.
- Dunkelberger, J.R.; Song, W.-C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50.
- Zipfel, P.F.; Skerka, C. Complement regulators and inhibitory proteins. Nat. Rev. Immunol. 2009, 9, 729–740.
- Collard, C.D.; Väkevä, A.; Morrissey, M.A.; Agah, A.; Rollins, S.A.; Reenstra, W.R.; Buras, J.A.; Meri, S.; Stahl, G.L. Complement activation after oxidative stress: Role of the lectin complement pathway. Am. J. Pathol. 2000, 156, 1549–1556.
- Tabassum, N.; Kheya, I.S.; Asaduzzaman, S.; Maniha, S.; Fayz, A.H.; Zakaria, A.; Noor, R. A review on the possible leakage of electrons through the electron transport chain within mitochondria. Life Sci. 2020, 6, 105–113.
- Wittmann, C.; Chockley, P.; Singh, S.K.; Pase, L.; Lieschke, G.J.; Grabher, C. Hydrogen peroxide in inflammation: Messenger, guide, and assassin. Adv. Hematol. 2012, 2012, 541471.
- Schoonbroodt, S.; Ferreira, V.; Best-Belpomme, M.; Boelaert, J.R.; Legrand-Poels, S.; Korner, M.; Piette, J. Crucial role of the amino-terminal tyrosine residue 42 and the carboxyl-terminal PEST domain of IκBα in NF-κB activation by an oxidative stress. J. Immunol. 2000, 164, 4292–4300.
- Tang, D.; Shi, Y.; Kang, R.; Li, T.; Xiao, W.; Wang, H.; Xiao, X. Hydrogen peroxide stimulates macrophages and monocytes to actively release HMGB1. J. Leukoc. Biol. 2007, 81, 741–747.
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181.
- Singer, M.; Sansonetti, P.J. IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of Shigella-induced colitis. J. Immunol. 2004, 173, 4197–4206.
- Filimon, A.; Preda, I.A.; Boloca, A.F.; Negroiu, G. Interleukin-8 in Melanoma Pathogenesis, Prognosis and Therapy—An Integrated View into Other Neoplasms and Chemokine Networks. Cells 2021, 11, 120.
- He, H.-Q.; Ye, R.D. The formyl peptide receptors: Diversity of ligands and mechanism for recognition. Molecules 2017, 22, 455.
- Kobayashi, S.D.; DeLeo, F.R. Role of neutrophils in innate immunity: A systems biology-level approach. Wiley Interdiscp. Rev. Syst. Biol. Med. 2009, 1, 309–333.
- Nguyen, G.; Green, E.; Mecsas, J. Neutrophils to the ROScue: Mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell Infect. Microbiol. 2017, 7, 373.
- Elsbach, P.; Weiss, J. Oxygen-dependent and oxygen-independent mechanisms of microbicidal activity of neutrophils. Immunol. Lett. 1985, 11, 159–163.
- Davies, M.J. Reactivity of Peroxidase-Derived Oxidants with Proteins, Glycoproteins and Proteoglycans. In Mammalian Heme Peroxidases; CRC Press: Boca Raton, FL, USA, 2021; pp. 53–77.
- Scieszka, D.; Lin, Y.-H.; Li, W.; Choudhury, S.; Yu, Y.; Freire, M. NETome: The molecular characterization of neutrophil extracellular traps (NETs). Cold Spring Harbor Lab. 2020.
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.; Fieschi, F. NADPH oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890.
- Demaurex, N.; Petheö, G.L. Electron and proton transport by NADPH oxidases. Philos. Trans. Royal Soc. B Biol. Sci. 2005, 360, 2315–2325.
- Waghela, B.N.; Vaidya, F.U.; Agrawal, Y.; Santra, M.K.; Mishra, V.; Pathak, C. Molecular insights of NADPH oxidases and its pathological consequences. Cell Biochem. Funct. 2021, 39, 218–234.
- Purushothaman, D.; Sarin, A. Cytokine-dependent regulation of NADPH oxidase activity and the consequences for activated T cell homeostasis. J. Exp. Med. 2009, 206, 1515–1523.
- Manea, S.-A.; Constantin, A.; Manda, G.; Sasson, S.; Manea, A. Regulation of Nox enzymes expression in vascular pathophysiology: Focusing on transcription factors and epigenetic mechanisms. Redox Biol. 2015, 5, 358–366.
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil extracellular trap formation: Physiology, pathology, and pharmacology. Biomolecules 2019, 9, 365.
- Roos, D. Chronic granulomatous disease. Br. Med. Bull. 2016, 118, 50.
- Mócsai, A.; Zhou, M.; Meng, F.; Tybulewicz, V.L.; Lowell, C.A. Syk is required for integrin signaling in neutrophils. Immunity 2002, 16, 547–558.
- Mócsai, A.; Ruland, J.; Tybulewicz, V.L.J. The SYK tyrosine kinase: A crucial player in diverse biological functions. Nat. Rev. Immunol. 2010, 10, 387–402.
- Kerrigan, A.M.; Brown, G.D. Syk-coupled C-type lectins in immunity. Trends Immunol. 2011, 32, 151–156.
- Chen, X.; Song, M.; Zhang, B.; Zhang, Y. Reactive oxygen species regulate T cell immune response in the tumor microenvironment. Oxid. Med. Cell. Longev. 2016, 2016.
- Fridovich, I. The biology of oxygen radicals: The superoxide radical is an agent of oxygen toxicity; superoxide dismutases provide an important defense. Science 1978, 201, 875–880.
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Nilsa, R.D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374.
- Forman, H.J.; Torres, M. Reactive oxygen species and cell signaling: Respiratory burst in macrophage signaling. Am. J. Respir. Crit. Care Med. 2002, 166, S4–S8.
- Nathan, C.; Cunningham-Bussel, A. Beyond oxidative stress: An immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 2013, 13, 349–361.
