Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Lindsay Dong and Version 1 by Nader Sheibani.
Cytochrome P450 (CYP) 1B1 belongs to the superfamily of heme-containing monooxygenases. Unlike other CYP enzymes, which are highly expressed in the liver, CYP1B1 is predominantly found in extrahepatic tissues, such as the brain, and ocular tissues including retina and trabecular meshwork. CYP1B1 metabolizes exogenous chemicals such as polycyclic aromatic hydrocarbons. CYP1B1 also metabolizes endogenous bioactive compounds including estradiol and arachidonic acid. These metabolites impact various cellular and physiological processes during development and pathological processes.
- CYP1B1
- iron homeostasis
- Cytochrome P450
1. Introduction
Cytochrome P450s (CYPs), named after the absorbance peak near 450 nm of their Fe(II)-carbon monoxide complex in rat liver microsomes [1], are a superfamily of heme-containing enzymes. The CYP superfamily includes approximately 9000 proteins classified into more than 800 families, which makes CYPs one of the largest and most functionally diverse protein superfamilies [2]. In humans, there are 57 putatively functional genes and 58 pseudogenes arranged into 18 CYP families and 43 subfamilies, distributed over most autosomal chromosomes [3]. In comparison, there are 108 functional and 88 pseudogenes representing CYPs in mice [4]. CYPs are membrane-bound enzymes, mostly found in the smooth endoplasmic reticulum and some in mitochondria. The enzymes are best known to catalyze a monooxygenation reaction (RH + O2 + NAD(P)H + H+ → ROH + H2O + NAD(P)+, where RH stands for a substrate with a hydroxylatable site [6][5]. CYPs are often referred to as poly-substrate monooxygenases.
CYPs also catalyze a variety of other reactions, such as reduction, desaturation, ester cleavage, and rearrangement of fatty acids [9][6]. CYP substrates include exogenous chemicals such as drugs, food toxicants, and carcinogens, as well as endogenous compounds, for example, steroids, prostaglandins, and bile acids. As the main site of metabolism of exogenous chemicals in humans, the liver expresses around 30 CYPs out of the 57 identified in humans [10][7]. Among CYP enzymes in the human liver, CYP3A4 is the most abundant and represents approximately 22.1% of the total CYP enzyme protein content [11][8]. Along with CYP3A4, CYP1A2, CYP2C9, CYP2C19, CYP2D6, and CYP3A5 are responsible for the biotransformation of about 80 percent of all marketed drugs [12][9]. CYP enzymes are also expressed throughout the body, and emerging evidence suggests that extrahepatic CYP enzymes, such as CYP1B1, have important roles in modulating tissue metabolic homeostasis, developmental processes, and contributing to carcinogenesis and other environmental diseases.
CYP1B1, one of the CYP enzymes, is mainly expressed in extrahepatic tissues [4] and is the only member of the CYP1B subfamily. It was initially identified from mouse embryonic fibroblasts (C3H10T1/2) following incubation with benzo(a)anthracene and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [13,14][10][11]. The human CYP1B1 was cloned from primary human skin keratinocytes shortly after [15][12]. Although CYP1B1 is assigned to the CYP1 family, CYP1B1 shows a low degree of genetic homology (~40%) with other CYP1 family enzymes, CYP1A1 and CYP1A2 [16][13], and is a significantly larger protein than other human CYPs. CYP1B1 is expressed in various adult tissues such as bone marrow, brain, breast, intestine, kidney, prostate, and ocular tissues. CYP1B1 expression is also found during development of the hindbrain, neural crest, and eyes in early human and mouse embryos [17,18][14][15] and has an important role in the generation and action of retinoic acid [19,20][16][17].
CYP1B1 metabolizes a range of compounds, including exogenous chemicals such as polycyclic aromatic hydrocarbons, dioxins, and aflatoxin B1. CYP1B1 also metabolizes endogenous bioactive compounds, including estradiol, arachidonic acid, vitamin A, and melatonin (summarevieweized in [21,27][18][19]). These metabolites are mediators of various cellular and physiological processes [28,29][20][21]. Thus, CYP1B1 expression and function could be important in the proper development and maintenance of metabolic homeostasis in various organs and tissues.
2. Iron Homeostasis
Iron absorption, transport, distribution, and storage are tightly regulated by several specific proteins and are discussed below. On average, a 70 kg man contains 3.5–4 g of iron. Most of the iron is intracellular and carried in molecules, such as the hemoglobin of red blood cells (about 2.0–2.5 g), ferritin in hepatocytes and macrophages (about 0.5–1 g), and in myoglobin, ferritin, and iron-containing enzymes in other types of cells (about 0.5 g in total). Only a few mg of iron is contained in blood plasma, and the majority of it is bound to transferrin [55][22]. Unlike most other essential nutrients, there are no regulated mechanisms for the excretion of iron in mammals. Iron excretion results from the exfoliation of dead skin, blood loss, and turnover of intestinal epithelial cells, which are independent of iron levels in the body. In humans, about 1–2 mg of iron is lost every day, and the loss is balanced by iron absorption, which occurs in the duodenum and proximal jejunum. Due to the lack of controlled iron excretion, systemic iron homeostasis is achieved mainly through the regulation of absorption, utilization, and recycling of iron [56][23]. Enterocytes of the proximal small intestine uptake dietary iron via divalent metal-iron transporter-1 (DMT1) (Figure 31A). Located on the apical membrane of enterocytes, DMT1 only transfers Fe2+, but most nonheme dietary iron exists as Fe3+. Thus, iron uptake through DMT1 requires a reduction in Fe3+, mediated by duodenal cytochrome B (DCYTB), an iron-regulated ferrireductase that is highly expressed in the apical membrane of duodenal enterocytes [57][24]. Dietary heme is imported by heme carrier protein (HCP1) into enterocytes and degraded by heme oxygenase 1 (HO1) to release Fe2+, which likely joins the same intracellular iron pool with nonheme iron [58][25]. Iron transfer from enterocytes into the circulation is mediated by ferroportin located at the basolateral membrane of enterocytes. Ferroportin, also known as iron-regulated transporter 1 or solute carrier family 40 number 1 (SLC40A1), is a transmembrane protein with 12 transmembrane alpha helices. Ferroportin is the only known cellular iron efflux transporter in vertebrates, and it is typically expressed in iron-exporting cells, including enterocytes, macrophages, and hepatocytes [59][26]. Due to its half-filled 3d5 electron configuration, Fe3+ is more stable and less water-soluble than Fe2+, which is water-soluble and reactive. Exported as Fe2+ through ferroportin, iron undergoes oxidation to Fe3+. Ferroportin cooperates with a membrane-bound ferroxidase hephaestin, which is located in the basolateral membrane of enterocytes and functions to convert Fe2+ to Fe3+ [60][27]. Hephaestin is a membrane-bound homolog of ceruloplasmin, which is an enzyme containing six atoms of copper and is produced in the liver. Secreted from the liver into the systemic circulation, ceruloplasmin functions to oxidize Fe2+ in the circulation into Fe3+ [61][28]. Almost all the iron in the circulation binds to transferrin, a 76–80 kDa bilobal glycoprotein that is produced predominantly by the liver. Transferrin contains two binding sites for Fe3+ and transports iron through the blood to various tissues. Transferrin-bound iron only accounts for 0.1% (3–4 mg) of the total iron in the body (3–4 g in an adult man) [62][29]. However, due to the rapid turnover rate of the transferrin-bound iron complex (about 10 times/day), transferrin forms an important iron pool (20–25 mg/day) to meet the daily demands of iron for physiological processes, including erythropoiesis [63,64][30][31]. Transferrin delivers iron through the circulation to cells expressing the transferrin receptors, such as retinal vascular EC [65][32].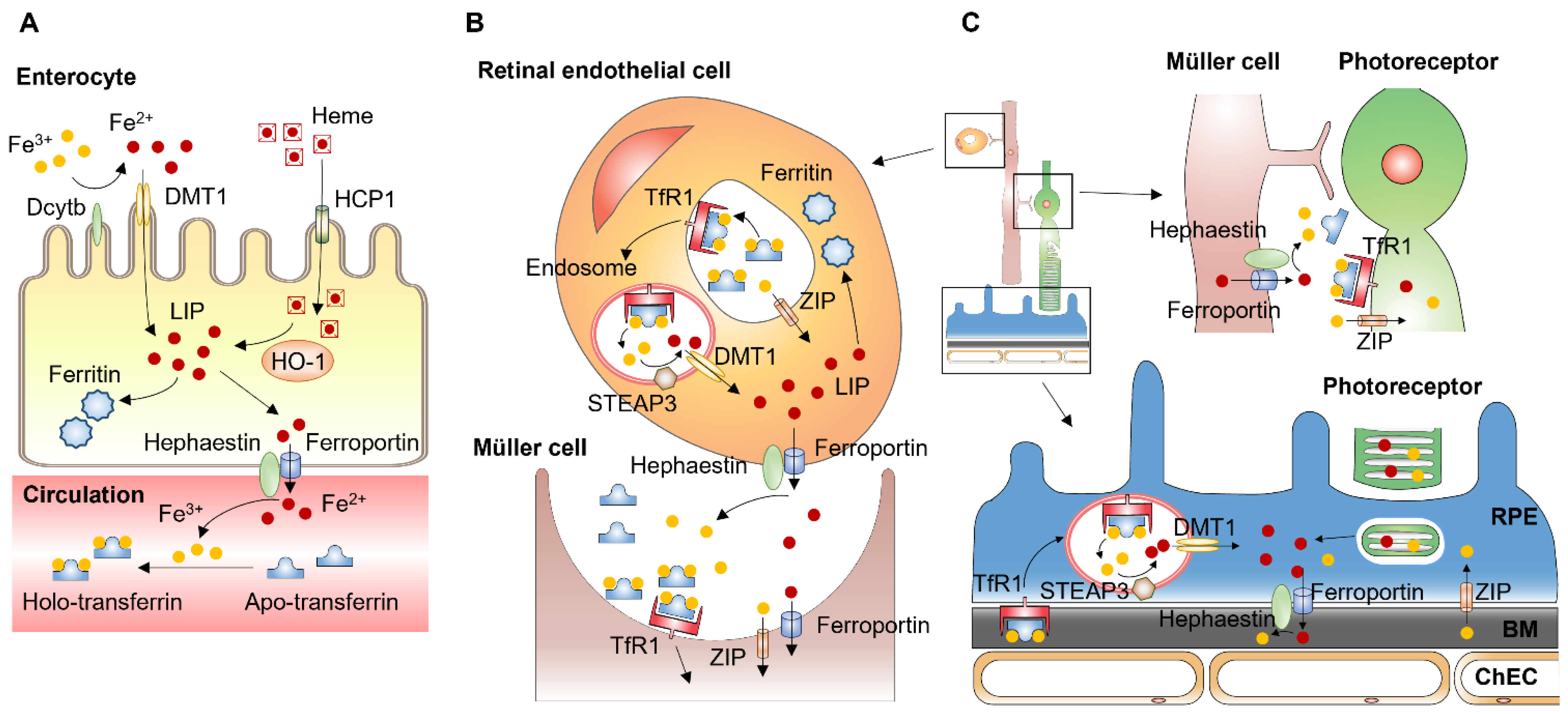
Figure 31. Systemic and local iron uptake and transport. (A) Enterocytes uptake dietary iron via divalent metal transporter-1 (DMT1) on the apical membrane. Iron uptake through DMT1 is mediated by duodenal cytochrome B (DCYTB), an enzyme that reduces Fe3+ to Fe2+. Dietary heme is imported by heme carrier protein (HCP1) into enterocytes and degraded by heme oxygenase 1 (HO1) to release Fe2+. Iron transfer from enterocytes into the circulation is mediated by ferroportin located at the basolateral membrane of enterocytes. Ferroportin cooperates with ferroxidase hephaestin converting Fe2+ to Fe3+, which binds to transferrin in the circulation. (B) Retinal endothelial cells (EC) express transferrin receptor 1 (TfR1) at the apical membrane. After binding of iron-loaded transferrin, the TfR undergoes clathrin-mediated endocytosis. Within the endosome, Fe3+ is released from transferrin and reduced to Fe2+ by six-transmembrane epithelial antigen of prostate 3 (STEAP3). Fe2+ is then transported from the endosome to cytosol by DMT1. Unbound Fe3+ can be transported into retinal EC via Zinc transporters (ZIP) such as ZIP8 and ZIP14. Exported from retinal EC, iron is imported by glial cells such as Müller cells. (C) Exported by Müller cells, iron can be imported by photoreceptors expressing transferrin receptor, Zip8 and Zip14. Photoreceptors export iron via phagocytosis of shed photoreceptor outer segments by retinal pigment epithelium (RPE) cells. RPE cells also import iron from the choroid via transferrin receptor [66,67][33][34]. BM: Bruch’s membrane.
Transferrin receptors are homodimeric transmembrane glycoproteins that mediate the uptake of transferrin-bound iron into the cells. Transferrin receptor 1 (TFR1, also known as CD71) is ubiquitously expressed in mammalian tissues and cells. Expression of TFR1 is regulated by intracellular iron levels. Under conditions of iron deficiency, iron-regulatory proteins (IRP) bind to the iron-responsive element (IRE) motifs in the 3′-untranslated region of TFR1 mRNA to prevent endonucleolytic cleavage, mediating post-transcriptional stabilization of TFR1 mRNA [68][35]. Transferrin receptor 2 (TFR2) is expressed primarily in the liver and erythroid precursors. TFR2 expression is not regulated by IRPs, as TFR2 does not have IRE motifs in its 5′ and 3′ untranslated regions, which indicates TFR2 expression is not regulated by intracellular iron status [69][36]. The binding affinity to iron-bound transferrin of TFR2 is 25-fold lower than that of TFR1, which suggests that TFR1 is the major receptor for cellular uptake of iron-bound transferrin. However, TFR2 plays an important role in iron homeostasis through the regulation of BMP signaling pathways. In hepatocytes, TFR2 is one of the auxiliary factors for BMP receptors modulating hepcidin expression, and TFR2 mutations result in reduced hepcidin production [70][37].
After binding of iron-loaded transferrin, the transferrin receptor undergoes clathrin-mediated endocytosis (Figure 31B). The acidic endosomal pH (~5.6), along with other factors such as conformational changes in transferrin, salt concentration, temperature, and chelators within the endosome, contribute to the release of Fe3+ from transferrin [71][38]. Fe3+ in the endosome is reduced to Fe2+ by an endosomal membrane ferric reductase, the six-transmembrane epithelial antigen of prostate 3 (STEAP3). The Fe2+ is then transported from the endosome to cytosol by the transmembrane protein DMT1 [72][39].
3. CYP1B1 and Regulation of Iron Homeostasis
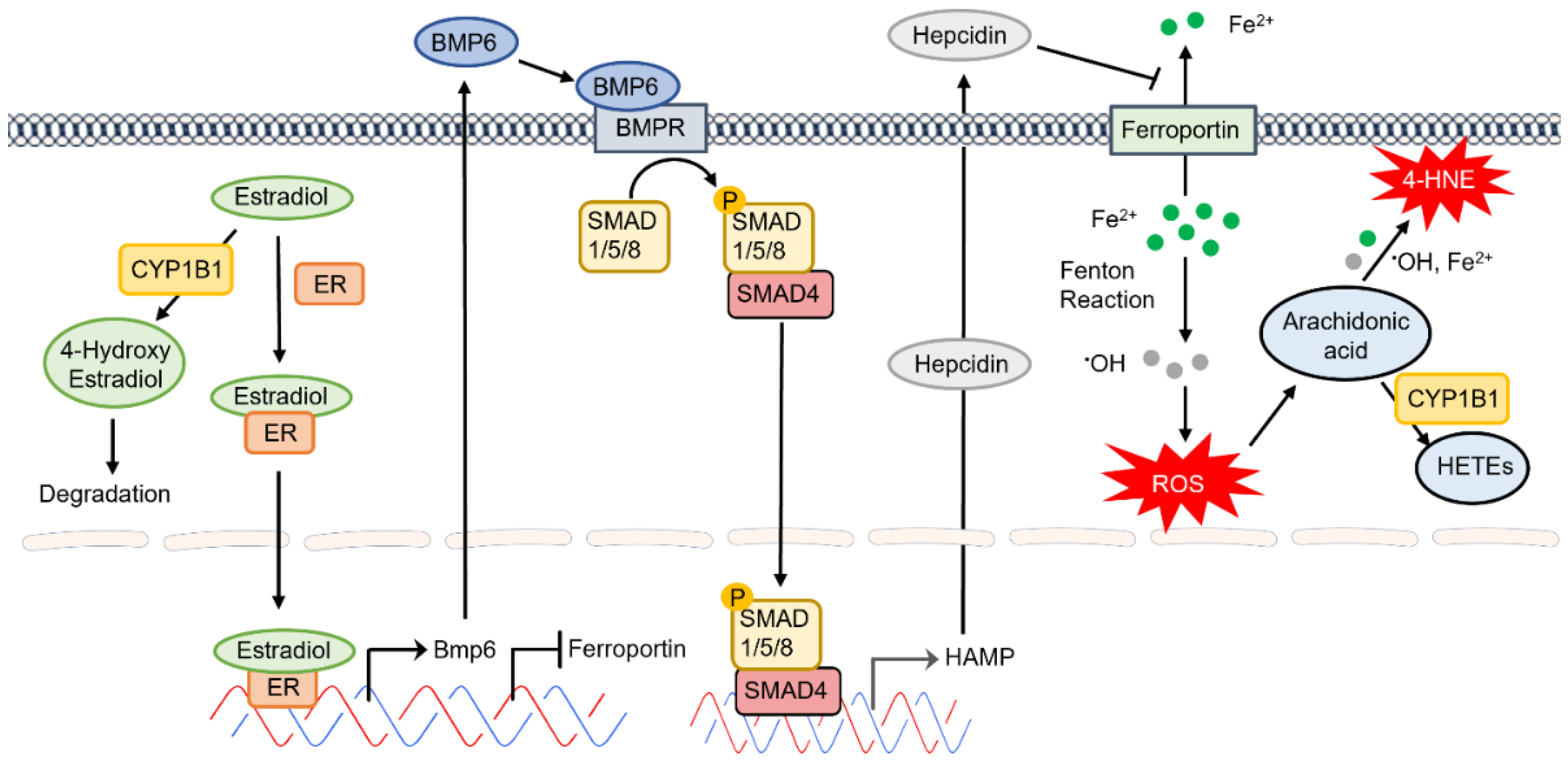
CYP1B1 expression in the eye is an important modulator of developmental processes. Mutations in CYP1B1 are associated with the development of primary congenital glaucoma in humans [118][40]. However, the underlying molecular and cellular mechanisms that regulate CYP1B1 expression and activity in these processes remain unknown. Mice deficient in CYP1B1 exhibit defects in the development and function of the conventional outflow pathway, including the trabecular meshwork and Schlemm’s canal. This defect was exacerbated in albino mice, suggesting a role for increased oxidative stress in these processes [119][41]. The ability of melanosomes to bind iron within living cells could contribute to the protection noted in pigmented mice [120][42]. Although weit haves noted significant constitutive expression of CYP1B1 in various ocular cell types, its expression is also induced by exposure to AhR agonists such as dioxin [13][10]. However, the normal regulatory mechanisms that keep CYP1B1 expression in check remain poorly understood. There have been numerous efforts toward identifying physiological substrates and metabolites of CYP1B1 in order to advance ourthe knowledge regarding the molecular and cellular mechanisms that impact CYP1B1 expression, activity, and function. We haveIt has shown that CYP1B1 expression has a significant impact on adhesive and migratory properties of various ocular cell types, including retinal vascular and trabecular meshwork cells [121][43]. How CYP1B1 expression and/or activity modulate these cell properties, which are likely linked to cellular redox state, needs further exploration. CYPs are generally considered as liver resident enzymes whose activation by exposure to various toxicants, including aromatic hydrocarbons, drive the detoxification and elimination of these chemicals. With advancements in genomic and transcriptomic studies, it is now recognized that CYP expression and activity play significant roles in the modulation of proper developmental and metabolic functional activities of various cell types and tissues. Estradiol and arachidonic acid are key endogenous substrates of CYP1B1, whose metabolites have significant impacts on various biological functions. CYP1B1 expression also has important roles in retinoic acid metabolism during early developmental processes. CYP1B1 is also involved in the metabolism of exogenous toxic chemicals, enhancing their elimination and minimizing their adverse effects on human health. Estrogens are metabolically converted to estrogenically inactive metabolites for elimination from the body. The hydroxylation of estrogens by CYP enzymes is the first step in their metabolism, mainly in the liver. 2-hydroxyestradiol, mainly catalyzed by CYP1A2 and CYP3A4 in the liver, and CYP1A1 in extrahepatic tissues, is the major metabolite of estradiol. However, in the estrogen target tissues which express a high level of CYP1B1; 4-hydroxyestradiol is the predominant estradiol metabolite [122][44]. 4-hydroxyestradiol produces free radicals from the oxidative-reductive cycling with the corresponding semiquinone and quinone forms, which cause cellular damage and could have adverse impact on tissue integrity and function [122][44]. In addition, estradiol regulates human CYP1B1 expression through estrogen receptor alpha [123][45]. Thus, the regulation of estrogen-metabolizing CYP enzymes by estrogen itself could contribute to local homeostasis of estrogens. Thus, the absence of CYP1B1 expression or inhibition of its activity may lead to accumulation of estradiol and enhanced estrogen receptor signaling, the consequences of which remain largely unexplored. A recent study by Kurmann et al. showed that estradiol inhibits the migratory activity of brain vascular pericytes [124][46]. Estradiol enhanced the barrier function of endothelial cells when cocultured with pericytes, which likely accounts for estradiol protection against blood–brain barrier disruption and antiangiogenic activity [124][46]. This notion is supported by ourthe studies demonstrating the mitigation of angiogenesis in Cyp1b1−/− mice [34][47], likely because of increased levels of estradiol in these mice. How these changes in estradiol metabolism impact the cellular redox state and increased oxidative stress that weit noted in various tissues of Cyp1b1−/− mice needs further exploration. The protective impact of estradiol on cardiovascular integrity and function has been known for quite some time. However, the underlying mechanisms and the cell-autonomous impact of estradiol on vascular cells are beginning to provide novel insight into the mechanisms involved, as discussed above. Estradiol signals through estrogen receptor in EC and regulates their proangiogenic properties [125,126][48][49]. One of the genes whose expression is increased by estradiol is BMP6 [127,128][50][51]. However, little is known about the autocrine and/or paracrine signaling of BMP6 in the retinal vasculature. BMP6 produced by liver SEC drives the expression of hepcidin in hepatocytes regulating systemic iron levels [129][52]. Given that retinal EC are the major regulators of iron homeostasis in the retina and express key iron regulatory proteins, wescholars propose that enhanced accumulation of estradiol in the absence of CYP1B1 could lead to increased BMP6 production by retinal EC and altered expression of hepcidin altering local iron levels. We hThe scholars hypothesize that increased hepcidin expression in retinal EC diminishes ferroportin expression in these cells, thus increasing intercellular iron levels leading to increased oxidative stress, lipid peroxidation, and cell death. This would mitigate angiogenesis and drive trabecular meshwork dysgenesis in Cyp1b1−/− mice as weit have previously demonstrated [32,34][47][53]. These possibilities are being presently explored in our laboratory and will provide novel insight into the regulatory pathways which mediate CYP1B1 activity in the eye (Figure 52). The impact of hepcidin on ferroportin expression in bovine retinal EC culture demonstrated decreased ferroportin expression and diminished iron export [130][54].
Figure 52. The proposed CYP1B1 regulation of intracellular iron levels and oxidative stress through estradiol metabolism, BMP6 signaling, hepcidin production, and ferroportin inhibition in the retinal endothelium.
CYP1B1 is also an important regulator of fatty acid homeostasis, and its expression is important in the modulation of PPARɣ and PPARα target genes and fatty acid metabolism. Arachidonic acid (AA) is oxidized by human CYP1B1 generating hydroxy eicosatetraenoic acids (HETEs), including 20-HETE and 12-HETE. However, the metabolism of AA by mouse CYP1B1 generates epoxy eicosatetraenoic acids (EETs) [21][18]. These metabolites impact various cellular and tissue functions, including cardiovascular, growth, apoptosis, vasodilation, and fibrosis. The differences in the catalytic efficiency of human and mouse CYP1B1 might contribute to their differences in AA metabolism. The potential pathophysiological impact of these AA metabolites will benefit from further exploration of their functions. WeScholars are conducting untargeted metabolomics studies to identify additional putative endogenous substrates of CYP1B1 with important physiological functions.
Vitamin A is critical for growth and development and supports the health of the immune system and vision. Retinol, retinal, and retinoic acid are the major forms of vitamin A. CYP1B1 catalyzes the oxidative metabolism of retinol to retinal and retinal to retinoic acid. Although the oxidation of retinol to retinoic acid is regulated by both human and mouse CYP1B1, neither can oxidize retinoic acid [21][18]. The interaction of retinoic acid with its receptors, including retinoic acid receptors and retinoid-X receptor (RXR), initiates signaling pathways with positive impacts on dyslipidemia, atherosclerosis, and cancer [131,132][55][56]. WeIt previously showed retinol, all-trans retinoic acid, and AM580 (an RXR agonist) failed to overcome the impact of CYP1B1 deficiency on retinal EC proangiogenic activity [34][47]. However, further delineating the impact of vitamin A metabolites on vision would be informative.
References
- Omura, T.; Sato, R. The carbon monoxide-binding pigment of liver microsomes. I. Evidence for its hemoprotein nature. J. Biol. Chem. 1964, 239, 2370–2378.
- Lepesheva, G.I.; Hargrove, T.Y.; Kleshchenko, Y.; Nes, W.D.; Villalta, F.; Waterman, M.R. CYP51: A major drug target in the cytochrome P450 superfamily. Lipids 2008, 43, 1117–1125.
- Elfaki, I.; Mir, R.; Almutairi, F.M.; Duhier, F.M.A. Cytochrome P450: Polymorphisms and Roles in Cancer, Diabetes and Atherosclerosis. Asian Pac. J. Cancer Prev. 2018, 19, 2057–2070.
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–141.
- Ortiz de Montellano, P.R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 2010, 110, 932–948.
- Guengerich, F.P. Common and Uncommon Cytochrome P450 Reactions Related to Metabolism and Chemical Toxicity. Chem. Res. Toxicol. 2001, 14, 611–650.
- Bovard, D.; Sandoz, A.; Luettich, K.; Frentzel, S.; Iskandar, A.; Marescotti, D.; Trivedi, K.; Guedj, E.; Dutertre, Q.; Peitsch, M.C.; et al. A lung/liver-on-a-chip platform for acute and chronic toxicity studies. Lab A Chip 2018, 18, 3814–3829.
- Almazroo, O.A.; Miah, M.K.; Venkataramanan, R. Drug Metabolism in the Liver. Clin. Liver Dis. 2017, 21, 1–20.
- Wu, Z.; Zhang, X.; Shen, L.; Xiong, Y.; Wu, X.; Huo, R.; Wei, Z.; Cai, L.; Qi, G.; Xu, Q.; et al. A Systematically Combined Genotype and Functional Combination Analysis of CYP2E1, CYP2D6, CYP2C9, CYP2C19 in Different Geographic Areas of Mainland China – A Basis for Personalized Therapy. PLoS ONE 2013, 8, e71934.
- Pottenger, L.H.; Christou, M.; Jefcoate, C.R. Purification and immunological characterization of a novel cytochrome P450 from C3H/10T1/2 cells. Arch. Biochem. Biophys. 1991, 286, 488–497.
- Savas, U.; Jefcoate, C.R. Dual Regulation of Cytochrome-P450ef Expression Via the Aryl-Hydrocarbon Receptor and Protein Stabilization in C3h/10t1/2 Cells. Mol. Pharmacol. 1994, 45, 1153–1159.
- Sutter, T.R.; Tang, Y.M.; Hayes, C.L.; Wo, Y.Y.; Jabs, E.W.; Li, X.; Yin, H.; Cody, C.W.; Greenlee, W.F. Complete cDNA sequence of a human dioxin-inducible mRNA identifies a new gene subfamily of cytochrome P450 that maps to chromosome 2. J. Biol. Chem. 1994, 269, 13092–13099.
- Murray, G.I.; Melvin, W.T.; Greenlee, W.F.; Burke, M.D. Regulation, Function, and Tissue-Specific Expression of Cytochrome P450 CYP1B1. Annu. Rev. Pharmacol. Toxicol. 2001, 41, 297–316.
- Choudhary, D.; Jansson, I.; Rezaul, K.; Han, D.K.; Sarfarazi, M.; Schenkman, J.B. Cyp1b1 protein in the mouse eye during development: An immunohistochemical study. Drug Metab. Dispos. 2007, 35, 987–994.
- Hakkola, J.; Pasanen, M.; Pelkonen, O.; Hukkanen, J.; Evisalmi, S.; Anttila, S.; Rane, A.; Mantyla, M.; Purkunen, R.; Saarikoski, S.; et al. Expression of CYP1B1 in human adult and fetal tissues and differential inducibility of CYP1B1 and CYP1A1 by Ah receptor ligands in human placenta and cultured cells. Carcinogenesis 1997, 18, 391–397.
- Chambers, D.; Wilson, L.; Maden, M.; Lumsden, A. RALDH-independent generation of retinoic acid during vertebrate embryogenesis by CYP1B1. Development 2007, 134, 1369–1383.
- Maguire, M.; Larsen, M.C.; Vezina, C.M.; Quadro, L.; Kim, Y.K.; Tanumihardjo, S.A.; Jefcoate, C.R. Cyp1b1 directs Srebp-mediated cholesterol and retinoid synthesis in perinatal liver; Association with retinoic acid activity during fetal development. PLoS ONE 2020, 15, e0228436.
- Li, F.; Zhu, W.; Gonzalez, F.J. Potential role of CYP1B1 in the development and treatment of metabolic diseases. Pharm. Ther. 2017, 178, 18–30.
- Shah, B.R.; Xu, W.; Mraz, J. Cytochrome P450 1B1: Role in health and disease and effect of nutrition on its expression. RSC Adv. 2019, 9, 21050–21062.
- Jennings, B.L.; George, L.W.; Pingili, A.K.; Khan, N.S.; Estes, A.M.; Fang, X.R.; Gonzalez, F.J.; Malik, K.U. Estrogen metabolism by cytochrome P450 1B1 modulates the hypertensive effect of angiotensin II in female mice. Hypertension 2014, 64, 134–140.
- Pingili, A.K.; Kara, M.; Khan, N.S.; Estes, A.M.; Lin, Z.; Li, W.; Gonzalez, F.J.; Malik, K.U. 6beta-hydroxytestosterone, a cytochrome P450 1B1 metabolite of testosterone, contributes to angiotensin II-induced hypertension and its pathogenesis in male mice. Hypertension 2015, 65, 1279–1287.
- Ganz, T. Iron and infection. Int. J. Hematol. 2018, 107, 7–15.
- Wallace, D.F. The Regulation of Iron Absorption and Homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62.
- Lane, D.J.R.; Bae, D.-H.; Merlot, A.M.; Sahni, S.; Richardson, D.R. Duodenal cytochrome b (DCYTB) in iron metabolism: An update on function and regulation. Nutrients 2015, 7, 2274–2296.
- Donovan, A.; Roy, C.N.; Andrews, N.C. The Ins and Outs of Iron Homeostasis. Physiology 2006, 21, 115–123.
- Ganz, T. Molecular Control of Iron Transport. J. Am. Soc. Nephrol. 2007, 18, 394–400.
- Duck, K.A.; Connor, J.R. Iron uptake and transport across physiological barriers. BioMetals 2016, 29, 573–591.
- Anderson, G.J.; Frazer, D.M.; McKie, A.T.; Vulpe, C.D. The ceruloplasmin homolog hephaestin and the control of intestinal iron absorption. Blood Cells Mol. Dis. 2002, 29, 367–375.
- Vaziri, N.D. Toxic effects of IV iron preparations in CKD patients. Nephrol. News Issues 2014, 28, 4–5.
- Schmaier, A.H. Transferrin: A blood coagulation modifier. Cell Res. 2020, 30, 101–102.
- Papanikolaou, G.; Pantopoulos, K. Systemic iron homeostasis and erythropoiesis. IUBMB Life 2017, 69, 399–413.
- Gnana-Prakasam, J.P.; Martin, P.M.; Smith, S.B.; Ganapathy, V. Expression and function of iron-regulatory proteins in retina. IUBMB Life 2010, 62, 363–370.
- Song, D.; Dunaief, J.L. Retinal iron homeostasis in health and disease. Front. Aging Neurosci. 2013, 5, 24.
- He, X.; Hahn, P.; Iacovelli, J.; Wong, R.; King, C.; Bhisitkul, R.; Massaro-Giordano, M.; Dunaief, J.L. Iron homeostasis and toxicity in retinal degeneration. Prog. Retin. Eye Res. 2007, 26, 649–673.
- Sanchez, M.; Galy, B.; Schwanhaeusser, B.; Blake, J.; Bähr-Ivacevic, T.; Benes, V.; Selbach, M.; Muckenthaler, M.U.; Hentze, M.W. Iron regulatory protein-1 and -2: Transcriptome-wide definition of binding mRNAs and shaping of the cellular proteome by iron regulatory proteins. Blood 2011, 118, e168–e179.
- Fleming, R.E.; Migas, M.C.; Holden, C.C.; Waheed, A.; Britton, R.S.; Tomatsu, S.; Bacon, B.R.; Sly, W.S. Transferrin receptor 2: Continued expression in mouse liver in the face of iron overload and in hereditary hemochromatosis. Proc. Natl. Acad. Sci. USA 2000, 97, 2214–2219.
- Worthen, C.A.; Enns, C.A. The role of hepatic transferrin receptor 2 in the regulation of iron homeostasis in the body. Front. Pharmacol. 2014, 5, 34.
- Abdizadeh, H.; Atilgan, A.R.; Atilgan, C. Mechanisms by Which Salt Concentration Moderates the Dynamics of Human Serum Transferrin. J. Phys. Chem. B 2017, 121, 4778–4789.
- Steere, A.N.; Byrne, S.L.; Chasteen, N.D.; Mason, A.B. Kinetics of iron release from transferrin bound to the transferrin receptor at endosomal pH. Biochim. Biophys. Acta Gen. Subj. 2012, 1820, 326–333.
- Stoilov, I.; Akarsu, A.N.; Sarfarazi, M. Identification of Three Different Truncating Mutations in Cytochrome P4501B1 (CYP1B1) as the Principal Cause of Primary Congenital Glaucoma (Buphthalmos) in Families Linked to the GLC3A Locus on Chromosome 2p21. Hum. Mol. Genet. 1997, 6, 641–647.
- Libby, R.T.; Smith, R.S.; Savinova, O.V.; Zabaleta, A.; Martin, J.E.; Gonzalez, F.J.; John, S.W. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science 2003, 299, 1578–1581.
- Kaczara, P.; Zaręba, M.; Herrnreiter, A.; Skumatz, C.M.; Ządło, A.; Sarna, T.; Burke, J.M. Melanosome-iron interactions within retinal pigment epithelium-derived cells. Pigment Cell Melanoma Res. 2012, 25, 804–814.
- Falero-Perez, J.; Song, Y.S.; Sorenson, C.M.; Sheibani, N. CYP1B1: A key regulator of redox homeostasis. Trends Cell. Mol. Biol. 2018, 13, 27–45.
- Tsuchiya, Y.; Nakajima, M.; Yokoi, T. Cytochrome P450-mediated metabolism of estrogens and its regulation in human. Cancer Lett. 2005, 227, 115–124.
- Tsuchiya, Y.; Nakajima, M.; Kyo, S.; Kanaya, T.; Inoue, M.; Yokoi, T. Human CYP1B1 is regulated by estradiol via estrogen receptor. Cancer Res. 2004, 64, 3119–3125.
- Kurmann, L.; Okoniewski, M.; Dubey, R.K. Estradiol Inhibits Human Brain Vascular Pericyte Migration Activity: A Functional and Transcriptomic Analysis. Cells 2021, 10, 2314.
- Tang, Y.; Scheef, E.A.; Wang, S.; Sorenson, C.M.; Marcus, C.B.; Jefcoate, C.R.; Sheibani, N. CYP1B1 expression promotes the proangiogenic phenotype of endothelium through decreased intracellular oxidative stress and thrombospondin-2 expression. Blood 2009, 113, 744–754.
- Sanchez, A.M.; Flamini, M.I.; Zullino, S.; Gopal, S.; Genazzani, A.R.; Simoncini, T. Estrogen receptor- promotes endothelial cell motility through focal adhesion kinase. Mol. Hum. Reprod. 2011, 17, 219–226.
- Oviedo, P.J.; Sobrino, A.; Laguna-Fernandez, A.; Novella, S.; Tarín, J.J.; García-Pérez, M.A.; Sanchís, J.; Cano, A.; Hermenegildo, C. Estradiol induces endothelial cell migration and proliferation through estrogen receptor-enhanced RhoA/ROCK pathway. Mol. Cell. Endocrinol. 2011, 335, 96–103.
- Ikeda, Y.; Tajima, S.; Izawa-Ishizawa, Y.; Kihira, Y.; Ishizawa, K.; Tomita, S.; Tsuchiya, K.; Tamaki, T. Estrogen Regulates Hepcidin Expression via GPR30-BMP6-Dependent Signaling in Hepatocytes. PLoS ONE 2012, 7, e40465.
- Ong, D.B.; Colley, S.M.; Norman, M.R.; Kitazawa, S.; Tobias, J.H. Transcriptional regulation of a BMP-6 promoter by estrogen receptor alpha. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2004, 19, 447–454.
- Canali, S.; Zumbrennen-Bullough, K.B.; Core, A.B.; Wang, C.Y.; Nairz, M.; Bouley, R.; Swirski, F.K.; Babitt, J.L. Endothelial cells produce bone morphogenetic protein 6 required for iron homeostasis in mice. Blood 2017, 129, 405–414.
- Zhao, Y.; Wang, S.; Sorenson, C.M.; Teixeira, L.; Dubielzig, R.R.; Peters, D.M.; Conway, S.J.; Jefcoate, C.R.; Sheibani, N. Cyp1b1 Mediates Periostin Regulation of Trabecular Meshwork Development by Suppression of Oxidative Stress. Mol. Cell. Biol. 2013, 33, 4225–4240.
- Hadziahmetovic, M.; Song, Y.; Ponnuru, P.; Iacovelli, J.; Hunter, A.; Haddad, N.; Beard, J.; Connor, J.R.; Vaulont, S.; Dunaief, J.L. Age-dependent retinal iron accumulation and degeneration in hepcidin knockout mice. Investig. Ophthalmol. Vis. Sci. 2011, 52, 109–118.
- Connolly, R.M.; Nguyen, N.K.; Sukumar, S. Molecular pathways: Current role and future directions of the retinoic acid pathway in cancer prevention and treatment. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2013, 19, 1651–1659.
- Jakel, H.; Fruchart-Najib, J.; Fruchart, J.C. Retinoic acid receptor-related orphan receptor alpha as a therapeutic target in the treatment of dyslipidemia and atherosclerosis. Drug News Perspect. 2006, 19, 91–97.
More
