Nanomaterials are popularly used in drug delivery, disease diagnosis and therapy. Among a number of functionalized nanomaterials such as carbon nanotubes, peptide nanostructures, liposomes and polymers, gold nanoparticles (Au NPs) make excellent drug and anticancer agent carriers in biomedical and cancer therapy application. Recent advances of synthetic technique improved the surface coating of Au NPs with accurate control of particle size, shape and surface chemistry. These make the gold nanomaterials a much easier and safer cancer agent and drug to be applied to the patient’s tumor. Although many studies on Au NPs have been published, more results are in the pipeline due to the rapid development of nanotechnology. The purpose of this review is to assess how the novel nanomaterials fabricated by Au NPs can impact biomedical applications such as drug delivery and cancer therapy. Moreover, this review explores the viability, property and cytotoxicity of various Au NPs.
- nanoscale materials
- gold nanoparticles
- functionalized nanomaterials
- drug delivery
- thermal therapy
- radiation therapy
- medical imaging
- gold nanoparticle-based cancer therapy
- and anticancer agent
1. Introduction
Gold nanoparticles (Au NPs) are effective radiosensitizers in medical applications such as drug delivery and cancer therapy. In biomedical and cancer therapy applications, Au NPs can act as a contrast agent and dose enhancer in image-guided nanoparticle-enhanced radiotherapy using kilovoltage cone-beam computed tomography [1][2]. With recent advances of synthesis and fabrication methods in nanomaterials, particle variables such as size, composition, morphology and surface chemistry can be controlled easily by precise technology [3]. Moreover, biocompatible surface coating can be added onto the NP surface to provide stabilization under physiological condition. The integration of functional ligands as coating through surface chemistry on the NPs enables them to perform multiple biomedical functions in the molecular or cellular level simultaneously. Applications of these nanomaterials include contrast agents in multimodal imaging, carriers in drug delivery and enhancers in cancer therapy. In this review, we will highlight various synthesis methods to fabricate Au NPs. We will explore different applications of nanomaterials in drug delivery and cancer therapy such as plasmid deoxynucleic acids vector delivery, ribonucleic acids delivery and gold nanoparticle-based therapy.
2. Application
Tremendous technological interest have been given to Au NPs due to their unique optical properties, ease of synthesis and chemical stability. The particles can be used in biomedical applications such as cancer treatment, biological imaging, chemical sensing, and drug delivery [4][5][6]. However, their potential toxicity and health impact need to be explored thoroughly, before they can be used in clinical settings [7]. Considerable interest has been given to nanostructures in the past few years due to their properties, such as safe delivery and ability to act as a therapeutic agent. Several therapeutic approaches have been reported to make use of NPs, such as in anticancer drug delivery, molecular diagnosis for disease detection and nanoscale immunotherapy. These areas show high potential for future clinical implementation [8]. Recent studies on NPs have given insight on how to develop new targeted therapies, systemic cancer treatments and identification of novel oncogenic targets [9]. Commonly used nanomaterials in biomedical application are Au NPs, liposomes, carbon nanotubes, polymeric micelles, graphene, ferrous or ferric oxide NPs, and quantum dots [10]. Human exposure to engineered nanomaterials is inevitable due to recent advances of NP-based applications. NPs provide plentiful advantages from industrial and consumer perspective. NPs are used in many applications and their properties determine their usage in application [11][12][13]. Some of the important properties of NPs are size, melting point, chemical reactivity and particle surface area. The size of NPs is generally below 100 nm and they have low melting point, high chemical reactivity and large external surface area [14]. Recently, NP-based drug delivery attracted increasing attention [15]. Au NPs are one of the popular NPs and have been widely studied in cancer theranostics. The application of Au NPs can be traced back to the middle age and that is why they are also known as potable gold [16]. Some of the unique properties of Au NPs prominent in medical application are the high x-ray absorption coefficient, localized surface plasmon resonance and radioactivity [17]. Au NPs also display amazing electronic and optical properties that can enable controllable interactions with organic molecules having electron-donating groups. With the advances of functionalized Au nanomaterials, their usages have been increased with the potential to be implemented in many more applications [18].
From the drug administration for patients, whose degradation, movement, drug accumulation in tumor and body excretion follow complex theories, which compromising of vascular morphology and blood flow rate. As the injected dose transverses into the patient’s body, the drug accumulation and kinetics fate are dictated by factors such as spatial diameter, geometry, number of and length of blood vessels. When the drug transports to the target destination, the efficacy of optimal dose uptake is reduced by the patient’s defense mechanism that tries to keep foreign materials such as viruses, drug chemicals, bacteria and sensor devices out of the body [19]. Biomaterials offer the ability to improve upon medical technologies through increased control of the type and concentration of immune signals delivered. With the advances of surface coating technology, surface functionalization of Au NPs becomes possible. Different functional groups such as PEG, ssDNA, antibody, peptide, drug, florescence marker and siRNA can be attached on the particle surface as shown in Figure 1. This makes Au NPs act as molecular sensors, therapeutic agents, and vehicles for imaging agent and drug delivery [20].
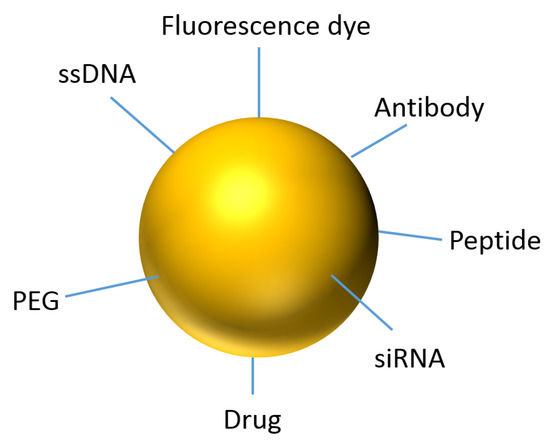
Figure 1. Representation of an Au NP for theranostics.
References
- B Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. Ca Cancer J. Clin. 2018, 68, 394–424.
- Asplund, J.; Kauppila, J.H.; Mattsson, F.; Lagergren, J. Survival Trends in Gastric Adenocarcinoma: A Population-Based Study in Sweden. Ann. Surg. Oncol. 2018, 25, 2693–2702.
- Amin, M.; Edge, S.; Greene, F.; Byrd, D.; Brookland, R.; Washington, M.; Gershenwald, J.; Compton, C.; Hess, K.R.; Sullivan, D.; et al. AJCC Cancer Staging Manual, 8th ed.; Springer International Publishing: Cham, Switzerland, 2017.
- Powell, A.; Wheat, J.; Patel, N.; Chan, D.; Foliaki, A.; Roberts, S.A.; Lewis, W.G. Value of individual surgeon performance metrics as quality assurance measures in oesophagogastric cancer surgery. Bjs Open 2020, 4, 91–100.
- Wang, K.; Yuen, S.T.; Xu, J.; Lee, S.P.; Yan, H.H.; Shi, S.T.; Siu, H.C.; Deng, S.; Chu, K.M.; Law, S.; et al. Whole-genome sequencing and comprehensive molecular profiling identify new driver mutations in gastric cancer. Nat. Genet. 2014, 46, 573–582.
- Carcas, L.P. Gastric cancer review. J. Carcinog. 2014, 13, 14.
- Piazuelo, M.B.; Correa, P. Gastric cáncer: Overview. Colomb Med. 2013, 44, 192–201.
- Ashley E. Russo; Vivian E. Strong; Gastric Cancer Etiology and Management in Asia and the West. Annual Review of Medicine 2019, 70, 353-367, 10.1146/annurev-med-081117-043436.
- John S Macdonald; Stephen R Smalley; Jacqueline Benedetti; Scott A. Hundahl; Norman C. Estes; Grant N. Stemmermann; Daniel G. Haller; Jaffer A. Ajani; Leonard L. Gunderson; J. Milburn Jessup; et al.James A. Martenson Chemoradiotherapy after Surgery Compared with Surgery Alone for Adenocarcinoma of the Stomach or Gastroesophageal Junction. New England Journal of Medicine 2001, 345, 725-730, 10.1056/nejmoa010187.
- David Cunningham; William H. Allum; Sally P. Stenning; Jeremy N. Thompson; Cornelis J.H. Van De Velde; Marianne Nicolson; J. Howard Scarffe; Fiona J. Lofts; Stephen J. Falk; Timothy J. Iveson; et al.David B. SmithRuth E. LangleyMonica VermaSimon WeedenYu Jo Chua Perioperative Chemotherapy versus Surgery Alone for Resectable Gastroesophageal Cancer. New England Journal of Medicine 2006, 355, 11-20, 10.1056/nejmoa055531.
- Cutsem, E.; Sagaert, X.; Topal, B.; Haustermans, K.; Prenen, H. Gastric Cancer. Lancet 2016, 388, 2654–2664.
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt signalling in cancer. Oncogene 2016, 36, 1461–1473.
- Giles, R.H.; van Es, J.H.; Clevers, H. Caught up in a Wnt storm: Wnt signaling in cancer. Biochim. Et. Biophys. Acta 2003, 1653, 1–24.
- Karl Willert; Jeffrey D. Brown; Esther Danenberg; Andrew W. Duncan; Irving L. Weissman; Tannishtha Reya; John R. Yates; Roel Nusse; Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature 2003, 423, 448-452, 10.1038/nature01611.
- Zachary Steinhart; Stephane Angers; Wnt signaling in development and tissue homeostasis. Development 2018, 145, dev146589, 10.1242/dev.146589.
- Vitezslav Bryja; Igor Červenka; Lukas Cajanek; The connections of Wnt pathway components with cell cycle and centrosome: side effects or a hidden logic?. Critical Reviews in Biochemistry and Molecular Biology 2017, 52, 614-637, 10.1080/10409238.2017.1350135.
- Dustin Flanagan; Chloe Austin; Elizabeth Vincan; Toby J. Phesse; Wnt Signalling in Gastrointestinal Epithelial Stem Cells. Genes 2018, 9, 178, 10.3390/genes9040178.
- Diane Slusarski; Julia Yang-Snyder; William B. Busa; Randall Moon; Modulation of Embryonic Intracellular Ca2+Signaling byWnt-5A. Developmental Biology 1997, 182, 114-120, 10.1006/dbio.1996.8463.
- Michael M. Kreusser; Johannes Backs; Integrated mechanisms of CaMKII-dependent ventricular remodeling. Frontiers in Pharmacology 2014, 5, 36, 10.3389/fphar.2014.00036.
- Kimberly A. Mulligan; C. Fuerer; Wendy Ching; Matt Fish; Karl Willert; Roeland Nusse; Secreted Wingless-interacting molecule (Swim) promotes long-range signaling by maintaining Wingless solubility.. Proceedings of the National Academy of Sciences 2011, 109, 370-377, 10.1073/pnas.1119197109.
