Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 3 by Vicky Zhou and Version 2 by Vicky Zhou.
Apoptosis, programmed cell death, has a central role in developmental biology and in maintaining the equilibrium of renewing tissues. A founding member of the Bcl-2 family of regulatory proteins for apoptosis is Bcl-2, which is encoded by the BCL2 gene. Caspase-3 shares typical features with all caspases, including the role of acting as a crucial mediator of apoptosis.
- apoptosis
- Bcl-2
- caspases
- caspase-3
Apoptosis
A multicellular organism consists of highly organized cells. The number of cells in this community is tightly regulated, by controlling not only the rate of cell division, but also the rate of cell death. If cells are no longer needed, they activate the intracellular biochemical events which lead to characteristic cell changes and the death program. The cell changes include blebbing, cell shrinkage, nuclear fragmentation, chromatin condensation, DNA fragmentation, and mRNA decay, and the process is called programmed cell death (PCD); although, it is more commonly referred to as apoptosis (from Ancient Greek, ἀπόπτωσις, apoptosis: “falling off”, as leaves from a tree) [1][2]. Unlike necrosis, a form of traumatic cell death resulting from acute cell damage, the controlled and highly regulated process of apoptosis provides benefits over the life cycle of the body. During apoptosis, cell fragments, also known as apoptotic bodies, are produced. The apoptotic bodies can be engulfed and removed by phagocytes before the contents of the cell can flow into and damage the surrounding cells [1].
In developmental biology, apoptosis plays a key role in the physiological process of selective cell deletion that is required to maintain a steady state of the cell in a constantly renewing tissue [3][4]. In various processes, such as embryonic development, the proper development and functioning of the immune system, normal cell metabolism, hormone-dependent atrophy, and chemically induced cell death, apoptosis is considered an essential component. In addition to its importance as a biological phenomenon, inappropriate apoptosis is regarded to be a factor in dysfunctional health conditions (neurodegenerative diseases, autoimmune disorders, ischemic damage, etc.) [5][6]. Defects in apoptotic processes have been associated with a number of diseases. For example, in cancer, uncontrolled cell proliferation is associated with insufficient apoptosis, and atrophy has been observed to be caused by excessive apoptosis. Because of the great therapeutic potential seen in the ability of apoptosis to modulate cell life or death, modern research continually focuses on the elucidation and analysis of signaling pathways that control cell cycle arrest and apoptosis. There are two well-known groups of features which characterize apoptosis. The first group of features are morphological: condensation of chromatin, reduction of cell volume, and nuclear fragmentation, which results in the formation of apoptotic bodies, also known as “little sealed sacs”. Another feature is the cleavage of DNA by Ca2+/Mg2+-dependent endonuclease into oligonucleosome-long fragments that can be identified by gel electrophoresis as a ladder pattern [7].
Phenomena considered to be endogenous activators of apoptosis include the absence of growth factors, the deficiency of the trophic hormone, glucocorticoid therapy, and the ablation of matrix attachment [8]. Exogenous activators are traumatic physical stimulators, such as radiation, toxins, chemotherapeutics, and infective pathogens such as bacterial toxins and viruses. Programmed cell death is controlled by apoptotic regulators, which either block the protective effect of inhibitors having a pro-apoptotic effect, or have an inhibitory, anti-apoptotic influence [9][10]. The genes that control apoptosis were firstly identified by studies in the nematode, C. elegans, the homologues of which function in humans to regulate apoptosis [11]. By encoding their own anti-apoptotic genes which prevent the target cells from passing away earlier, some of the viruses have found a protective method to combat apoptosis.
Apoptosis is a highly regulated process that cannot be stopped once it has started [12]. The initiation of apoptosis goes through one of two pathways: intrinsic or extrinsic pathways [1][13]. In the inner pathway, or as it is more commonly known, the intrinsic or mitochondrial pathway, the cell sensing the cellular stress kills itself. In the outer or so-called extrinsic pathway, the tumor necrosis factor (TNF) pathway, and the first apoptosis signal (Fas) pathway caused by the signals from other cells, the cell kills itself.
Intrinsic Pathway
The intrinsic pathway of apoptosis is a cellular response to a change in the intracellular environment caused by stress. The sources of stress that cause cellular changes can be exogenous or endogenous. The exogenous triggers are toxins, radiation, fatty acids, etc. The endogenous stressors include DNA damage, endoplasmic reticulum stress, mitochondrial translocation, p53 activation, overexpression of BH3-only proteins, Ca2+ homeostasis, and/or imbalance in the redox status [14]. As the signals caused by both types of triggers influence mitochondria, the intrinsic pathway of apoptosis is known as the mitochondrial pathway (Figure 1).
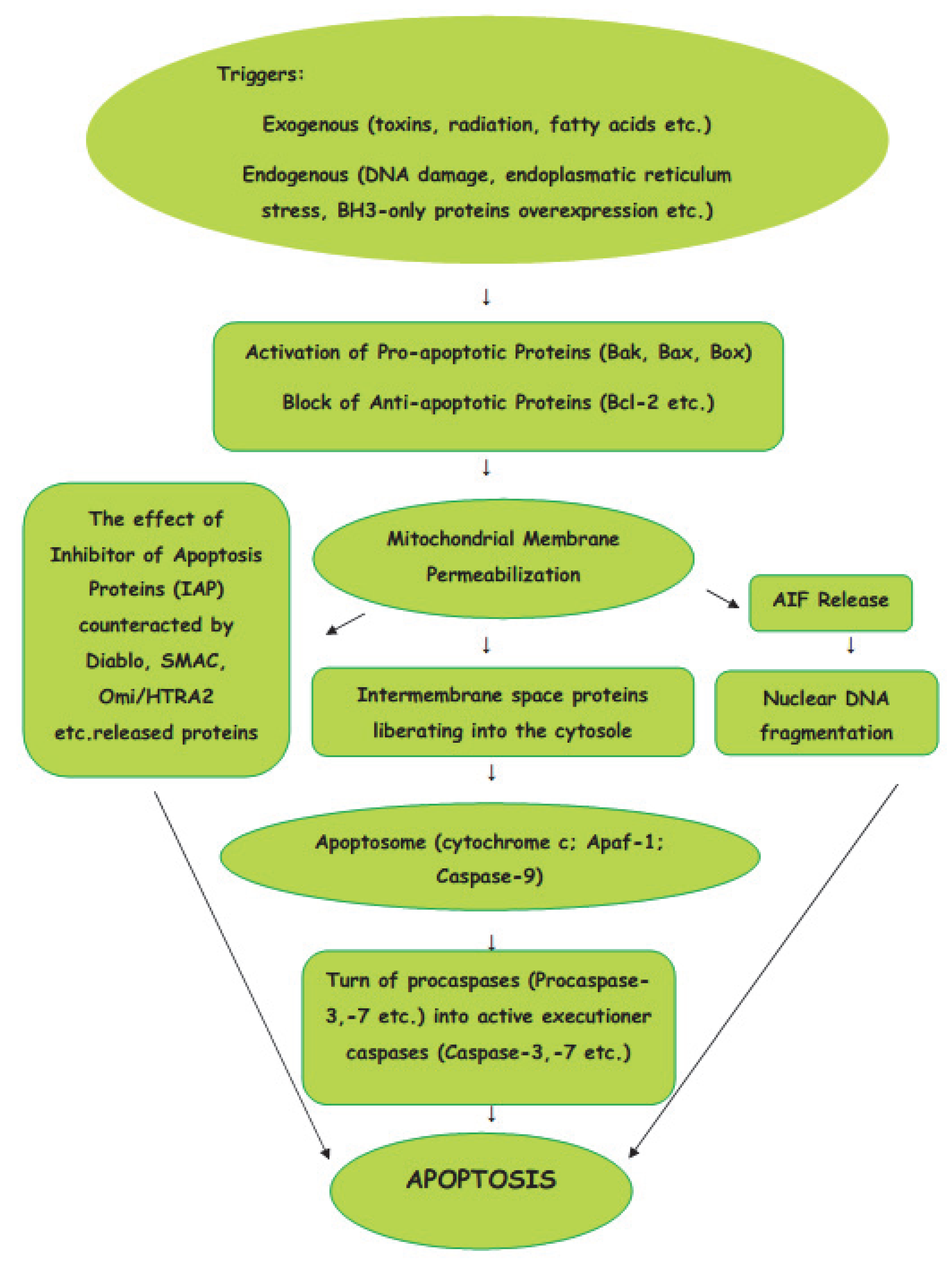
Figure 1. Intrinsic (mitochondrial) pathway of apoptosis.
Mitochondria are the basic “energy stations” of the cell, where, through oxidative phosphorylation, the energy is converted to ATP molecules.Mitochondria can be affected by signals in a variety of ways, such as by translocation to the outer mitochondrial matrix, or by post-transduction modification of mitochondrial proteins and channel formation. The effects of the signals causes the selective permeability of the mitochondrial inner or outer membrane, which causes the proteins in the intermembrane space to be liberated into the cytosole. The Bcl-2 protein family is involved in the process of the formation of macropores in the outer mitochondrial membrane. The active pro-apoptotic proteins, Bak, Bax, and Bok, undergo hetero–homo oligomerization, leading to the formation of macropores which enable proteins from the mitochondrial intermembrane space to enter the cytosole [15]. From mitochondrial intermembrane space proteins, cytochrome c, after cytosolic release, participates in the formation of a protein complex known as apoptosome, which cleaves Pro-caspase-9 to active Caspase-9. In turn, the activation of Caspase-9 activates the Pro-caspase-3 into effector Caspase-3. Thus, the cascade of caspases is initiated. The effect of IAP (inhibitor of apoptosis proteins), which ordinarily prevents the activation of Caspase-3, may be counteracted by some proteins, such as Arts, Diablo, Second Mitochondria-Derived Activator of Caspase (SMAC), and High-Temperature Requirement Protein A2 (Omi/HTRA2), released from damaged mitochondria. The interaction of IAPs, SMACs, and Omi/HTRA2 of members of the Bcl family is central to the pathway of intrinsic apoptosis. It has been demonstrated recently that Endonuclease-G (EndoG), the nuclease which is specifically activated by apoptotic stimuli, is also capable of inducing nucleosomal fragmentation of DNA independently of DNA Fragmentation Factor (DFF)/Caspase-Activated DNAse (CAD) and Caspase [16].
Extrinsic Pathway
The signals from the extracellular environment which designate the death of the cell cause the extrinsic pathway of apoptosis (Figure 2).
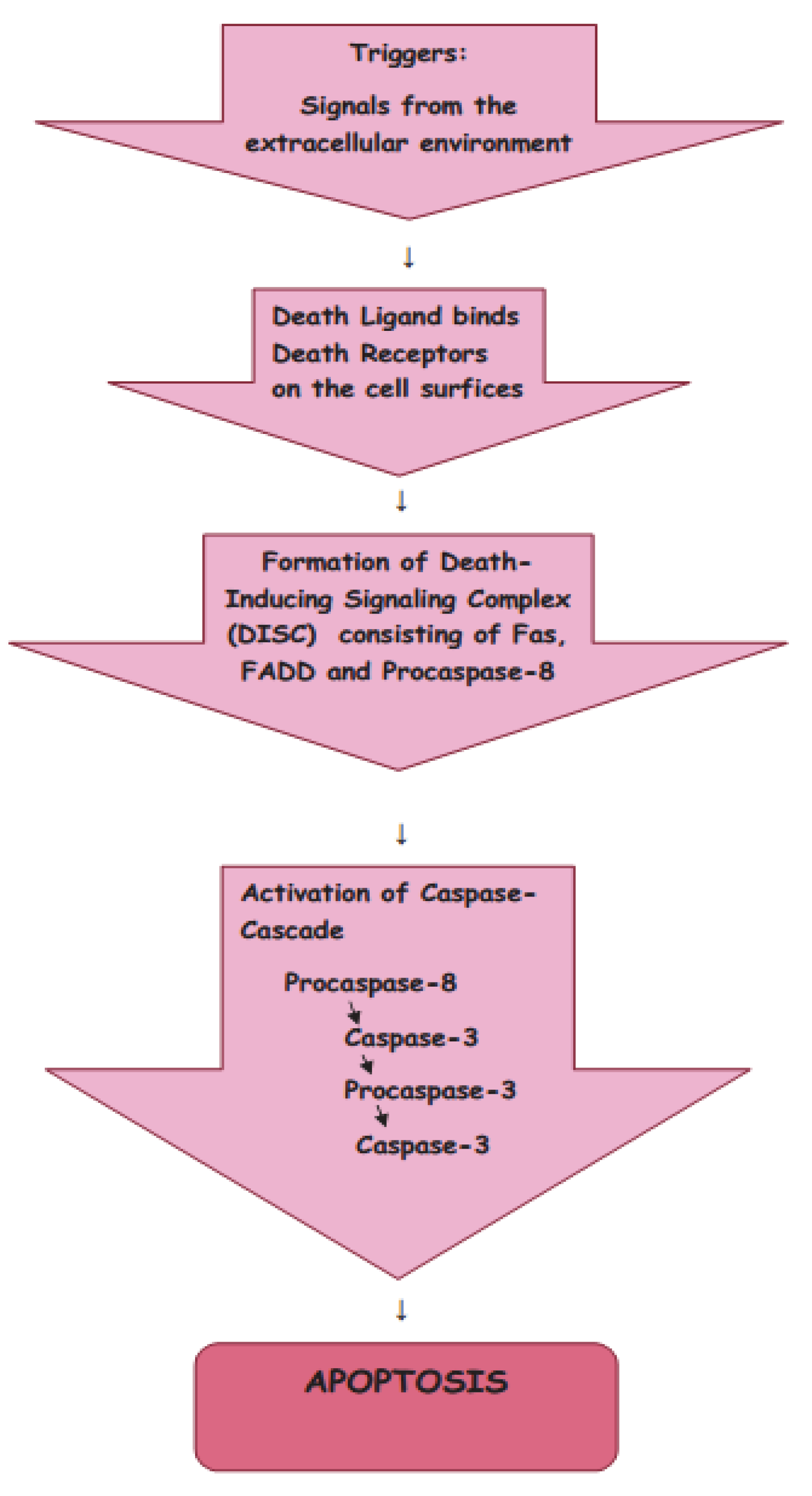
Figure 2. Extrinsic pathway of apoptosis.
There are two main theories of the extrinsic pathways of apoptosis: the TNF (tumor necrosis factor-induced) pathway and the Fas (Fas ligand-mediated model) pathway [17][18]. These pathways involve receptors of the TNF receptor family that are associated with external signals, and both pathways induce cell death by activating caspases, which then activate executioner caspases; after which, the cells are killed by degrading proteins indiscriminately.
Death receptors (DRs), the cell surface receptors, transmit apoptotic signals initiated by specific ligands, and have a major role in instructive apoptosis.
TNF Pathway
The TNF pathway involves receptor systems. The Tumor Necrosis Factor Receptor family includes cell surface receptors, death receptors (DRs), which convey apoptotic signals initiated by particular ligands with a major role in instructive apoptosis [19]. Apoptosis of the cell in the TNF pathway is caused by the activation of death caspases activated by the DRs. The apoptotic signal is transmitted from DRs to the death machinery by adapter molecules, such as Daxx containing Death Domains, Fas-Associated via Death Domain (FADD), and Tumor Necrosis Factor Receptor-1-Associated Death Domain (TRADD).
Fas Pathway
The Fas pathway is triggered by cytotoxic stress. The Fas receptor is a transmembrane protein belonging to the TNF family which binds the FasL (Fas ligand) [17]. When the interaction occurs between FasL and Fas, the death-inducing signaling complex (DISC), consisting of Fas, FADD, and Procaspase-8, is formed, and by interaction with the regulator FLICE Inhibitory Protein (FLIP), the co-factor function of FADD is blocked. Following recruitment by FADD, the oligomerization of Procaspase-8 activates it by self-cleavage; after which, Caspase-3 and Caspase-7 are activated by Caspase-8, causing cell apoptosis.
Other Pathways of Apoptosis
In addition to the caspase-dependent intrinsic and extrinsic apoptotic pathways, the caspase-independent pathway, mediated by apoptosis-inducing factor (AIF), has been described [20][21]. Apart from apoptotic activation by caspase or by AIF-mediated pathways, studies have demonstrated that apoptosis can also be induced by dual pathways: via the activation of AIF and caspase cascade simultaneously [22]. It has been shown that the modulation of mitochondrial activity may lead to the activation of cell death signals, which results in the release of cytochrome c and AIF in the cytoplasm [23][24]. Cytochrome c binds to the apoptotic protease-activating factor and to deoxy-ATP, activating Caspase-9 and causing the activation of the caspase cascade. This kind of signaling pathway is responsible for the hydrolysis of several key cytoplasmic proteins required for cell survival.
Recently, a nuclear pathway associated with apoptosis has been proposed [25]. Precisely, ZIP Kinase, after initiating apoptosis from nuclear Promyelocytic Leukemia Oncogenic Domains, combines with Daxx and Prostate Apoptosis Response Protein-4, a nuclear, caspase-independent apoptosis pathway.
Additionally, as the Jun N-terminal kinases (JNK) can also promote apoptosis by increasing the expression of pro-apoptotic genes through the transactivation of c-Jun/AP1-dependent or p53/73 protein-dependent mechanisms, the pathway is known as the JNK-mediated apoptosis pathway [26].
Some members of the Bcl-2 family of proteins inhibit apoptosis, whereas other factors, such as Fas receptors and caspases, promote apoptosis. The Bcl-2 family proteins have been found to include members that either promote or inhibit apoptosis [27]. In apoptosis, pro- and anti-apoptotic signaling proteins both have essential roles [28].
References
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Apoptosis: Programmed cell death eliminates unwanted cells. In Molecular Biology of the Cell, 5th ed.; Alberts, B., Ed.; Garland Science: New York, NY, USA, 2008; p. 1115.
- Green, D. Means to an End: Apoptosis and Other Cell Death Mechanisms; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2011.
- Ucker, P.S. Death by suicide: One way to go in mammalian cellular development. New Biol. 1991, 3, 103–109.
- Wyllie, A.H. Cell death: A new classification separating apoptosis from necrosis. In Cell Death in Biology and Pathology; Bowen, I.D., Lockshin, R.A., Eds.; Chapman and Hall: London, UK, 1981; pp. 9–34.
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516.
- Moujalled, D.; Strasser, A.; Liddell, J.R. Molecular mechanisms of cell death in neurological diseases. Cell Death Differ. 2021, 28, 2029–2044.
- Wyllie, A.H. Apoptosis (the 1992 Frank Rose Memorial Lecture). Br. J. Cancer 1993, 67, 205–208.
- Jan, R.; Chaudhry, G.E. Understanding apoptosis and apoptotic pathways targeted cancer therapeutics. Adv. Pharm. Bull. 2019, 9, 205–218.
- Vaux, D.L. A boom time for necrobiology. Curr. Biol. 1993, 3, 877–878.
- Milliman, C.L.; Korsmeyer, S.J.; Wang, K.; Yin, X.M.; Chao, D.T. BID: A Novel BH3 Domain-only Death Agonist. Genes Dev. 1996, 10, 2859–2869.
- Arvanitis, M.; Li, D.D.; Lee, K.; Mylonakis, E. Apoptosis in C. elegans: Lessons for cancer and immunity. Front. Cell. Infect. Microbiol. 2013, 3, 67.
- Raychaudhuri, S. A minimal model of signalling network elucidates cell-to cell stochastic variability in apoptosis. PLoS ONE 2010, 5, e11930.
- Lossi, L. The concept of intrinsic versus extrinsic apoptosis. Biochem. J. 2022, 479, 357–384.
- Wang, Z.; Figueiredo-Pereira, C.; Oudot, C.; Vieira, H.L.A.; Brenner, C. Mitochondrion: A common organelle for distinct cell deaths? Int. Rev. Cell Mol. Biol. 2017, 331, 245–287.
- Urbani, A.; Prosdocimi, E.; Carrer, A.; Checchetto, V.; Szabò, I. Mitochondrial ion channels of the inner membrane and their regulation in cell death signaling. Front. Cell Dev. Biol. 2021, 8, 620081.
- Cagnol, S.; Mansour, A.; Van Obberghen-Schilling, E.; Chambard, J.C. Raf-1 activation prevents caspase 9 processing downstream of apoptosome formation. J. Signal Transduct. 2011, 2011, 834948.
- Wajant, H. The Fas signaling pathway: More than a paradigm. Science 2002, 296, 1635–1636.
- Azzwali, A.A.A.; Azab, A.E. Apoptosis: Insight into Stages, Extrinsic and Intrinsic Pathways. Clin. Med. 2019, 7, 80–82.
- Muntané, J. Harnessing tumor necrosis factor receptors to enhance antitumor activities of drugs. Chem. Res. Toxicol. 2011, 24, 1610–1616.
- Susin, S.A.; Lorenzo, H.K.; Zamzami, N.; Marzo, I.; Snow, B.E.; Brothers, G.M.; Mangion, J.; Jacotot, E.; Costantini, P.; Loeffler, M.; et al. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature 1999, 397, 441–446.
- Aral, K.; Aral, C.A.; Kapila, Y. The role of caspase-8, caspase-9, and apoptosis inducing factor in periodontal disease. J. Periodontol. 2019, 90, 288–294.
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner caspase-3, -6, and-7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J. Biol. Chem. 2001, 276, 7320–7326.
- Chen, C.; Zhang, J.; Guo, Z.; Shi, X.; Zhang, Y.; Zhang, L.; Yu, Q.; Han, L. Effect of Oxidative Stress on AIF-mediated Apoptosis and Bovine Muscle Tenderness during Postmortem Aging. J. Food Sci. 2020, 85, 77–85.
- Talanian, R.V.; Allen, H. Roles of caspases in inflammation and apoptosis: Prospects as drug discovery targets. Annu. Rep. Med. Chem. 1998, 33, 273–282.
- Bojarska-Junak, A.; Sieklucka, M.; Hus, I.; Wąsik-Szczepanek, E.; Kusz, M.L.; Surdacka, A.; Chocholska, S.; Dmoszyńska, A.; Roliński, J. Assessment of the pathway of apoptosis involving PAR-4, DAXX and ZIPK proteins in CLL patients and its relationship with the principal prognostic factors. Folia Histochem. Cytobiol. 2011, 49, 98–103.
- Dey, D.K.; Chang, S.N.; Vadlamudi, Y.; Park, J.G.; Kang, S.C. Synergistic therapy with tangeretin and 5-fluorouracil accelerates the ROS/JNK mediated apoptotic pathway in human colorectal cancer cell. Food Chem. Toxicol. 2020, 143, 111529.
- Zamzami, N.; Brenner, C.; Marzo, I.; Susin, S.A.; Kroemer, G. Subcellular and submitochondrial mode of action of Bcl-2 like oncoproteins. Oncogene 1998, 16, 2265–2282.
- Nalepa, G.; Zukowska-Szczechowska, E. Caspases and apoptosis: Die and let live. Wiad. Lec. 2002, 55, 100–106.
More
