Inflammatory bowel disease (IBD) is a gastrointestinal disease that involves chronic mucosal or submucosal lesions that affect tissue integrity. Although IBD is not life-threatening, it sometimes causes severe complications, such as colon cancer. The exact etiology of IBD remains unclear, but several risk factors, such as pathogen infection, stress, diet, age, and genetics, have been involved in the occurrence and aggravation of IBD. Immune system malfunction with the over-production of inflammatory cytokines and associated oxidative stress are the hallmarks of IBD. Dietary intervention and medical treatment suppressing abnormal inflammation and oxidative stress are recommended as potential therapies. Thymol, a natural monoterpene phenol that is mostly found in thyme, exhibits multiple biological functions as a potential adjuvant for IBD. The purpose of this review is to summarize current findings on the protective effect of thymol on intestinal health in the context of specific animal models of IBD, describe the role of thymol in the modulation of inflammation, oxidative stress, and gut microbiota against gastrointestinal disease, and discuss the potential mechanism for its pharmacological activity.
- gastrointestinal disease
- gut health
- natural anti-oxidant
1. Introduction
2. Pharmacokinetics and Pharmacological Properties of Thymol
Thymol is the secondary metabolite produced by the aromatization of γ-terpinene to p-cymene, followed by the hydroxylation of p-cymene in plants [18][19] (Figure 1), including Thymus zygis [19][20], Thymbra capitata [20][21], Thymus vulgaris [21][22], Satureja thymbra [22][23], Nigella sativa seeds [23][24], and Monarda didyma [24][25]. Pure thymol has low solubility in water, high volatility, and a strong bitter/irritating taste [25][26]; thus, it is usually encapsulated in electrospun nanofibers to enhance its water solubility and high temperature stability [26][27]. Emulsification is also used for thymol processing. Both encapsulation and emulsification have been shown to improve the anti-oxidant activity of thymol [27][28].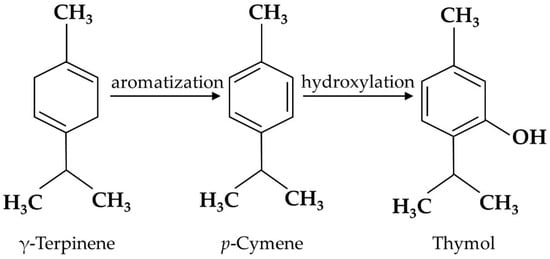
3. Thymol Protects Intestinal Barrier Function against IBD
The intestinal barrier, mainly composed of intestinal epithelial cells (IECs) and immune cells, maintains the balance between the luminal contents and mucosa. The disturbance of this balance has been associated with gastrointestinal diseases, such as IBD [41][42]. Although the exact pathogenesis of IBD remains unclear, a “leaky gut” with impaired intestinal barrier function is the main feature. The intestinal barrier is the first line of defense against pathogen infection, and injury to the intestinal barrier aggravates the disease. Thus, understanding how thymol protects the intestinal barrier is important for relieving IBD. Thymol has exhibited a protective function for the intestinal barrier in both in vivo and in vitro studies, as illustrated in Table 1, and its specific mechanism is shown in Figure 2. Thymol attenuates weaning stress-induced diarrheal and intestinal barrier dysfunction in weanling pigs by reducing the serum diamine oxidase level, an indicator of intestinal integrity, and increasing the expression of the tight junction protein zonula occludens-1 (ZO-1) and occludins [42][43]. Thymol alleviates dextran sulfate sodium (DSS)-induced intestinal damage and increases tight junction claudin-3 expression [43][44]. Increased plasma endotoxin and D-lactic acid levels are markers of increased intestinal permeability. The latest study found that dietary thymol reduced the plasma endotoxin and D-lactic acid concentrations on days 7 and 14 post-weaning [44][45]. The intestinal mucus layer is the first line of defense maintaining bacterial symbiosis with the host and preventing bacterial penetration into epithelial cells [45][46]. Thymol increases mucus secretion to relieve ethanol-induced ulcer mucosal damage in rats [46][47]. In IPEC-J2 cells, thymol alleviates lipopolysaccharide (LPS)-induced decrease in trans-epithelial electrical resistance (TEER), indicating an increase in the integrity of the single cell layer [47][48]. In Caco-2 cells, thymol increases the integrity of the tight junction and up-regulates cyclooxygenase-1 (COX1) activity to maintain GIT homeostasis, which is beneficial for intestinal health [48][49]. In addition, thymol changes the expressions of 120 and 59 genes in the oxyntic and pyloric mucosa, respectively, which are associated with gastric epithelium proliferation and maturation activities in weaned pigs [28][29].
| Model | Thymol Dose | Effects | Ref. |
|---|---|---|---|
| In vivo | |||
| Weaned pigs | 50 mg/kg diet | Enrichment of 120 and 59 gene sets in oxyntic and pyloric mucosa↓ |
[28][29] |
| Ethanol-induced acute ulcer | 10–100 mg/kg diet | Mucosal damage↓ Amount of mucus↑ |
[46][47] |
| Chicken infected with Clostridium perfringens |
30 mg/kg diet | Intestinal lesions and mortality↓ Lactobacillus salivarius and L. johnsonii↓ L. crispatus, L. agilis, and Escherichia coli↑ |
[49][50] |
| Weaned pigs | 100 mg/kg diet | Expression of ZO-1 and occludins in jejunal mucosa↓ Enterococcus genus and E. coli↓ Plasma diamine oxidase concentration↓ Weaning-induced intestinal OS↓ |
[42][43] |
| In vitro | |||
| IPEC-J2 | 50 μM | TEER↑ Cell permeability↓ ZO-1and actin staining↑ |
[47][48] |
| Caco-2 cells | 15 mg/L | COX1 transcription↑ COX1:COX2 ratio↑ TEER↑ |
[48][49] |
4. Thymol Alleviates Intestinal Inflammation in IBD
The dysregulation of innate and adaptive immune responses leads to chronic intestinal inflammation in IBD patients [50][51][52][51,52,53]. Transcription factor nuclear factor κB (NF-κB) is the key mediator regulating the inflammatory response. The activation of NF-κB signaling induces the expression of pro-inflammatory cytokines, including tumor necrosis factor-α (TNF-α), inductible nitric oxide synthase (iNOS), interleukin (IL-1β), and IL-6 [53][54]. The over-production and accumulation of inflammatory cytokines cause intestinal epithelial apoptosis and the disruption of intestinal homeostasis, resulting in the dysfunction of the intestinal epithelial barrier in IBD patients [54][55]. Currently, the suppression of inflammation is the mainstay of IBD treatment. Thymol shows strong anti-inflammatory properties in in vivo and in vitro studies [55][56]. A schematic diagram of thymol’s action is depicted in Figure 3. Toll-like receptors (TLRs) are the main sensors used to detect various dangerous signals and activate innate immune responses. The classic TLR signaling pathway activates NF-κB to regulate the expressions of a series of cytokines [56][57]. In mice and macrophages, thymol inhibits TLR4 expression and then inhibits the activation of NF-κB signaling, which reduces the production of inflammatory cytokines, such as TNF-α and IL-1β [57][58][58,59]. NF-κB is a master mediator of inflammatory responses. Inactive NF-κB binds to IκB, an inhibitory subunit of NF-κB, and presents in the cytoplasm. When activated by a variety of signals, such as cytokine receptors and pattern-recognition receptors (PRRs), IκB is phosphorylated and degraded to release RelA (p65) from the NF-κB complex. p65 is then translocated to the nucleus to induce pro-inflammatory cytokine expression as a transcription factor [59][60]. Thymol has been shown to inhibit NF-κB activation by reducing p65 translocation and abundance in the colons of acetic acid-induced colitis rats and in LPS-activated macrophages, respectively, with decreased cytokine production [60][61][61,62]. The activation of NF-κB induces the expressions of iNOS and COX-2, which further promote vigorous inflammation. In ulcerative colitis rats, thymol reduces the COX-2 expression and nitric oxide (NO) levels produced by iNOS in the rats’ colon [62][63]. These studies showed the anti-inflammatory function of thymol through the inhibition of the NF-κB signaling pathway.
