Primary headaches are a large group of diseases where the headache is not a symptom of another known disease. Tension-type headache affects approximately 80% of the general population, and the prevalence of migraine is estimated at 10–12%. Clinical data and experience to date have demonstrated that botulinum toxin may be an effective prophylactic treatment for chronic headache types. It has been used in neurology for the treatment of dystonia and blepharospasm. Now it has been approved to treat chronic migraine and has been shown to confer significant benefit in refractory cases. Botulinum toxin is effective in pain control through its interaction with the SNARE complex, which inhibits the release of neurotransmitters, such as glutamate, substance P and calcitonin gene-related peptide. OnabotulinumtoxinA is effective not only in headache frequency and pain intensity but in other parameters, including quality of life.
- chronic migraine
- onabotulinumtoxinA
- headaches
- botulinum toxin
1. Introduction
2. Botulinum Toxin in the Management of Chronic Migraine
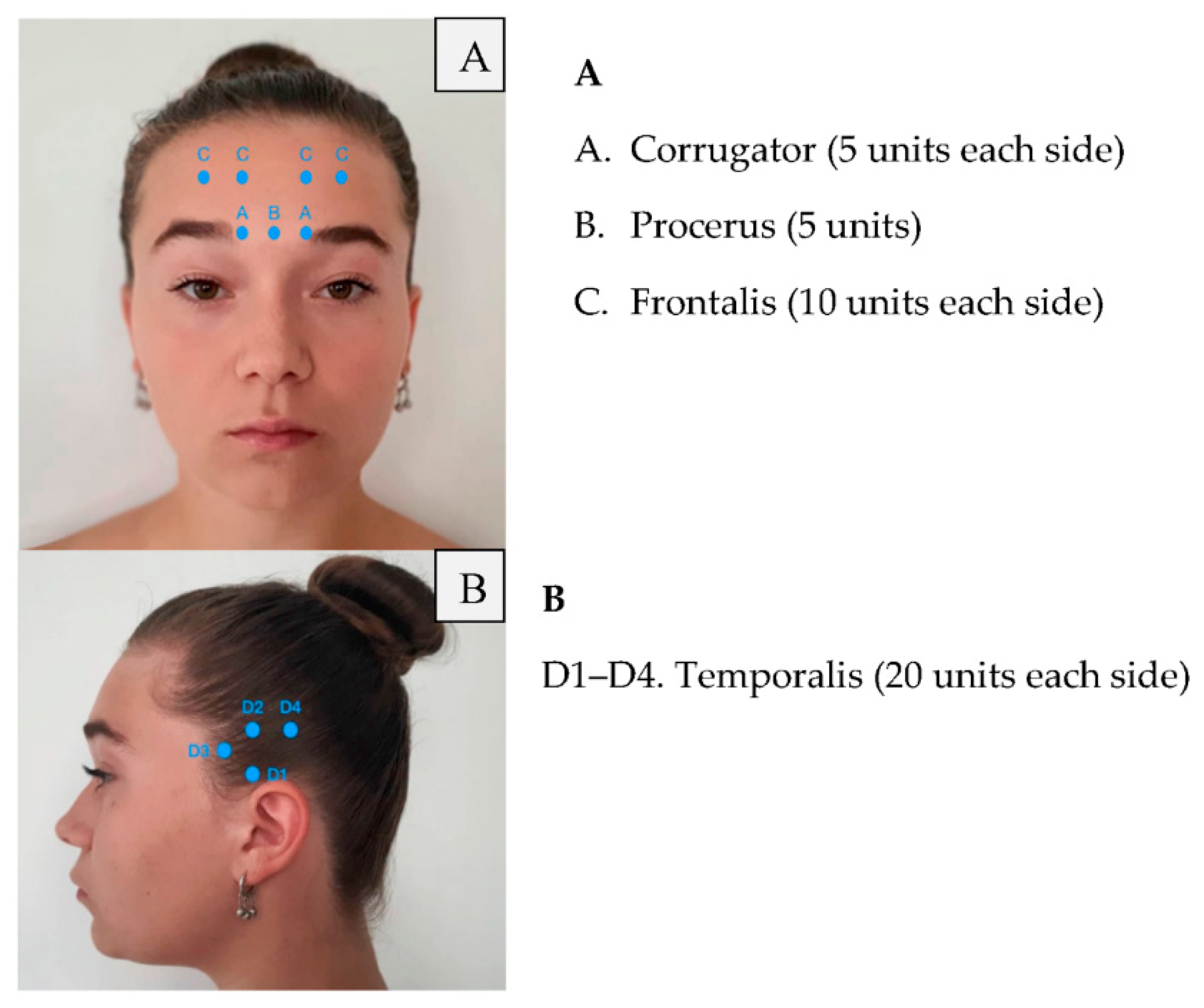
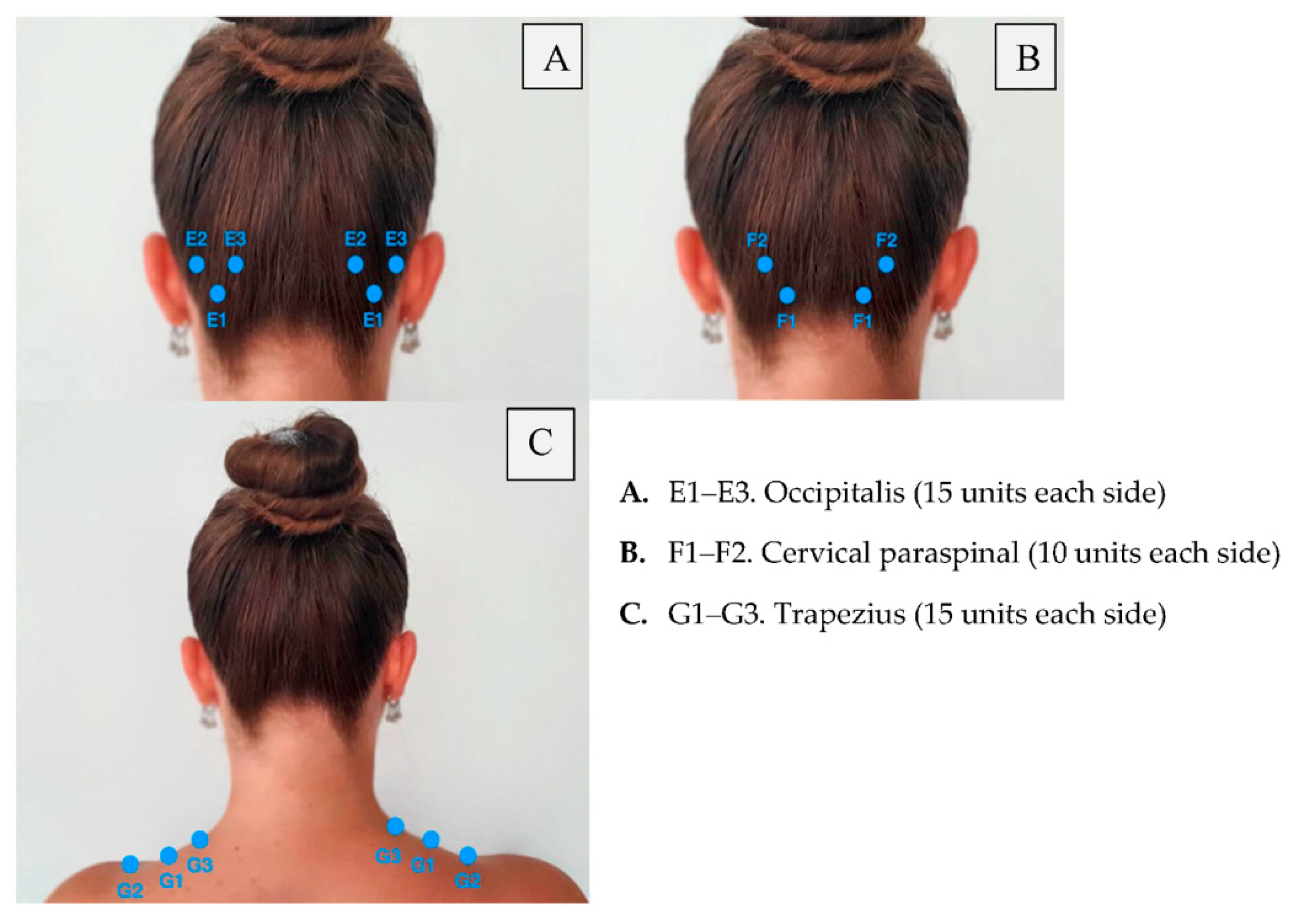
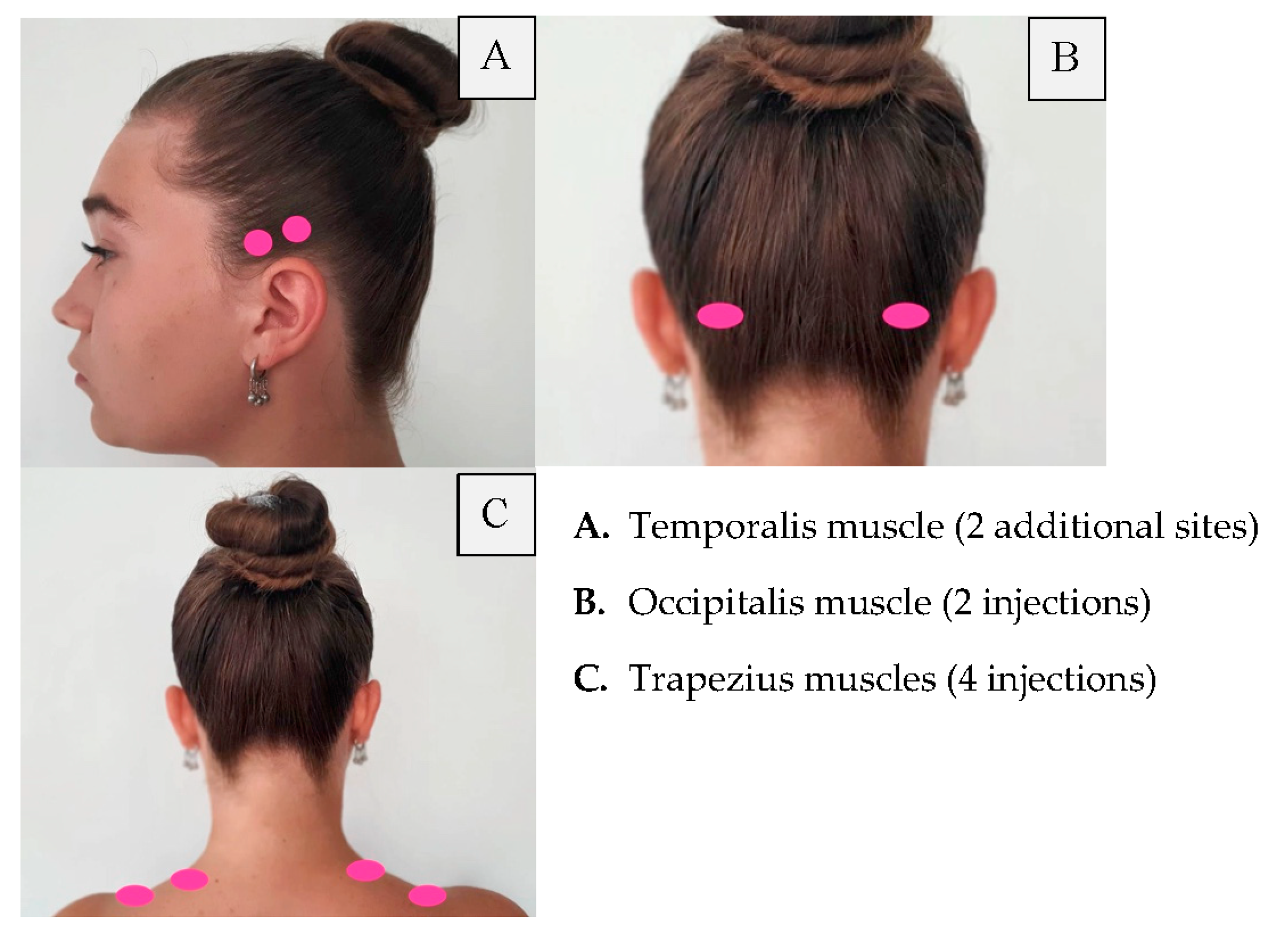
| Area of Injection | Recommended Dose | |
|---|---|---|
| Frontalis Corrugator Procerus Occipitalis Temporalis Trapezius Cervical paraspinal muscle group |
20 units (4 sites) 10 units (2 sites) 5 units (1 site) 30 units (6 sites) + 10 units in 2 sites (follow the pain areas—optional injections) 40 units (8 sites) + 10 units in 2 sites (follow the pain areas—optional injections) 30 units (6 sites) + 20 units in 4 sites (follow the pain areas—optional injections) 20 units (4 sites) | Summary: 155–195 units |
References
- Headache Classification Commitee of the International Headache Society, International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211.
- Bahra, A.; Evans, R.W. The secondary Headaches. Cephalalgia 2021, 41, 427–430.
- Steiner, T.J.; Stovner, L.J.; Vos, T. GBD 2015: Migraine is the third cause of disability in under 50s. J. Headache Pain 2016, 17, 104.
- Bigal, M.E.; Lipton, R.B. Modifiable risk factors for migraine progression. Headache 2006, 46, 1334–1343.
- Burch, R.C.; Buse, D.C.; Lipton, R.B. Migraine: Epidemiology, Burden and Comorbidity. Neurol. Clin. 2019, 37, 631–649.
- Aurora, S.K.; Dodick, D.W.; Turkel, C.C.; DeGryse, R.E.; Silberstein, S.D.; Lipton, R.B.; Diener, H.C.; Brin, M.F.; PREEMPT 1 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PRREMPT 1 trial. Cephalalgia 2010, 30, 793–803.
- Diener, H.C.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; DeGryse, R.E.; Lipton, R.B.; Silberstein, S.D.; Brin, M.F.; PREEMPT 2 Chronic Migraine Study Group. OnabotulinumtoxinA for treatment of chronic migraine: Results from the double-blind, randomized, placebo-controlled phase of the PRREMPT 2 trial. Cephalalgia 2010, 30, 804–814.
- Adams, A.M.; Serrano, D.; Buse, D.C.; Reed, M.L.; Marske, V.; Fanning, K.M.; Lipton, R.B. The impact of chronic migraine: The Chronic Migraine Epidemiology and Outcomes (CaMEO) Study methods and baseline results. Cephalalgia 2015, 35, 563–578.
- Robertson, C.E.; Garza, I. Critical analysis of the use of onatobotulinumtoxinA (botulinum toxin type A) in migraine. Neuropsychiatr. Dis. Treat. 2012, 8, 35–48.
- Wang, Y.F. OnabotulinumtoxinA injection in the treatment of chronic migraine. Prog. Brain Res. 2020, 255, 171–206.
- Lipton, R.B.; Cady, R.K.; Stewart, W.F.; Wilks, K.; Hall, C. Diagnostic lessons from the spectrum study. Neurology 2002, 14, 27–31.
- Jackson, J.; Kuriyama, A.; Hayashino, Y. Botulinum toxin A for prophylactic treatment of migraine and tension headaches in adults: A meta-analysis. J. Am. Med. Assoc. 2012, 307, 1736–1745.
- Silberstein, S.D.; Stark, S.R.; Lucas, S.M.; Christie, S.N.; Degryse, R.E.; Turkel, C.C. Botulinum toxin type A for the prophylactic treatment of chronic daily headache: A randomized, double-blind, placebo-controlled trial. Mayo Clin. Proc. 2005, 80, 1126–1137.
- Vo, A.; Satori, R.; Jabbari, B.; Green, J.; Killgore, W.D.; Labutta, R.; Campbell, W.W. Botulinum toxin type-A in the prevention of migraine: A double-blind controlled trial. Aviat. Space Environ. Med. 2007, 78, 113–118.
- Blumenfeld, A.; Silberstein, S.D.; Dodick, D.W.; Aurora, S.K.; Turkel, C.C.; Binder, W.J. Method of injection of onabotulinumtoxinA for chronic migraine: A safe, well-tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache 2010, 50, 1406–1418.
- Dodick, D.W.; Mauskop, A.; Elkind, A.H.; DeGryse, R.E.; Brin, M.F.; Silberstein, S.D. Botulinum toxin type a for the prophylaxis of chronic daily headache: Subgroup analysis of patients not receiving other prophylactic medications: A randomized double-blind, placebo-cotrolled study. Headache 2005, 45, 315–324.
