CD38 is a major mammalian NAD+ glycohydrolase (NADase), expresses on T cells following activation and appears to be an essential modulator of intracellular NAD+ levels. The enzymatic activity of CD38 in the process of generating the second messenger cADPR utilizes intracellular NAD+, and thus limits its availability to different
NAD+ consuming enzymes (PARP, ART, and sirtuins) inside the cells.
- CD38
- NAD+, T cell differentiation
- metabolism
- chromatin remodeling
1.Introduction
In addition to various co-stimulatory and co-inhibitory T cell receptors, ectonucleotidases that regulate the extracellular concentration of nucleotides, are also considered pivotal in modulating T cell response [11,12]. It has been shown that ectonucleotidases like CD39 and CD73 can promote an immunosuppressive microenvironment in various diseases like cancer, autoimmunity, and allergy through generation of adenosine by sequential cleavage of extracellular ATP to AMP and AMP to adenosine [11]. By doing this conversion, CD39 and CD73 impinge on purinergic signaling in T cells by limiting the availability of purinergic mediator ATP, and hence mitigate the pro-inflammatory response of T cells [12]. This demonstrates the emerging role of ectonucletidases as key regulator in determining the generation of inflammatory vs. immunosuppressive T cell response.
In recent years, CD38, another critical ectonucleotidase has gained prominence as an important regulator of T cell activation and function [13,14]. CD38 is a multifunctional transmembrane ectoenzyme that belongs to nicotinamide adenine dinucleotide (NAD+) glycohydrolase/adenosine 5’-diphosphate-ribosyl cyclase gene family. The enzymatic activity of CD38 not only catalyzes the cyclization of NAD+ to cyclic ADP-ribose (cADPR), but also hydrolyzes cADPR to form ADP-ribose (ADPR) [15]. Interestingly, it has been shown that a small amount of NAD+ gets cyclized by CD38 to produce cADPR, while the majority is hydrolyzed to ADPR [16]. This observation led to the proposition that the major enzymatic activity of CD38 is NAD+ glycohydrolase (NADase), but not ADP-ribosyl cyclase. In addition to NAD+, CD38 has also been shown to hydrolyze nicotinamide adenine dinucleotide phosphate (NADP) into nicotinic acid adenine dinucleotide phosphate (NAADP) via a base-exchange reaction [17]. However, the reaction requires an acidic pH and high (millimolar) concentration of nicotinic acid, the conditions can only be attained in vitro but hardly possible in vivo [17]. In fact, this notion was further supported by the observation that shRNA mediated knockdown of CD38 in Jurkat T cells had no effect in altering the intracellular concentration of NAADP, suggesting the dispensable role of CD38 in generating NAADP [18].
Numerous studies suggest that cADPR generated by the enzymatic action of CD38 acts as a second messenger for intracellular Ca2+ mobilization in several cells [19,20]. This indicates a plausible involvement of CD38 in regulating T cell activation [14,21], given the unequivocal role of Ca2+ signaling in triggering T cell activation. In fact, it has been reported that the expression of CD38 accompanies T cell activation and predominantly localizes to the immune synapse in close contact with T cell receptor (TCR) [22]. Moreover, the NAD+ glycohydrolase (NADase) activity of CD38 which determines the intracellular level of NAD+ [16], a principal metabolite regulating diverse biochemical and cellular processes further evinces the pivotal role of CD38 in regulating T cell functionality. Here in, we will focus on how CD38 is involved in regulating T cell-mediated immunity.
2. CD38− NAD+ Axis in Health and Diseases
CD38 was discovered as a cell surface marker present on the thymocytes and activated T cell surface and initially termed as T10 [23,24]. The enzymatic activities of CD38 generating ADPR and cADPR were described by Berthelier et al. and De Flora et al. [25,26]. A decade later, this molecule drew attention after Edward Chini and colleagues unearthed the role of CD38 as a major NAD+ catabolizing enzyme having a number of pathophysiological implications in aging, infection, and tumorigenesis [15].
High expression of CD38 has often been found to be associated with several hematological malignancies [27,28]. For example, the pathogenic role of CD38 have been implicated in multiple myeloma (MM), where tumor cells exhibit high surface expression of CD38 [29,30]. Likewise, CD38 expression is reported in other hematological tumors including B cell-chronic lymphocytic leukemia, acute myeloid leukemia, acute lymphocyte leukemia, and acute promyelocytic leukemia [31]. Owing to high CD38 expression, therapeutic interventions targeting CD38 are being devised for various hematological malignancies. Recently, monoclonal antibody targeting CD38 has been approved by FDA for the treatment of patients with refractory MM [27,28,32,33]. Conversely to hematological tumors, malignant cells from solid tumors do not express CD38. However, emerging studies are indicating that immune cells of both lymphoid and myeloid origin present at solid tumor sites exhibit high cell surface expression of CD38, which negatively correlates with the prognosis of the disease [13,34,35].
In contrast to causal attribution of CD38 in hematological malignancies many intriguing pieces of evidence suggest that CD38 is an essential component that serves to combat various infections by triggering innate immune response. A study in mice with Listeria monocytogenes infection has shown that upregulation of CD38 on neutrophils and macrophages is essential for their recruitment to the site of infection and efficient pathogen clearance [36]. In accord with this observation, an earlier study in C57BL/6 mice with Mycobacterium avium infection also implicated the role of CD38 in mounting protective immune response against the pathogen [37]. Mechanistically, CD38 has been shown to facilitate signaling pathways that lead to the production of pro-inflammatory cytokines from DC and macrophages [38–41], which appears to be instrumental in restraining infectious burden. Recent findings also indicate that the expression of CD38 can act as a negative regulator of immune cell function. In multiple myeloma, CD38 is implicated in promoting more aggressive immunosuppressive MDSCs and Treg [42]. A similar observation was also reported in the cases of esophageal and colorectal cancer (CRC) patients, where expression of CD38 potentiates the suppressive function of MDSCs and hence is associated with poor survival of patients [35,43]. These studies thus demonstrate that apart from acting as an adhesion molecule through interaction with CD31 on endothelial cells, CD38 could also tinker with the cellular events leading to distinctive functional outcome by immune cells.
Though, much efforts have been made to elucidate the role of CD38 in B cell malignancies and innate immune cells, its relative contribution in modulating T cell response is still limiting. Earlier studies reported the expression of CD38 on human early T cell precursors and on CD4+CD8+ double positive thymocytes [44]. In contrast, mature T cells have low level of CD38 but its expression is enhanced by various lymphocytes activators [45,46]. In fact, a number of studies from Fabio Malavasi’s group reported that in vitro cross-linking of CD38 with specific monoclonal antibodies on human T cells are capable of inducing its activation, proliferation and cytokine secretion through triggering different signaling events [47–49]. Owing to these facts, CD38 has long been considered as the activation marker for T cells. Most recently, a transient increase in the frequency of both CD4+ and CD8+CD38+HLA−DR+ T cells was observed in the blood sample from patient with COVID-19 during the viral clearance phase (day 7–9) [52] . This population (CD4+ and CD8+ CD38+HLA−DR+ T cells) has been shown to be positively corelated with the improved outcome of the patient [52]. However, CD38 has also been characterized as a marker of terminally exhausted T cells, which are refractory to the PD1 blockade mediated functional rejuvenation [50,51]. In agreement with this observation, a study from our group also reported that expression of CD38 caused metabolic aberration and compromised anti-tumor response by T cells [13]. These intriguing evidences suggest a complex role of CD38 in regulating T cell response through intervening multiple cellular and molecular pathways.
3. CD38 Mediated Signaling in Activated T Cells
The importance of CD38 in regulating T cell function is increasingly appreciated owing to their multifunctional enzymatic activity (both NADase and ADP-ribosyl cyclase), which can deplete intracellular NAD+ level and generates key signaling mediator, cADPR in T cells concomitantly [14]. However, in lymphocytes, CD38 is present on the plasma membrane in a type II conformation, with its catalytic domain exposed extracellularly [53,54]. This observation aroused the question of how CD38 metabolizes intracellular NAD+ and generates cADPR, an intracellular second messenger, while its catalytic domain faces outside. In a study by Zhao et al., this issue was addressed and they found that CD38 could be positioned in the plasma membrane in a type III orientation, with its C-terminal catalytic domain would be facing the cytoplasm [55]. Therefore, the type III conformation of CD38 appears to be crucial for its intracellular signaling activity and hence could be important for mediating the cADPR induced intracellular Ca2+ signaling.
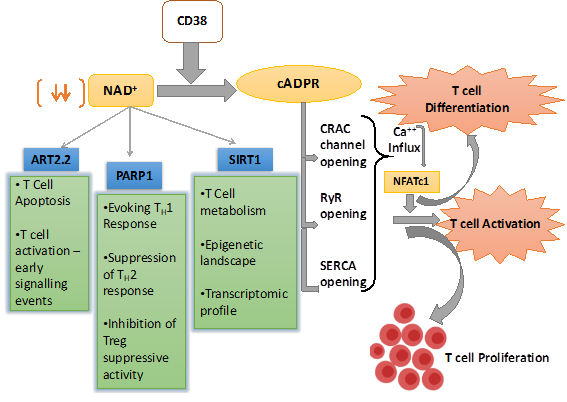
Ca2+ signaling is known to play a vital role in T cell activation and differentiation [56]. The engagement of TCR with its cognate antigen in the context of MHC results in an increase in intracellular Ca2+ concentration through store-operated calcium entry (SOCE) and activation of calcium release-activated calcium (CRAC) channels [56]. In T cells, the surge in Ca2+ concentration following TCR stimulation is predominantly triggered by 1,4,5-inositol triphosphate (IP3), which has been shown to mobilize Ca2+ from ER lumen through binding with IP3 receptors (IP3Rs) on ER membrane [57–59]. Although IP3Rs were found to be important for antigen triggered Ca2+ release in T cells, it could not explain prolonged (>1 h) Ca2+ signaling by CRAC as IP3 levels returned to near basal levels within 10 mins following TCR stimulation [60–62]. This led to the possibility of other mechanisms operating in parallel to the IP3-IP3R axis in mobilizing Ca2+ from ER following TCR stimulation. Studies by Geuse et al. had shown that ryanodine receptors (RyRs), another Ca2+ release channel also contributed to Ca2+ release from ER lumen through binding to the second messenger cADPR in a TCR stimulation dependent way. Using high-performance liquid chromatography analysis, they showed that stimulation of the T-cell receptor/CD3 (TCR/CD3) complex resulted in the activation of a soluble ADP-ribosyl cyclase and a sustained increase in intracellular levels of cADPR. Increased cADPR significantly and specifically stimulated type-3 ryanodine receptor, indicating a direct modulatory effect of cADPR on Ca2+ channel opening [63,64] and hence T cell activation and proliferation. Furthermore, the CD38 mediated cADPR production could indirectly induce increase in intracellular Ca2+ level in T cells by inhibition of the sarcoendoplasmic reticulum Ca2+ ATPase (SERCA), which facilitates calcium entry into ER from cytosol [56,65]. These studies together provide direct evidences that T cell activation, proliferation, and differentiation could be regulated by the CD38 dependent cADPR-RyR axis owing to its ability to modulate intracellular Ca2+ signaling.
In addition to mobilizing Ca2+ from ER, the role of CD38 induced cADRP-RyR axis in regulating T cell functionality has also been reported. It was shown that splenocytes from CD38 deficient mice with M. avium infection were skewed towards Th2 type and secreted lower IFNγ , which correlated with their compromised ability to limit mycobacterial burden [37]. This is in agreement with the earlier observation showing that human T cells upon CD38 ligation in vitro secreted several pro-inflammatory cytokines, including IFN-γ, IL-6, GM-CSF, and IL-10 [48]. It can be conceivable that the reported effect of CD38 in regulating cytokines production by T cells could be mediated through the activation of NFATc1 by cADPR-RyR axis induced Ca2+ signaling [66]. It has been shown that activation-induced Ca2+ influx in T cells results in nuclear localization of NFATc1 that drives the expression of several genes associated with T cell functionality, including the expression of various cytokines genes [67]. Chromatin immunoprecipitation (ChIP) assay has revealed that NFATc1 has a putative binding site in the regulatory region of IL-2, IL-4, and IFN-γ in T cells, and hence can control their expression [67]. Therefore, it seems that CD38 can act as an upstream regulator of intracellular Ca2+ signaling that could activate NFATc1 and hence determine the functionality of the T cells. Apart from regulating Ca2+ signaling, an association between activation-induced expression of CD38 and triggering of MAPK pathway has also been demonstrated where PTK, CD3-z/ZAP-70/PLC-g1 played a significant role [68]. Considering these findings, CD38-cADPR-Ca2+axis in T cells must be explored in more detail to further unravel the underlying mechanisms driving T cell functionality (Figure 1).
Figure 1. Schematic representation of nicotinamide adenine dinucleotide (NAD+) utilizing pathways inside the T cell and their overall effect on T cell response. CD38 is the major mammalian NAD+ glycohydrolase (NADase) which metabolizes NAD+ and generates cyclic ADP-ribose (cADPR), which promotes T cell activation and proliferations through facilitating Ca2+ signaling. CD38 expression also depletes NAD+ level and hence affect the enzymatic activity of different NAD+ consuming enzymes like SIRT1, PARP1, and ART2.2, which play pivotal role in T cell fate determination.
4. CD38− NAD+ Axis in Regulating T cell Fate and Function
CD38 has been identified as a critical modulator of NAD+ metabolism owing to its NADase activity [15,16]. NAD+ is a crucial cellular metabolite being, directly and indirectly, involved in a plethora of signaling pathways. Intracellular NAD+ level dependent regulation of various signaling cascades is shown to be mediated through two important enzymes, Poly (ADP-ribose) polymerase (PARP) and sirtuins (Sirt), which utilize NAD+ as substrate [69–71]. PARP is a family of proteins involved in several cellular processes such as DNA repair, genomic stability, and programmed cell death [72]. Sirt (Sirt1-6) are a class of proteins that possess either mono-ADP-ribosyltransferase, or deacylase activity, including deacetylase, desuccinylase, demalonylase, demyristoylase, and depalmitoylase activity [73]. Several studies reveal that overexpression of CD38 leads to the depletion of intracellular NAD+ levels and thus has a profound influence on the activity of the NAD+ consuming enzymes (PARP and Sirt), which regulate cellular homeostasis [16].
Alongside the PARPs and Sirt, whose activity is principally governed by the availability of the intracellular NAD+, there exists another class of NAD+ consuming enzyme named as ADP-ribosyl transferases or ARTs that act as extracellular NAD+ sensors. There are two major isoforms of ARTs—ART2.1 and ART2.2, which are reported to play a critical role in T cell activation and fate determination [74–77]. In addition, directly interfering early events of TCR signaling through producing cADPR, the NADase activity of CD38 could also have a profound influence on various aspects of T cell activation and differentiation. In the next few sections, we will be elaborating, how CD38 dependent modulation of NAD+ levels affect different cellular events in T cells, which in turn dictate the functional and phenotypic outcome of T cells.
5. CD38−NAD+ Axis in T Cell Immuno-Metabolism
Given the role of CD38 in modulating intracellular NAD+ levels, its involvement in regulating the metabolic commitment of T cells is becoming apparent [1,2]. Several studies are shedding light on CD38−NAD+-Sirt1 axis as an important metabolic checkpoint having enormous contribution in varied aspects of cellular energy metabolisms, including glycolysis, oxidative phosphorylation (OXPHOS), glutaminolysis, which are inherently associated with dictating T cell functional fate [2-4].
A recent study from our group illustrated that the ablation of the surface expression of the CD38 in CD4 T cells exhibited intrinsically higher levels of NAD+, which contributed to the rewiring of metabolic commitment and altered mitochondrial dynamics that renders T cells more effective in terms of anti-tumor immunity [2]. The study further reported that although targeting CD38 concomitantly enhanced Sirt1 activity, but the metabolic changes were independent of Sirt1, as Sirt1 deficiency had minimal effect on metabolic changes observed in CD38−/− CD4 T cells [2]. This indicated the possibility that other CD38 dependent pathways could play an essential role in this process. One possibility could be the involvement of other Sirt like Sirt3, as it is reported that CD38 promotes the age-related decline of NAD+ which causes mitochondrial dysfunction and reduced OXPHOS in Sirt3 dependent manner [5]. The metabolic dysfunctionality observed in the CD38 expressing tumor-infiltrating CD4 T cells [2] could also be mediated by the loss of anti-oxidant potential of T cells. Studies published by our group and others have shown that expression of CD38 inversely regulates the anti-oxidant potential of cells, and loss of CD38 in CD4 T cells significantly increased the expression of various anti-oxidant genes including Trx1, Trx2, Sod1, Sod2, and Nrf2 [2,6]. Recently, it has been shown that elevated ROS generation in T cells as a result of diminished anti-oxidant (glutathione) level, led to compromised activation of mTOR and reduced expression of NFAT and Myc, an important transcription factor drives glutaminolysis in T cells [7]. Therefore, further investigation in this direction is needed to delineate the Sirt1 independent role of CD38 in regulating the metabolic features of T cells.
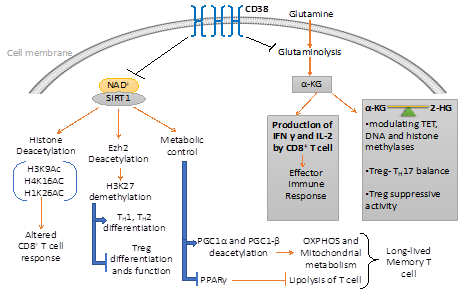
NAD+-Sirt1 axis, which is regulated by the expression of CD38 plays a vital role in determining the metabolic commitment of T cells. Importantly, Sirt1 has shown to promote oxidative phosphorylation and mitochondrial metabolism [8-11], which has appeared to be crucial in the differentiation of memory T cell [12,13]. Sirt1 mediated regulation of mitochondrial metabolism is mainly attributed to proteins belonging to peroxisome proliferators γ co-activator 1 (PGC-1) family, PGC1α and PGC1β [11,14]. The activity of PGC1α and PGC1β are known to be promoted via Sirt1 mediated deacetylation. The activation of these proteins facilitates mitochondrial biogenesis and subsequently OXPHOS [14]. Thus, it might be hypothesized that hyperactivity of CD38 alleviates Sirt1 activity and hence perturb mitochondrial biogenesis and OXPHOS, which impinge on memory T cell differentiation. It should also be noted that Sirt1 could promote the differentiation of long-lived T cells with heightened anti-tumor potential through inhibiting the activity of PPARγ [15,16], which suppresses the induction of lipolysis in T cells [16], a prerequisite for memory T cell differentiation [16,17]. Therefore, it seems that the expression of CD38 on T cells by limiting intracellular NAD+ levels and Sirt1 activity exerts metabolic perturbations, which ultimately affects T cell differentiation and functionality. Further investigation is thus needed to delineate the intricate mechanisms which would be useful in devising drugable target to improve the metabolic fitness and hence the functionality of T cells (Figure 2).
Figure 2. CD38 mediated regulation of metabolic pathways and chromatin modifications in T cells. CD38 affects the differentiation and effector response of T cells through modulating the metabolic pathways and epigenetic landscape of T cells. On one hand, CD38 curtails the availability of NAD+ to Sirt1 and hence attenuates its enzymatic activity which regulates different metabolic and epigenetic pathways in T cells. On the other hand, CD38 inversely regulates glutaminolysis pathways, which not only regulate effector cytokine production in T cells but also produce α-ketoglutarate (α-KG) and 2-hydroxyglutarate (2-HG), important epigenetic modifiers.
6. CD38−NAD+ Axis and T Cell Epigenetic Modifications
CD38, by virtue of its NADase activity, has shown to perturb cellular homeostasis of different NAD+ consuming enzymes reported to act as epigenetic modifiers and hence can alter the functional fate of T cells [18]. Emerging evidence suggests that CD38-NAD+ axis has a profound influence in regulating the intracellular levels of various metabolites including α-ketoglutarate (α-KG), 2-hydroxyglutarate (2-HG), and signaling mediator like ROS, which are reported to play a pivotal role in orchestrating the epigenetic landscape of T cells [2,19,20]. Thus, a detailed discussion of these pathways is of utmost importance in the aspect of T cell differentiation, development, and function.
6.1. Metabolites Mediated Epigenetic Regulation
As discussed in the previous section, elevated expression of CD38 on T cells inversely regulates glutaminolysis, a predominant pathway of yielding α-KG, which act as a co-factor of histone and DNA demethylases [2,19]. Recently, it has been reported that α-KG mediated H3K27 demethylation can be linked to the increased effector cytokines (IFN-γ and IL-2) production by mouse CD8+ T cell [21]. The study arouses the possible association of CD38 dependent metabolic rewiring as a critical cellular event regulating the epigenetic modification of T cells and hence their functional state. This is supported by the recent studies showing that expression of CD38 facilitates T cell exhaustion at the tumor site, which is refractory to restore their functionality by immune checkpoint blockade therapy [22]. This phenomenon is in part due to the extensive epigenetic remodeling of CD38 expressing stable exhausted T cells (CD38+PD1+CD101+ T cells) [22]. Although the detailed mechanisms underpinning CD38 dependent epigenetic modification of T cells has not been fully explored, altered metabolic commitment of CD38 expressing T cells could play an essential role in this process.
Several studies have reported that a balance between intracellular level of a-KG and 2-HG, a metabolite produced by isocitrate dehydrogenase 1 and 2 (IDH1/2), are capable of altering histone methylation and chromatin accessibility in various cell types [19,23]. α-KG and 2-HG are mutually antagonistic in nature and are found to modulate epigenetic modification of T cells via affecting the activity of ten-eleven Translocases (TET), DNA, and histone methylases [19]. The intricate balance between α-KG and 2-HG in instilling epigenetic modification has recently been implicated in fate determination of Th17 and Treg [23]. This is in accordance with the early observation showing that glutaminolysis derived α-KG negatively regulates Treg differentiation [24]. The mechanism could be of further importance in explaining the elevated expression of CD38, particularly on Treg with heightened suppressive activity [25]. It is possible that CD38 mediated negative regulation of glutaminolysis, and hence the production of α-KG and subsequently 2-HG, maintains demethylation state of FoxP3 promotor that results in increased Treg stability.
6.2. Sirt1 Dependent Epigenetic Regulation
NAD+-Sirt1 axis, which is inversely regulated by CD38 expression, has been reported to be an important epigenetic modifier owing to its deacetylation activity [26]. The Sirt1 induced epigenetic regulation can be achieved in three distinct mechanisms, viz., a) regulation of chromatin structure by histone deacetylation, b) regulating the activity of transcription factor by deacetylation, and c) regulation of other epigenetic enzymes by deacetylation [26].
Sirt1 can deacetylate lysine residues of different histone marks, including H3K9Ac, H4K16Ac, and H1K26Ac, as silencing Sirt1 using RNAi approach in human cells led to a global increase in H3K9Ac and H4K16Ac [27]. In human CD8 memory T cells, increased histone acetylation at H3K9 (H3K9Ac), is associated with the activate transcription of EOMES, PRF1, and GZMB loci [28]. However, whether Sirt1 has any role in regulating the acetylation of H3K9 at EOMES, PRF1, and GZMB loci of memory CD8 T cells has not been fully explored and thus warrants further investigations.
The role of Sirt1 on imparting distinctive epigenetic signature on T cells could also be mediated through regulating the activity of epigenetic enzymes. It has recently been demonstrated that CD38 ablation mediated elevation of Sirt1 in CD8 T cells from SLE patients is capable of deacetylating enhancer of zeste homolog 2 (Ezh2), an enzyme catalyzing methylation of H3K27 which ultimately causes transcriptional repression [29]. The study further reported that Sirt1 mediated deacetylation of Ezh2 rendered it inactive, resulting in increased transcription of T-bet, EOMES, and Runx3 in CD8 T cells due to reduced Ezh2 mediated H3K27me3 in these gene loci [29]. The study, thus, pointed out the role of CD38−NAD+-Sirt1 axis mediated regulation of Ezh2 in determining the cytotoxic potential of CD8 T cells. Ezh2 mediated H3K27 tri-methylation is also reported to regulate Th1, Th2 and Treg differentiation. Ezh2 and increased H3K27 tri-methylation inhibits Th1 and Th2 differentiation as it facilitates the silencing of genes encoding lineage-specific cytokines (like Ifng and il13 for Th1 and Th2, respectively) and transcription factors (T-bet and GATA3 for Th1 and Th2, respectively) [30].
Conversely, expression of Ezh2 promotes Treg cell stability and function, as genetic ablation of Ezh2, specifically in FoxP3 expressing T cells, has shown to suppress Treg cell signature gene FoxP3 [31]. From a recent clinical study, it was found that T cells from Rheumatoid arthritis (RA) patients exhibited lower Ezh2, which regulated T cell differentiation through promoting epigenetic modification [32]. From in vitro studies, it was concluded that attenuation of Ezh2 led to the downregulation of RUNX1, and promoted SMAD7 which synergistically dampened the TGFβ signaling events, essential for generation of Tregs [32]. Therefore, it seems reasonable to argue that in addition to directly influencing FoxP3 activity and stability, Sirt1 could indirectly affect the fate of Treg via regulating the enzymatic activity of Ezh2 [33,34] (Figure 2).
7. Conclusion
From the above discussion, the multifaceted roles of CD38 in T cell differentiation, development, and different aspects of T cell health is quite evident. In addition to controlling the different aspects of T cell activation by interplaying with the TCR downstream signaling pathways, the competition of CD38 with several post-translational and epigenetic modifiers for occupancy of NAD+ has been shown to be one of the key dictating factors driving discrete T cell fates. Even this intricate balance appears to be decisive in regulating the suppressive potential of Treg. It can be speculated from the existing studies that Sirt1 mediated deacetylation of FoxP3, a post-translational event that diminishes the suppressive potential of Treg could be instrumental in regulating the differential suppressive activity between CD38hi and CD38lo Treg. In addition to Sirt1 axis, CD38 mediated metabolic rewiring could also play a crucial role in this context through orchestrating the cellular balance of -KG and 2-HG; key glutaminolysis derived metabolites have shown to regulate epigenetic modification. Therefore, it seems that although CD38 expression during activation of T cells might be necessary for mediating early activation events, its stabilization could have a differential effect in defining the functional outcome of different T cell subsets.
It is also evident from recent studies that CD38 has a crucial role in driving stable exhaustion of T cells, which is refractory to the PD-1 mediated functional rejuvenation. Although the precise mechanism(s) yet to decipher, it seems that epigenetic modification could play a pivotal role in inducing the stable exhausted phenotype of CD38hi T cells. The notion can be supported by the fact that decreased deacetylase activity of Sirt1 in CD38hi T cells attenuates the enzymatic activity of histone methyltransferase Ezh2 which has a profound effect in determining the functionality and survival of T cells. Depleting CD38 levels in these cells by administration of an antibody against CD38 along with immune checkpoint blockade (anti-PD1) could potentially rejuvenate these cells from being exhausted. These could result in a better manifestation of the anti-tumor property of the tumor-infiltrating T cells in the advanced stage of a tumor, and in the resolution of any chronic infections, which also induce stable exhaustion phenotype of T cells. However, it should be noted in this context that CD38 antibodies which are available for clinical evaluation including Daratumumab, Isatuximab, MOR202, and TAK-079, cause depletion of target cells through multiple mechanisms including antibody dependent cellular cytotoxicity (ADCC), complement dependent cytotoxicity (CDC), antibody dependent cellular phagocytosis (ADCP), etc. [27,147]. Therefore, clinical use of these antibodies to restore the functionality of CD38+ exhausted T cells in solid tumors or other chorionic infections might cause an adverse effect due to depletion of T cells. Considering this fact, prudent selection of CD38 antibodies specifically targeting the NADase/glycohydrolase activity without triggering target cell cytotoxicity would be extremely important to garner T cell mediated tumor killing and exploiting this strategy for improved clinical outcomes in solid tumors. The future of CD38 research thus far has much vital information to offer, unraveling some novel downstream mechanisms, and this could emerge as one of the principal pharmacological tools in the hands of the scientific and medical fraternity to modulate functionality T cells in varied disease scenario.
References
- Hogan, K.A.; Chini, C.C.S.; Chini, E.N. The Multi-faceted Ecto-enzyme CD38: Roles in Immunomodulation, Cancer, Aging, and Metabolic Diseases. Front Immunol 2019, 10, 1187, doi:10.3389/fimmu.2019.01187.
- Chatterjee, S.; Daenthanasanmak, A.; Chakraborty, P.; Wyatt, M.W.; Dhar, P.; Selvam, S.P.; Fu, J.; Zhang, J.; Nguyen, H.; Kang, I., et al. CD38-NAD(+)Axis Regulates Immunotherapeutic Anti-Tumor T Cell Response. Cell Metab 2018, 27, 85-100 e108, doi:10.1016/j.cmet.2017.10.006.
- Chang, H.C.; Guarente, L. SIRT1 and other sirtuins in metabolism. Trends Endocrinol Metab 2014, 25, 138-145, doi:10.1016/j.tem.2013.12.001.
- Geltink, R.I.K.; Kyle, R.L.; Pearce, E.L. Unraveling the Complex Interplay Between T Cell Metabolism and Function. Annu Rev Immunol 2018, 36, 461-488, doi:10.1146/annurev-immunol-042617-053019.
- Camacho-Pereira, J.; Tarrago, M.G.; Chini, C.C.S.; Nin, V.; Escande, C.; Warner, G.M.; Puranik, A.S.; Schoon, R.A.; Reid, J.M.; Galina, A., et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab 2016, 23, 1127-1139, doi:10.1016/j.cmet.2016.05.006.
- Hu, Y.; Wang, H.; Wang, Q.; Deng, H. Overexpression of CD38 decreases cellular NAD levels and alters the expression of proteins involved in energy metabolism and antioxidant defense. J Proteome Res 2014, 13, 786-795, doi:10.1021/pr4010597.
- Mak, T.W.; Grusdat, M.; Duncan, G.S.; Dostert, C.; Nonnenmacher, Y.; Cox, M.; Binsfeld, C.; Hao, Z.; Brustle, A.; Itsumi, M., et al. Glutathione Primes T Cell Metabolism for Inflammation. Immunity 2017, 46, 675-689, doi:10.1016/j.immuni.2017.03.019.
- Vellinga, T.T.; Borovski, T.; de Boer, V.C.; Fatrai, S.; van Schelven, S.; Trumpi, K.; Verheem, A.; Snoeren, N.; Emmink, B.L.; Koster, J., et al. SIRT1/PGC1alpha-Dependent Increase in Oxidative Phosphorylation Supports Chemotherapy Resistance of Colon Cancer. Clin Cancer Res 2015, 21, 2870-2879, doi:10.1158/1078-0432.CCR-14-2290.
- Abraham, A.; Qiu, S.; Chacko, B.K.; Li, H.; Paterson, A.; He, J.; Agarwal, P.; Shah, M.; Welner, R.; Darley-Usmar, V.M., et al. SIRT1 regulates metabolism and leukemogenic potential in CML stem cells. J Clin Invest 2019, 129, 2685-2701, doi:10.1172/JCI127080.
- Brenmoehl, J.; Hoeflich, A. Dual control of mitochondrial biogenesis by sirtuin 1 and sirtuin 3. Mitochondrion 2013, 13, 755-761, doi:10.1016/j.mito.2013.04.002.
- Tang, B.L. Sirt1 and the Mitochondria. Mol Cells 2016, 39, 87-95, doi:10.14348/molcells.2016.2318.
- van der Windt, G.J.; Everts, B.; Chang, C.H.; Curtis, J.D.; Freitas, T.C.; Amiel, E.; Pearce, E.J.; Pearce, E.L. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 2012, 36, 68-78, doi:10.1016/j.immuni.2011.12.007.
- Buck, M.D.; O'Sullivan, D.; Klein Geltink, R.I.; Curtis, J.D.; Chang, C.H.; Sanin, D.E.; Qiu, J.; Kretz, O.; Braas, D.; van der Windt, G.J., et al. Mitochondrial Dynamics Controls T Cell Fate through Metabolic Programming. Cell 2016, 166, 63-76, doi:10.1016/j.cell.2016.05.035.
- Kelly, T.J.; Lerin, C.; Haas, W.; Gygi, S.P.; Puigserver, P. GCN5-mediated transcriptional control of the metabolic coactivator PGC-1beta through lysine acetylation. J Biol Chem 2009, 284, 19945-19952, doi:10.1074/jbc.M109.015164.
- Picard, F.; Kurtev, M.; Chung, N.; Topark-Ngarm, A.; Senawong, T.; Machado De Oliveira, R.; Leid, M.; McBurney, M.W.; Guarente, L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature 2004, 429, 771-776, doi:10.1038/nature02583.
- Chakraborty, P.; Vaena, S.G.; Thyagarajan, K.; Chatterjee, S.; Al-Khami, A.; Selvam, S.P.; Nguyen, H.; Kang, I.; Wyatt, M.W.; Baliga, U., et al. Pro-Survival Lipid Sphingosine-1-Phosphate Metabolically Programs T Cells to Limit Anti-tumor Activity. Cell Rep 2019, 28, 1879-1893 e1877, doi:10.1016/j.celrep.2019.07.044.
- O'Sullivan, D.; van der Windt, G.J.W.; Huang, S.C.; Curtis, J.D.; Chang, C.H.; Buck, M.D.; Qiu, J.; Smith, A.M.; Lam, W.Y.; DiPlato, L.M., et al. Memory CD8(+) T Cells Use Cell-Intrinsic Lipolysis to Support the Metabolic Programming Necessary for Development. Immunity 2018, 49, 375-376, doi:10.1016/j.immuni.2018.07.018.
- Chen, Y.; Zander, R.; Khatun, A.; Schauder, D.M.; Cui, W. Transcriptional and Epigenetic Regulation of Effector and Memory CD8 T Cell Differentiation. Front Immunol 2018, 9, 2826, doi:10.3389/fimmu.2018.02826.
- Lu, C.; Thompson, C.B. Metabolic regulation of epigenetics. Cell Metab 2012, 16, 9-17, doi:10.1016/j.cmet.2012.06.001.
- Kietzmann, T.; Petry, A.; Shvetsova, A.; Gerhold, J.M.; Gorlach, A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol 2017, 174, 1533-1554, doi:10.1111/bph.13792.
- Suzuki, J.; Yamada, T.; Inoue, K.; Nabe, S.; Kuwahara, M.; Takemori, N.; Takemori, A.; Matsuda, S.; Kanoh, M.; Imai, Y., et al. The tumor suppressor menin prevents effector CD8 T-cell dysfunction by targeting mTORC1-dependent metabolic activation. Nat Commun 2018, 9, 3296, doi:10.1038/s41467-018-05854-6.
- Philip, M.; Fairchild, L.; Sun, L.; Horste, E.L.; Camara, S.; Shakiba, M.; Scott, A.C.; Viale, A.; Lauer, P.; Merghoub, T., et al. Chromatin states define tumour-specific T cell dysfunction and reprogramming. Nature 2017, 545, 452-456, doi:10.1038/nature22367.
- Xu, T.; Stewart, K.M.; Wang, X.; Liu, K.; Xie, M.; Ryu, J.K.; Li, K.; Ma, T.; Wang, H.; Ni, L., et al. Metabolic control of TH17 and induced Treg cell balance by an epigenetic mechanism. Nature 2017, 548, 228-233, doi:10.1038/nature23475.
- Klysz, D.; Tai, X.; Robert, P.A.; Craveiro, M.; Cretenet, G.; Oburoglu, L.; Mongellaz, C.; Floess, S.; Fritz, V.; Matias, M.I., et al. Glutamine-dependent alpha-ketoglutarate production regulates the balance between T helper 1 cell and regulatory T cell generation. Sci Signal 2015, 8, ra97, doi:10.1126/scisignal.aab2610.
- Feng, X.; Zhang, L.; Acharya, C.; An, G.; Wen, K.; Qiu, L.; Munshi, N.C.; Tai, Y.T.; Anderson, K.C. Targeting CD38 Suppresses Induction and Function of T Regulatory Cells to Mitigate Immunosuppression in Multiple Myeloma. Clin Cancer Res 2017, 23, 4290-4300, doi:10.1158/1078-0432.CCR-16-3192.
- Jing, H.; Lin, H. Sirtuins in epigenetic regulation. Chem Rev 2015, 115, 2350-2375, doi:10.1021/cr500457h.
- Rifai, K.; Judes, G.; Idrissou, M.; Daures, M.; Bignon, Y.J.; Penault-Llorca, F.; Bernard-Gallon, D. SIRT1-dependent epigenetic regulation of H3 and H4 histone acetylation in human breast cancer. Oncotarget 2018, 9, 30661-30678, doi:10.18632/oncotarget.25771.
- Araki, Y.; Fann, M.; Wersto, R.; Weng, N.P. Histone acetylation facilitates rapid and robust memory CD8 T cell response through differential expression of effector molecules (eomesodermin and its targets: perforin and granzyme B). J Immunol 2008, 180, 8102-8108, doi:10.4049/jimmunol.180.12.8102.
- Katsuyama, E.; Suarez-Fueyo, A.; Bradley, S.J.; Mizui, M.; Marin, A.V.; Mulki, L.; Krishfield, S.; Malavasi, F.; Yoon, J.; Sui, S.J.H., et al. The CD38/NAD/SIRTUIN1/EZH2 Axis Mitigates Cytotoxic CD8 T Cell Function and Identifies Patients with SLE Prone to Infections. Cell Rep 2020, 30, 112-123 e114, doi:10.1016/j.celrep.2019.12.014.
- Tumes, D.J.; Onodera, A.; Suzuki, A.; Shinoda, K.; Endo, Y.; Iwamura, C.; Hosokawa, H.; Koseki, H.; Tokoyoda, K.; Suzuki, Y., et al. The polycomb protein Ezh2 regulates differentiation and plasticity of CD4(+) T helper type 1 and type 2 cells. Immunity 2013, 39, 819-832, doi:10.1016/j.immuni.2013.09.012.
- Yang, X.P.; Jiang, K.; Hirahara, K.; Vahedi, G.; Afzali, B.; Sciume, G.; Bonelli, M.; Sun, H.W.; Jankovic, D.; Kanno, Y., et al. EZH2 is crucial for both differentiation of regulatory T cells and T effector cell expansion. Sci Rep 2015, 5, 10643, doi:10.1038/srep10643.
- Xiao, X.Y.; Li, Y.T.; Jiang, X.; Ji, X.; Lu, X.; Yang, B.; Wu, L.J.; Wang, X.H.; Guo, J.B.; Zhao, L.D., et al. EZH2 deficiency attenuates Treg differentiation in rheumatoid arthritis. J Autoimmun 2020, 108, 102404, doi:10.1016/j.jaut.2020.102404.
- van Loosdregt, J.; Vercoulen, Y.; Guichelaar, T.; Gent, Y.Y.; Beekman, J.M.; van Beekum, O.; Brenkman, A.B.; Hijnen, D.J.; Mutis, T.; Kalkhoven, E., et al. Regulation of Treg functionality by acetylation-mediated Foxp3 protein stabilization. Blood 2010, 115, 965-974, doi:10.1182/blood-2009-02-207118.
- Beier, U.H.; Wang, L.; Bhatti, T.R.; Liu, Y.; Han, R.; Ge, G.; Hancock, W.W. Sirtuin-1 targeting promotes Foxp3+ T-regulatory cell function and prolongs allograft survival. Mol Cell Biol 2011, 31, 1022-1029, doi:10.1128/MCB.01206-10.