Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Conner Chen and Version 1 by Tapas Mandal.
Mono-, few-, and multi-layer graphene can be synthesized using various methods, including micromechanical exfoliation, chemical vapour deposition, and chemical methods such as oxidizing graphene into graphene oxide and then reducing it chemically or thermally.
- 2D nanosheet
- nanomaterial
- layered material
1. Introduction
The sixth element is truly fascinating due to its allotropic forms [1,2][1][2]. Its allotropes are as soft as graphite [3,4][3][4] and as hard as diamond [5]. Graphite is three-dimensional structure that is made up of stacking multiple one-atom-thick layers, which are formed from strong sp2 hybridized carbon atoms arranged in a hexagonal lattice [6,7][6][7]. This two-dimensional monatomic thick crystal structure consists of single atomic sheet of graphite, and is called “graphene” [8]. Recently, graphene, an allotrope of carbon, has become a hot topic of research due to its good physico-chemical properties [9,10][9][10]. When stacked, graphene forms different types of graphene, such as monolayer graphene [11], few-layer graphene [12] and multilayer graphene [13]. The properties of graphene are dependent on the number of stacked layers; for example, monolayer graphene has better properties than few-layer graphene and multi-layer graphene [14,15][14][15].
Scientists described the theoretical existence of single layer graphene more than 80 years ago [16]. Then, the practical existence of two-dimensional graphene was considered physically impossible [17]. However, in 2004, Geim et al. isolated single-sheet graphene via the scotch tape method and demonstrated its properties experimentally [18]. This was the first time researchers came to know about the remarkable properties of graphene [19]. Since then, there has been an exponential rise in studies of graphene-based materials to determine the various applications of their properties, such as in biomedical applications [20,21][20][21].
Due to the expert attention paid to graphene-based materials, high-performance materials were successfully produced. However, achieving these high performance levels involves various challenges, especially in relation to monolayer graphene [22,23][22][23]. One of these challenges is the synthesis of monolayer or few-layer graphene in bulk with high purity, which is an is extremely difficult process [24]. Other challenges include the restacking of monolayer graphene in few-layer and multi-layer graphene [25,26][25][26]. This restacking results in the decreased performance of the graphene-based devices. Therefore, the control of lateral size and aggregation states, in addition to the process of the oxidation of graphene in graphene oxide, is essential for developing graphene-based high-performance devices [27,28][27][28].
After achieving the synthesis of monolayer graphene, which exhibits good characteristics for a roadmap of graphene-based devices, the characterization of the order of stacking in graphene became a subject of interest for researchers [29,30][29][30]. These characterization techniques include atomic force microscopy (AFM) [31], Raman spectra [32], Raman mapping [33], and Transmission electron microscopy (TEM) [34]. Among these techniques, Raman spectra and TEM are methods frequently used to determine whether the synthesized material is monolayer graphene, few-layer graphene, or multi-layer graphene [32,34][32][34]. After characterization, the graphene material can be used for different applications, such as biosensors [35], tissue engineering [36], drug carriers [37], and other biomedical applications.
2. Production of Mono-, Few-, and Multi-Layer Graphene
Graphene, especially monolayer graphene, has received a great deal of attention since 2004 due to its good mechanical, electrical, and thermal properties [18]. However, the synthesis of monolayer graphene is extremely difficult and expensive [38,39][38][39]. Therefore, synthesizing few-layer graphene or multi-layer graphene is also a subject of interest [40]. A general scheme represents monolayer, few-layer, and multi-layer graphene (Figure 1). Mono-, few-, and multi-layer graphene can be synthesized using various methods, including micromechanical exfoliation [41], chemical vapour deposition [42], and chemical methods such as oxidizing graphene into graphene oxide [43] and then reducing it chemically [44] or thermally [45]. A few methods provide high-quality, large-scale few-layer and multi-layer graphene but small amounts of monolayer graphene, such as the chemical method; however, the purity and defect density remain matters of concern [46,47][46][47]. Similarly, large-scale monolayer graphene can be synthesized by chemical vapour deposition, but purity still is a topic of concern [48]. The various methods for producing mono-, few-, and multi-layer graphene are as follows.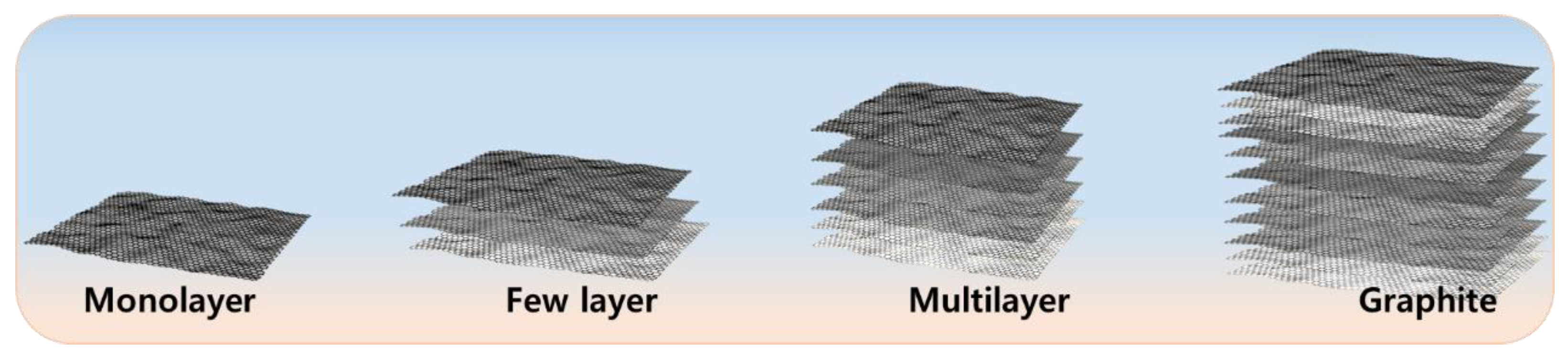
Figure 1.
Schematic overview of monolayer, few-layer and multi-layer graphene.
2.1. Synthesis of Few- to Multi-Layer Graphene
2.1.1. Exfoliation of Graphite
Graphite is an abundant material and well known for its lubricating properties [49,50][49][50]. However, it has poor mechanical, electrical, and thermal properties. Graphite can be exfoliated into different types of graphene, but we must first overcome the weak van der Waals forces which hold the different types of graphene into graphite [51]. A general scheme of the process for obtaining different layers of graphene is shown in Figure 2.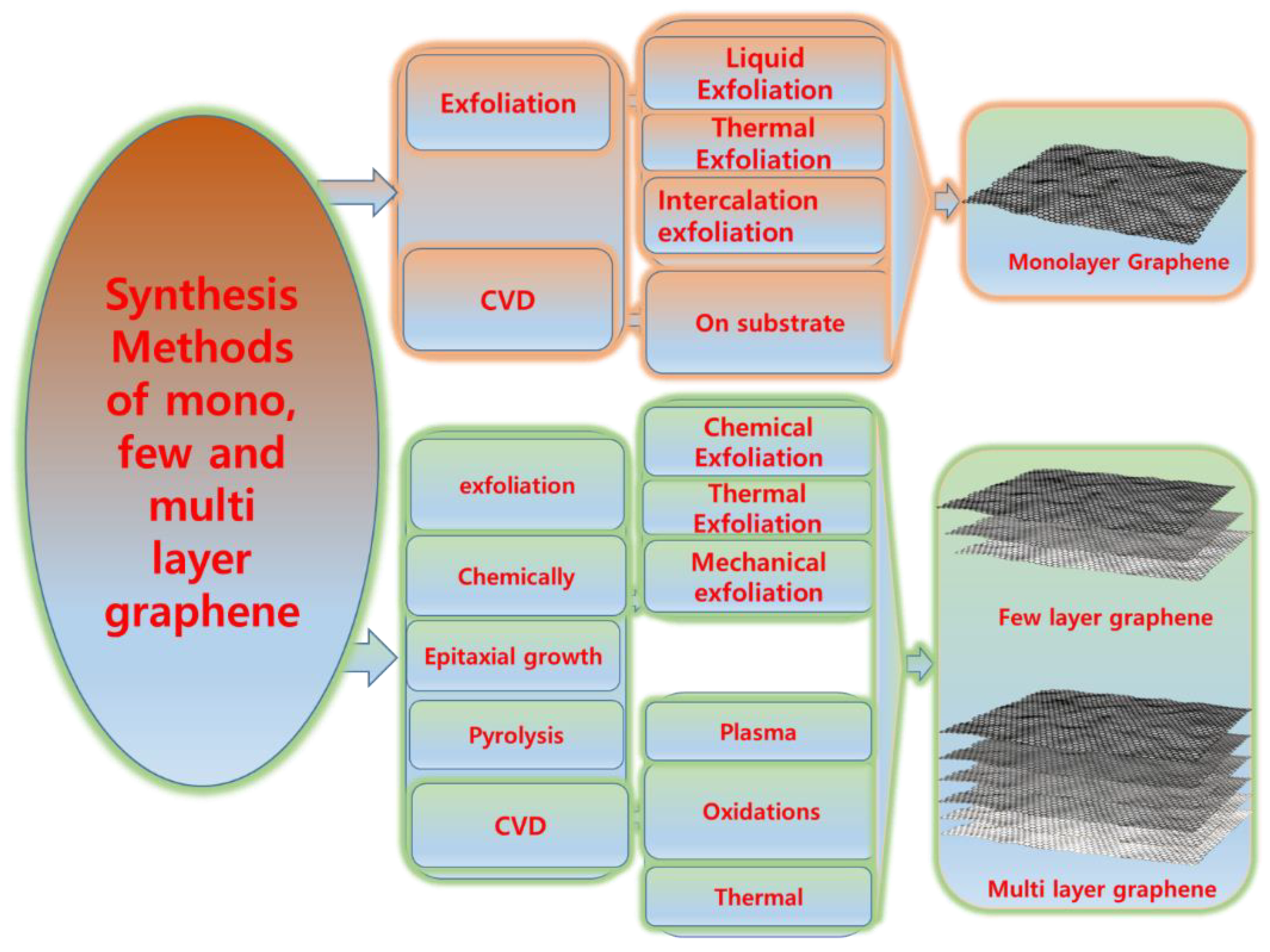
Figure 2.
Schematic overview of the process for obtaining different layers of graphene.
- (a)
-
There are several ways to overcome these weak interactions, and the most promising among them is the sonication of graphite in different solvents; however, the yields of multi-layer and monolayer graphene [52] are very poor in this process.
- (b)
-
Another promising strategy to obtain graphene from graphite involves oxidizing graphite by various methods, such as Hummer’s method, and reducing it chemically or thermally to obtain a large-scale yield [53]. However, the redox results of graphite into graphene mostly provide high yields of few-layer graphene or multi-layer graphene.
- (c)
-
Another method of producing few- and multi-layer graphene involves the exfoliation of graphite via graphite intercalation [54,55][54][55]. Different types of chemicals can be inserted to graphite interlayer space, thereby increasing the interlayer distance of adjacent graphene sheets in graphite. This phenomenon also changes the properties of graphene, since the increase in interlayer spacing affects electronic coupling between adjacent graphene sheets in graphite [56].
- (d)
-
Another way to exfoliate graphite into few- and multi-layer graphene is via ball milling [57]. This is a way of exfoliating graphite via mechanical exfoliation. Ball milling has been extensively used in the past to reduce the particle size of a material [58]. Scientists thus propose ball milling as a way to mechanically exfoliate graphite in small-size nano-graphite, increasing the mixing time to obtain few- or multi-layer graphene. Thus, ball milling is a promising technique for exfoliating graphite into graphene. The advantage of using ball milling to produce graphene is its low production cost, its easy handling, and its ability to produce graphene at large scale.
- (e)
-
The plasma synthesis method is another significant way to produce graphene with few-to-multiple layers. Microwave plasmas produced by surface waves at a stimulation frequency of 2.45 GHz and under atmospheric pressure conditions were successfully used to produce highly structured and stable self-standing graphene sheets [16]. There were also investigations into how the addition of hydrogen affects the density of the carbon precursor (C2, C) and the structural soundness of synthetic graphene sheets. Changes in the sp3/sp2 ratio and the C2 and C number densities were shown to be correlated [59]. Microwave-driven plasmas were used to control oxygen functions and the sp2/sp3 carbon ratio (~15) to a high degree [60].
2.1.2. Synthesis of Monolayer Graphene
- (a)
-
In 2004, for the first time, Geim and Novoselov developed a method of synthesizing graphene using micromechanical cleavage as “scotch tape” via mechanical exfoliation [18]. This was for the first time in history that any scientist experimentally synthesized monolayer graphene. After synthesizing the monolayer graphene, these scientists further demonstrated its outstanding properties [61]. However, due to the uneven thickness of the graphene flakes and its high production costs, the mechanical exfoliation method was not suitable for the mass production of graphene that might be used to study graphene-based devices.
- (b)
-
Another method of producing monolayer graphene is the chemical vapour deposition method [62]. Monolayer graphene can be grown epitaxially on a silicon carbide substrate, and can be used for various applications, such as transistors. The size of the monolayer graphene grown depends on the size of the silicon wafer. The surface of the silicon wafer also influences the properties of the synthesized monolayer graphene.
References
- Dong, H.; Zhang, Z.; Feng, Z.; Kang, J.; Wu, D.; Wang, Q.; Li, J.; Su, R. Origins of low lattice thermal conductivity in 2D carbon allotropes. J. Mater. Res. Technol. 2021, 11, 1982–1990.
- Liu, W.-D.; Yu, Y.; Dargusch, M.; Liu, Q.; Chen, Z.-G. Carbon allotrope hybrids advance thermoelectric development and applications. Renew. Sustain. Energy Rev. 2021, 141, 110800.
- Mailian, A.; Panosyan, Z.; Yengibaryan, Y.; Mailian, M. Identification of Carbon Allotropes in Tribolayers Obtained by Rubbing of Graphite. Mater. Today Proc. 2017, 4, 6842–6848.
- Kabir, H.; Zhu, H.; May, J.; Hamal, K.; Kan, Y.; Williams, T.; Echeverria, E.; McIlroy, D.N.; Estrada, D.; Davis, P.H.; et al. The sp2-sp3 carbon hybridization content of nanocrystalline graphite from pyrolyzed vegetable oil, comparison of electrochemistry and physical properties with other carbon forms and allotropes. Carbon 2019, 144, 831–840.
- Liu, Y.; Jiang, X.; Fu, J.; Zhao, J. New metallic carbon: Three dimensionally carbon allotropes comprising ultrathin diamond nanostripes. Carbon 2018, 126, 601–610.
- Zhang, X.; Schneider, R.; Müller, E.; Gerthsen, D. Practical aspects of the quantification of sp2-hybridized carbon atoms in diamond-like carbon by electron energy loss spectroscopy. Carbon 2016, 102, 198–207.
- Theye, M.-L.; Paret, V. Spatial organization of the sp2-hybridized carbon atoms and electronic density of states of hydrogenated amorphous carbon films. Carbon 2002, 40, 1153–1166.
- Mandal, T.K.; Hou, Y.; Gao, Z.Y.; Ning, H.; Yang, W.S.; Gao, M.Y. Graphene oxide-based sensor for ultrasensitive visual detection of fluoride. Adv. Sci. 2016, 3, 1600217.
- Bin Hamid, M.A.; Chan, K.T.; Raymond Ooi, C.H.; Zainuddin, H.; Mohd Shah, N.; Shahrol Nidzam, N.N. Structural stability and electronic properties of graphene/germanene heterobilayer. Results Phys. 2021, 28, 104545.
- Zhang, H.; Zhang, B.; Gao, Q.; Song, J.; Han, G. A review on microstructures and properties of graphene-reinforced aluminum matrix composites fabricated by friction stir processing. J. Manuf. Process. 2021, 68, 126–135.
- Wu, Y.-C.; Shao, J.-L.; Zhan, H. Damage and self-healing characteristics of monolayer graphene enhanced Cu under ballistic impact. Mech. Mater. 2021, 155, 103736.
- Graf, D.; Molitor, F.; Ensslin, K.; Stampfer, C.; Jungen, A.; Hierold, C.; Wirtz, L. Spatially Resolved Raman Spectroscopy of Single- and Few-Layer Graphene. Nano Lett. 2007, 7, 238–242.
- Liu, B.; Cao, S.; Gao, N.; Cheng, L.; Liu, Y.; Zhang, Y.; Feng, D. Thermosetting CFRP interlaminar toughening with multi-layers graphene and MWCNTs under mode I fracture. Compos. Sci. Technol. 2019, 183, 107829.
- Zhang, Y.Y.; Gu, Y.T. Mechanical properties of graphene: Effects of layer number, temperature and isotope. Comput. Mater. Sci. 2013, 71, 197–200.
- Mag-isa, A.E.; Kim, S.-M.; Kim, J.-H.; Oh, C.-S. Variation of thermal expansion coefficient of freestanding multilayer pristine graphene with temperature and number of layers. Mater. Today Commun. 2020, 25, 101387.
- Tsyganov, D.; Bundaleska, N.; Henriques, J.; Felizardo, E.; Dias, A.; Abrashev, M.; Kissovski, J.; Botelho do Rego, A.M.; Ferraria, A.M.; Tatarova, E. Simultaneous Synthesis and Nitrogen Doping of Free-Standing Graphene Applying Microwave Plasma. Materials 2020, 13, 4213.
- Mukri, M.-R.; Elias, M.S.; Aziz, M.; Tanemura, M.; Mohd Yusop, M.Z. Structural Modification of Pristine Graphene Network Towards Nanoporous Graphene Membrane: A Review. J. Appl. Membr. Sci. Technol. 2018, 22.
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669.
- Geim, A.K.; Novoselov, K.S. The rise of graphene. In Nanoscience and Technology; Macmillan Publishers Ltd.: London, UK, 2009; pp. 11–19.
- Shende, P.; Pathan, N. Potential of carbohydrate-conjugated graphene assemblies in biomedical applications. Carbohydr. Polym. 2021, 255, 117385.
- Song, S.; Shen, H.; Wang, Y.; Chu, X.; Xie, J.; Zhou, N.; Shen, J. Biomedical application of graphene: From drug delivery, tumor therapy, to theranostics. Colloids Surf. B Biointerfaces 2020, 185, 110596.
- Lin, L.; Peng, H.; Liu, Z. Synthesis challenges for graphene industry. Nat. Mater. 2019, 18, 520–524.
- Urban, K.W. The challenges of graphene. Nat. Mater. 2011, 10, 165–166.
- Reina, G.; González-Domínguez, J.M.; Criado, A.; Vázquez, E.; Bianco, A.; Prato, M. Promises, facts and challenges for graphene in biomedical applications. Chem. Soc. Rev. 2017, 46, 4400–4416.
- Jeong, J.H.; Kim, Y.H.; Roh, K.C.; Kim, K.-B. Effect of thermally decomposable spacers on graphene microsphere structure and restacking of graphene sheets during electrode fabrication. Carbon 2019, 150, 128–135.
- Xie, Q.; Zhang, Y.; Zhao, P. Facile fabrication of honeycomb-like restacking-inhibited graphene architecture with superior electrochemical performance for energy storage. Mater. Lett. 2018, 225, 93–96.
- Kota, M.; Park, H.S. Restacking-inhibited nitrogen-incorporated mesoporous reduced graphene oxides for high energy supercapacitors. Ceram. Int. 2018, 44, 3195–3200.
- Xu, G.; Yuan, J.; Geng, X.; Dou, H.; Chen, L.; Yan, X.; Zhu, H. Caterpillar-like graphene confining sulfur by restacking effect for high performance lithium sulfur batteries. Chem. Eng. J. 2017, 322, 454–462.
- Tambe, P. Synthesis and characterization of acid treated reduced graphene oxide. Mater. Today Proc. 2022, 49, 1294–1297.
- Fu, H.; Gao, B.; Liu, Z.; Liu, W.; Wang, Z.; Wang, M.; Li, J.; Feng, Z.; Reza Kamali, A. Electrochemical performance of honeycomb graphene prepared from acidic graphene oxide via a chemical expansion method. J. Electroanal. Chem. 2022, 920, 116545.
- Hauquier, F.; Alamarguy, D.; Viel, P.; Noël, S.; Filoramo, A.; Huc, V.; Houzé, F.; Palacin, S. Conductive-probe AFM characterization of graphene sheets bonded to gold surfaces. Appl. Surf. Sci. 2012, 258, 2920–2926.
- Agarwal, P.B.; Paulchowdhury, P.; Mukherjee, A.; Lohani, P.; Thakur, N.K. Optimization of oxygen plasma based etching of single layered graphene through Raman and FESEM characterization. Mater. Today Proc. 2022, 48, 616–618.
- Shtepliuk, I.; Ivanov, I.G.; Pliatsikas, N.; Iakimov, T.; Jamnig, A.; Sarakinos, K.; Yakimova, R. Probing the uniformity of silver-doped epitaxial graphene by micro-Raman mapping. Phys. B Condens. Matter 2020, 580, 411751.
- Pelaez-Fernandez, M.; Bermejo, A.; Benito, A.M.; Maser, W.K.; Arenal, R. Detailed thermal reduction analyses of graphene oxide via in-situ TEM/EELS studies. Carbon 2021, 178, 477–487.
- Wang, Z.; Yu, H.; Zhao, Z. Silk fibroin hydrogel encapsulated graphene filed-effect transistors as enzyme-based biosensors. Microchem. J. 2021, 169, 106585.
- GV, Y.D.; Prabhu, A.; Anil, S.; Venkatesan, J. Preparation and characterization of dexamethasone loaded sodium alginate-graphene oxide microspheres for bone tissue engineering. J. Drug Deliv. Sci. Technol. 2021, 64, 102624.
- Karimi, S.; Namazi, H. Fe3O4@PEG-coated dendrimer modified graphene oxide nanocomposite as a pH-sensitive drug carrier for targeted delivery of doxorubicin. J. Alloys Compd. 2021, 879, 160426.
- Sharif, S.; Ahmad, K.S.; Rehman, F.; Bhatti, Z.; Thebo, K.H. Two-dimensional graphene oxide based membranes for ionic and molecular separation: Current status and challenges. J. Environ. Chem. Eng. 2021, 9, 105605.
- Ikram, R.; Jan, B.M.; Ahmad, W. Advances in synthesis of graphene derivatives using industrial wastes precursors; prospects and challenges. J. Mater. Res. Technol. 2020, 9, 15924–15951.
- Rotte, N.K.; Naresh, V.; Muduli, S.; Reddy, V.; Srikanth, V.V.S.; Martha, S.K. Microwave aided scalable synthesis of sulfur, nitrogen co-doped few-layered graphene material for high-performance supercapacitors. Electrochim. Acta 2020, 363, 137209.
- Salussolia, G.; Barbieri, E.; Pugno, N.M.; Botto, L. Micromechanics of liquid-phase exfoliation of a layered 2D material: A hydrodynamic peeling model. J. Mech. Phys. Solids 2020, 134, 103764.
- Hwang, G.; Kim, T.; Shin, J.; Shin, N.; Hwang, S. Machine learnings for CVD graphene analysis: From measurement to simulation of SEM images. J. Ind. Eng. Chem. 2021, 101, 430–444.
- Yildiz, G.; Bolton-Warberg, M.; Awaja, F. Graphene and graphene oxide for bio-sensing: General properties and the effects of graphene ripples. Acta Biomater. 2021, 131, 62–79.
- Guo, J.; Mao, B.; Li, J.; Wang, X.; Yang, X. Rethinking the reaction pathways of chemical reduction of graphene oxide. Carbon 2021, 171, 963–967.
- Barkauskas, J.; Gaidukevič, J.; Niaura, G. Thermal reduction of graphite oxide in the presence of nitrogen-containing dyes. Carbon Lett. 2021, 31, 1097–1110.
- Wadekar, P.H.; Ghosh, A.; Khose, R.V.; Pethsangave, D.A.; Mitra, S.; Some, S. A novel chemical reduction/co-precipitation method to prepare sulfur functionalized reduced graphene oxide for lithium-sulfur batteries. Electrochim. Acta 2020, 344, 136147.
- Vázquez-Sánchez, P.; Rodríguez-Escudero, M.A.; Burgos, F.J.; Llorente, I.; Caballero-Calero, O.; González, M.M.; Fernández, R.; García-Alonso, M.C. Synthesis of Cu/rGO composites by chemical and thermal reduction of graphene oxide. J. Alloys Compd. 2019, 800, 379–391.
- Demetriou, G.; Biancalana, F.; Abraham, E.; Ji, W.; Wang, Y.; Kar, A.K. Direct observation of an irradiance dependent nonlinear refraction in CVD single layer graphene. Opt. Commun. 2021, 481, 126535.
- Jiang, X.; Song, J.; Chen, S.; Su, Y.; Fan, H.; Zhang, Y.; Hu, L. In-situ fabricated bulk metallic glass/graphite composites with a 3D lubricating layer: Tribological properties under dry sliding and in seawater. Tribol. Int. 2020, 148, 106301.
- Liu, Q.; Pang, M.; Chen, J.; Liu, G.; Zhang, L. Microstructure and properties characterization of Ti-containing Ni60/Graphite self-lubricating composite coatings applied on 300 M ultra-high strength steel by laser cladding. Mater. Chem. Phys. 2021, 266, 124554.
- Ferreira, M.P.; da Nova Mussel, W.; Dutra, P.R.; Ângela de Barros Correia Menezes, M.; Pedrosa, T.A. Physical-chemical exfoliation of pristine graphite flakes. Radiat. Phys. Chem. 2021, 188, 109652.
- Tene, T.; Guevara, M.; Viteri, E.; Maldonado, A.; Pisarra, M.; Sindona, A.; Vacacela Gomez, C.; Bellucci, S. Calibration of Fermi Velocity to Explore the Plasmonic Character of Graphene Nanoribbon Arrays by a Semi-Analytical Model. Nanomaterials 2022, 12, 2028.
- Aixart, J.; Díaz, F.; Llorca, J.; Rosell-Llompart, J. Increasing reaction time in Hummers’ method towards well exfoliated graphene oxide of low oxidation degree. Ceram. Int. 2021, 47, 22130–22137.
- Rozmanowski, T.; Krawczyk, P. Methanol electrooxidation at NiCl2–FeCl3–graphite intercalation compound affected by ozone treatment. J. Phys. Chem. Solids 2021, 157, 110223.
- Yamamoto, H.; Matsumoto, K.; Hagiwara, R. Stage-number dependence of intercalated species for fluorosilicate graphite intercalation compounds: Pentafluorosilicate vs. hexafluorosilicate. J. Fluor. Chem. 2021, 242, 109714.
- Cahen, S.; Vangelisti, R. Chemical vapor transport for intercalation reactions: Synthesis of a 1st stage DyCl3 graphite intercalation compound. J. Solid State Chem. 2021, 299, 122185.
- Suvarna, K.S.; Binitha, N.N. Graphene preparation by jaggery assisted ball-milling of graphite for the adsorption of Cr(VI). Mater. Today Proc. 2020, 25, 236–240.
- Tenorio Gonzalez, F.N.; Barajas Rosales, I.R.; Vera Serna, P.; Sánchez de Jesus, F.; Bolarin Miró, A.M.; Garrido Hernández, A.; Kusý, M. Reducing the crystallite and particle size of SrFe12O19 with PVA by high energy ball milling. J. Alloys Compd. 2019, 771, 464–470.
- Tsyganov, D.; Bundaleska, N.; Tatarova, E.; Dias, A.; Henriques, J.; Rego, A.; Ferraria, A.; Abrashev, M.V.; Dias, F.M.; Luhrs, C.C.; et al. On the plasma-based growth of ‘flowing’ graphene sheets at atmospheric pressure conditions. Plasma Sources Sci. Technol. 2016, 25, 015013.
- Tatarova, E.; Dias, A.; Henriques, J.; Abrashev, M.; Bundaleska, N.; Kovacevic, E.; Bundaleski, N.; Cvelbar, U.; Valcheva, E.; Arnaudov, B.; et al. Towards large-scale in free-standing graphene and N-graphene sheets. Sci. Rep. 2017, 7, 10175.
- Zhao, C.; Hong, Y.; Chu, X.; Dong, Y.; Hu, Z.; Sun, X.; Yan, S. Enhanced ferroelectric properties of P(VDF-TrFE) thin film on single-layer graphene simply adjusted by crystallization condition. Mater. Today Energy 2021, 20, 100678.
- Wu, Y.; Wang, S.; Komvopoulos, K. A review of graphene synthesis by indirect and direct deposition methods. J. Mater. Res. 2020, 35, 76–89.
More
