You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Kuan O. Tan and Version 2 by Conner Chen.
Adenovirus is formed of an icosahedral protein shell measuring 90 nm and harboring linear double-stranded DNA (dsDNA) that belongs to the genus Mastadenovirus of the Adenoviridae family. Most people have been infected by adenovirus, leading to lifelong immunity.
- oncolytic adenovirus
- immune system
- tumor microenvironment (TME)
- cancer therapy
1. Adenovirus: Structure, Genome, and Serotype
Adenovirus is formed of an icosahedral protein shell measuring 90 nm and harboring linear double-stranded DNA (dsDNA) that belongs to the genus Mastadenovirus of the Adenoviridae family [1][6]. The icosahedral capsid outer shell is composed of three primary types of proteins including hexon, penton, and fiber [2][7]. Interestingly, the hexon, which comprises the majority of the adenovirus capsid, is made up of up to 240 homotrimers to encapsidate the adenovirus [2][7]. In addition, the fibers arise from the 12 vertices of the icosahedron, whereas the penton base is located at the base of each fiber [3][8]. The genome of the virus encodes approximately 35 proteins that are produced in two phases, the early and late phases. The early phase corresponds to the start of viral DNA replication seven hours after infection, whereas the late phase refers to the incidence of post-DNA replication. The first 20 synthesized proteins have regulatory functions that allow the virus to gain control of the cells, followed by viral DNA replication activity.
In contrast, the proteins synthesized at a later stage are mainly structural proteins of the virus [4][9]. As early as one day post-infection, the virions are assembled at the nucleus of the host cell, and for several days, the host cells lyse and release the infectious virus. As reported in a previous study, more than 57 serotypes and over 100 genotypes of human adenoviruses consist of 7 species from A to G (HADV-A to HADV-G) [4][9]. Species A, B, C, D, E, and F are widely circulating and have been associated with human infection outbreaks [1][5][6,10]. Serotype refers to the ability of specific antisera to neutralize infected cells. Despite the diversity of its serotype, the structure and protein activities of all serotypes are similar. However, specific protein activities lead up to the distinctive characteristics of each serotype. For instance, the packaging domain of the adenovirus is serotype specific [6][11]. In a previous study, it was reported that IVa2 and 22K proteins complement the activity of their corresponding human adenovirus C5 protein, but not L1 52/55K protein of human adenovirus 17 serotypes [7][12]. This suggests that 52/55K protein is important for serotype specificity of adenovirus DNA packaging.
2. Adenovirus: Pathogenicity and Immunogenicity
Most people have been infected by adenovirus, leading to lifelong immunity. In general, adenovirus species C, consisting of Ad 1, 2, 5, and 6, is the most common to infect humans, especially in early childhood, consequently causing infection of the upper respiratory tract [8][13]. In addition, adenovirus species B, such as Ad 3, 7, 11, 14, 16, 21, 34, 35, and 50, contributes to 5% of common cold cases, involving infection of the upper respiratory, gastrointestinal, and urinary tract [4][9]. In most adenovirus infections, the symptoms are minor, but they might be fatal for immunocompromised individuals [4][9]. However, despite low pathogenicity, some adenoviruses result in severe symptoms, especially in children. For instance, human adenovirus (HAdV) types F40 and F41 are a leading cause of diarrhea and diarrhea-related mortality in infants and toddlers worldwide [9][14]. Adenovirus can be propagated, replicated, and grown to achieve high viral titers of up to 1 × 1013 virus particles per mL [10][15]. Immunocompetent individuals can develop minor, self-limiting clinical pathologies due to the immunogenicity of adenovirus. The host immune response to adenoviral infection limits the use of adenovirus in gene therapy; however, immune stimulation such as the induction of a systemic pro-inflammatory state, the recruitment of cytotoxic immune cell populations to the infection sites to eradicate virus-containing cells, and the alerting of adjacent uninfected cells of viral infection is beneficial for the development of immunotherapies against cancer [11][16]. Additionally, oncolytic adenoviruses are designed to precisely deliver therapeutic transgenes to tumor cells without affecting healthy cells [12][17]. Both replicative and nonreplicating adenoviruses (Figure 1) are used as in situ cancer vaccines due to their high immunogenicity [13][18].
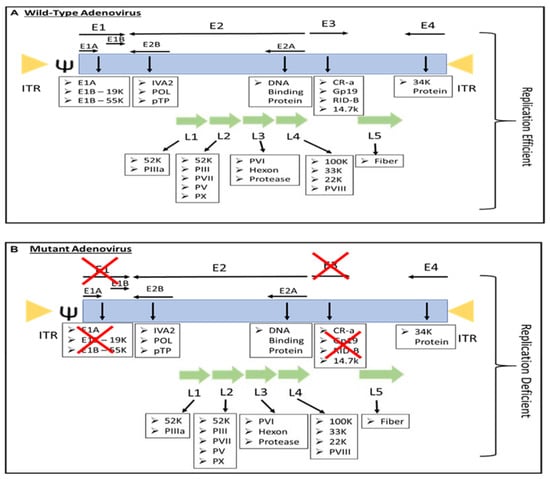
Figure 1. The genome of (A) replication-efficient adenovirus and (B) replication-deficient adenovirus. ITR—inverted terminal repeat; Ψ—viral packaging element; K—glycoprotein; P—pre-protein, X—Deleted.
2.1. Innate Immune Responses
3.1. Innate Immune Responses
Due to pathogen-associated molecular patterns (PAMPs) components of the viral capsid and viral nucleic acids, adenovirus infections elicit an innate immune response comprised of cellular components such as pattern recognition receptors (PRR) [13][18]. The interaction of adenovirus fiber and knob capsid proteins with the coxsackie and adenovirus receptor as well as the v integrins induces activation of nuclear factor-kB, leading to the expression of chemokines and interleukins (ILs) [14][19]. In addition, according to a prior study, an innate immune response to capsid proteins is elicited immediately after intravenous administration in a murine model for up to six hours [4][9]. However, the response was lethal at high dosages of more than 1010 viral particles. This leads to the stimulation of biphasic synthesis of pro-inflammatory proteins, including IL-6, TNF-α, IFN-γ, IL-1β, and IL-12, along with chemokines [4][9].
In addition, following viral entry, PRRs include Toll-like receptor (TLR) 9 in endosomes, and cytosolic sensors such as DNA-dependent activator of IFN-regulatory factors (DAIs) or cytosolic inflammasome (NALP3) recognize viral DNA [15][16][17][20,21,22]. For this, the interferon response is stimulated [18][23]. The interferon response eliminates the virions from the cells and inhibits the E1A transcription, subsequently blocking the replication of adenovirus [15][19][20,24].
2.2. Adaptive Immune Responses
3.2. Adaptive Immune Responses
Adenovirus infection that causes upper respiratory infections induces anti-adenovirus serotype-specific antibodies and cross-reactive T cell responses [20][25]. As reported in a previous study, serotype 5 is known to be the most prevalent adenovirus strain, which has a seroprevalence of approximately 50% in North America and close to 100% in Africa [21][26]. In addition, the seroprevalence of adenovirus serotype 35 ranges from 3% to 22% in the United States [21][26].
Adenovirus is strongly immunogenic, consisting of the three major capsid protein antigens, which are the hexon, penton base, and fiber. The majority of neutralizing antibodies are targeted against the capsid proteins and non-conserved loops that differ across serotypes and are exposed on the surface of hexon [22][27]. Interestingly, the presence of adenovirus-specific CD4+ T cells in peripheral blood lymphocytes has been reported in nearly all individuals of all ages, where the CD4+ T cell epitopes are in the hexon region [23][28]. In addition, cytotoxic T lymphocytes (CTL) specific to the hexon were also reported to be protective against adenovirus infections in humans [24][29].
