Hearing loss negatively impacts the well-being of millions of people worldwide. Systemic delivery of ototherapeutics has limited efficacy due to severe systemic side effects and the presence of the blood–labyrinth barrier that selectively limits or enables transfer of molecules between plasma and inner ear tissues and fluids. Local drug delivery into the middle and inner ear would be preferable for many newly emerging classes of drugs. Although the cochlea is a challenging target for drug delivery, recent technologies could provide a safe and efficacious delivery of ototherapeutics. Local drug delivery routes include topical delivery via the external auditory meatus, retroauricular, transtympanic, and intracochlear delivery.
- drug delivery
- blood–labyrinth barrier
- otoprotective therapeutics
- local delivery
- cochlea
1. Introduction
2. Anatomy
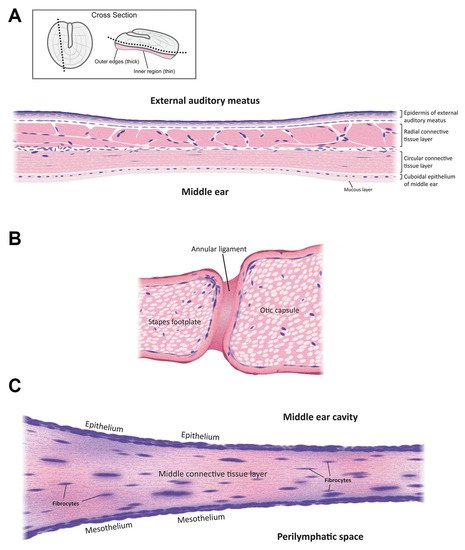
The mammalian ear is a complex sensory organ critical to hearing and maintaining balance (Figure 1). The outer ear includes an external auditory meatus (ear canal) that is slightly curved [7]. The outer and middle ear are separated by the semitransparent tympanic membrane that is thinnest in the center (50–70 µm) and thickest (~100–120 µm) near the peripheral rim [8]. This membrane is concave, with its deepest point projecting into the middle ear cavity. The outer and innermost layers of the tympanic membrane are extensions of the epidermis lining the external auditory meatus and the mucosal epithelial layer lining the middle ear cavity. The middle, extracellular layer of the tympanic membrane (Figure 2A) is formed of radial and circular connective tissue comprised of collagen and elastic fibers and innervated by several cranial nerves [9].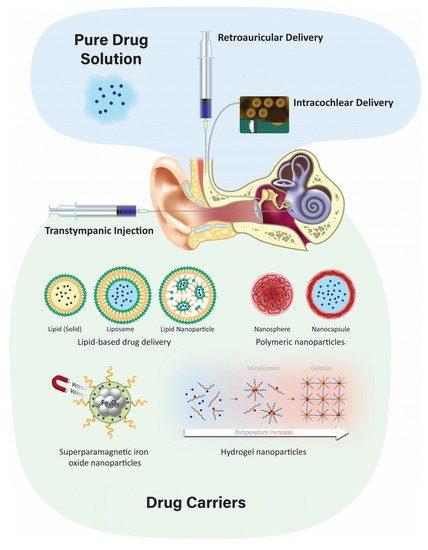
3. Delivery Routes
3.1. Systemic Delivery
Systemic delivery of therapeutics to the inner ear typically involves delivery via the vasculature and crossing the tight junction-coupled blood–labyrinth barrier (BLB). The BLB is fundamentally similar to the blood–brain barrier (BBB) and is well described elsewhere [24][17]. Paracellular flux across the BLB is not thought to occur under normal physiological conditions but may arise during inflammation, as for the BBB [25][18]. Drug transport across the BLB could involve similar mechanisms to that across the BBB [24,25,26][17][18][19]. These include (i) diffusion of lipophilic molecules (e.g., solvents) across cellular membranes; (ii) transcellular flux of hydrophilic drugs (e.g., aminoglycosides) via permeation of non-selective cation channels [27,28][20][21]; translocation via substrate transporters [29][22]; or transcytosis through the cell [26][19].
3.2. Topical Delivery via the External Auditory Meatus
Topical delivery of drugs to the ear canal is used to treat inflammation of the outer and middle ear. Antibacterial and antifungal ointments (or drops) are applied directly to the ear canal up to three times a day. The advantages of this administration route are that it is inexpensive, and it can be performed as an outpatient procedure or at home by the patient. There are a few disadvantages, including apparently limited therapeutic application, as it cannot currently be used for drug delivery to the inner ear as the tympanic and round window membranes are thought to act as barriers, as well as loss of drug from the middle ear via the Eustachian tube [32][23]. However, ototoxic drugs like gentamicin are not used clinically as ear drops as they can cause serious side effects such as hearing loss and tinnitus when used by patients with a perforated tympanic membrane [33][24], suggesting that topically-applied therapeutics can reach the inner ear. Moreover, concerns exist regarding the efficacy of topically-administered drugs against bacteria that have infected the middle ear and mastoid cavities [34][25].3.3. Transtympanic Delivery
It is a minimally-invasive injection that is typically performed in a clinic setting. The major advantage of transtympanic delivery is that it provides a potential direct route of administration to the inner ear. Typically, transtympanic delivery of drugs is injected onto the round window (and round window niche) and relies on the permeation of compounds through the RWM into the perilymphatic scala tympani in the cochlea. Permeation of the RWM is dependent on various factors, including the drug size, charge, lipophilicity, concentration, and formulation of the substances [18][16]. Following administration to the middle ear, local absorption takes place via the round window and the oval window into the cochlear perilymph and subsequent diffusion into the vestibular system. Previous studies have demonstrated that prolonging the contact duration of the drug formulation with the RWM can increase drug levels in the inner ear, but the pharmacokinetics for individual drugs remain complex to interpret [35][26]. Permeation of drugs across the round window membrane depends on passive diffusion and active transport [31][27]. Manipulations of the RWM have been shown to increase the entry of drugs following transtympanic administration. A variety of agents have been screened that are used to improve drug penetration in other biological systems for their capacity to increase entry into the inner ear. Some of these agents include benzyl alcohol, dimethyl sulfoxide, saponin, caprate, and Poloxamer 407. Benzyl alcohol is commonly used as a preservative in drug formulations and has been found to be the most effective permeabilizing agent [36][28].3.4. Retroauricular Delivery
Microcomputed tomography scanning of human temporal bone has revealed multiple large air-filled tuberculae, divided by bony septae. These air-filled spaces known as retroauricular microchannels are located behind the posterior wall of the ear canal and are rich in blood supply [40][29]. These microchannels have been utilized to deliver drugs to the middle or inner ear via parenteral retroauricular injections [41][30]. A study in a group of 20 healthy adults given a retroauricular injection of adrenaline found a significant drop in pressure in the middle ear cavity compared to the control group. It is thought that these microchannels enabled the adrenaline to enter the mastoid mucosa to induce vascular constriction and mucosal decongestion, leading to decreased middle ear pressure [42][31].3.5. Intracochlear Application
Intracochlear delivery bypasses the tympanic, oval, and round window membranes by infusing drugs directly into perilymph within the cochlea, presumptively providing greater control over drug concentrations within the cochlea [14][32]. Intracochlear injection through the RWM provides higher and more sustained drug levels compared to transtympanic injection onto the RWM [16][14]. However, the perforation of the RWM caused by the needle can release inner ear pressure, allowing cerebrospinal fluid (CSF) to enter the scala tympani via the cochlear aqueduct [45][33]. Other intracochlear drug delivery methods include infusion via an osmotic minipump, or a catheter built into the electrode array of a cochlear implant with an implantable peristaltic pump attached to it [18,46][16][34]. The osmotic pump is implanted subcutaneously with a cannula threaded into the middle ear cavity and inserted through the RWM or through the bone directly into the cochlea via a cochleostomy. These pumps can provide continuous infusion of drugs for up to 6 weeks [47][35]. Osmotic pump delivery of betamethasone to treat a vestibular disorder in guinea pigs showed a shorter duration of recovery than in controls of untreated with no osmotic pump and saline delivered through osmotic pump groups [48][36]. Osmotic pump delivery of an anti-apoptotic agent, Z-VAD-FMK, infused into the cochlea of guinea pigs for 14 days revealed less noise-induced hearing loss and lower hair cell loss compared to noise-exposed, untreated ears [49][37]. However, osmotic pumps are limited in that drug delivery cannot be stopped, nor can the flow rate be changed once the pump is started, and the volume of vehicle plus drug infused over time can exceed the volume of perilymph in the inner ear [47][35]. Recently, a micropump with a better automated control of drugs for intracochlear delivery at safe and slower flow rates without increasing the volume of perilymph in the cochlea has been described [50][38]. Surgical implants, including cochlear implants, can rehabilitate severe-to-profound sensorineural hearing loss. Recently, intracochlear controlled release drug delivery in combination with cochlear implants has been developed. This can improve the performance of cochlear implants by preventing fibrosis induced by insertion trauma, protecting the neuronal structures and by providing controlled drug release [51][39]. However, potential limitations include an enhanced risk of infection, particularly meningitis, associated with the surgery [52][40]; damage to the facial nerve; and loss of residual hearing. An implantable peristaltic pump connected to a cochlear implant electrode array has been developed for long-term delivery and effective dose-control in non-human primates (macaques). The infusion time ranged from 2–24 h to reach maximum peak concentrations, demonstrating feasibility [46][34].4. Localized Inner Ear Delivery Methods
Localized drug delivery to the cochlea has the advantages of targeted delivery with higher bioavailability and minimal systemic side effects. The ideal delivery system would be a carrier system that delivers pharmacotherapeutics across the intact tympanic, oval, and/or round window membranes to efficaciously treat inner ear disorders. Before a carrier system is chosen, advanced knowledge of the preferred drug and its physicochemical properties is required to determine the appropriate formulation and delivery method. In the field of drug discovery, Lipinski’s rule of five is frequently used to predict the absorption and solubility properties of a drug [53[41][42],54], and includes molecular weight, lipophilicity, polar surface area, hydrogen bonding, and charge [55][43]. These properties assist in the design and screening of new candidate drugs by predicting if a chemical compound has the appropriate bioavailability and pharmacokinetics. The rule states that ideal candidate drugs will have a logP ≤ 5 (the partition coefficient of a molecule between aqueous and lipophilic phases [usually water and octanol]), a molecular mass ≤500, ≤10 hydrogen bond acceptors, and ≤5 hydrogen bond donors. Molecules that fail to follow one or more of these rules may have difficulty with bioavailability [56][44]. Given the uncertainty of how drugs are metabolized in the inner ear, it is paramount to determine the physicochemical properties of candidate drugs for inner ear delivery. These may differ from the well-established Lipinski rule of five for systemic drug development. Thus far, much of our knowledge of these physicochemical properties for inner ear delivery are based on a few empirical studies of selected agents such as local anesthetics, corticosteroids (e.g., dexamethasone), monoclonal antibodies, growth factors, apoptosis inhibitors, and vectors for gene therapy under clinical investigation [57,58][45][46]. Many more studies are required to establish the optimal physicochemical properties for efficacious inner ear therapeutics, e.g., the ability to cross the blood–labyrinth barrier, the tympanic, oval, or round window membranes, as well as intracochlear epithelial barriers.4.1. Developing Different Injectable Solutions like Hydrogels
These hydrogels are fluid-like at room temperature and quickly gelate at body temperature to promote the sustained release of encapsulated drugs, increasing drug contact time with the RWM [61][47]. Hydrogels have numerous advantages, including increased biocompatibility, adjustable biodegradability, low toxicity, and good swelling behavior [62][48]. The degree of swelling is a critical parameter, with a higher concentration of polymers leading to greater swelling and slower drug release [63][49]. In the middle ear, hydrogels are typically injected near the RWM and RWM niche. This enables prolonged diffusion of the released drug across the RWM at appropriate therapeutic concentrations [64][50]. Hydrogels have been developed in several formulations for inner ear drug delivery, including polymers such as chitosan or PEG-based hydrogel, Poloxamer 407, and hyaluronic acid [65,66,67,68][51][52][53][54]. Injectable PEG-based hydrogel has been shown to be an effective and safe method for inner ear delivery. In guinea pigs, the dexamethasone concentrations in perilymph were maintained for at least 10 days for the PEG hydrogel as compared to the control sample of free dexamethasone [69][55]. Hyaluronic acid, when applied to the RWM of guinea pigs prior to delivering an adenovirus, provides an atraumatic and feasible method of delivering transgene into the inner ear [70][56].4.2. Poloxamer 407 and Its Mechanism
Poloxamer 407, also known as Pluronic® F-127, is the primary polymer for formulating hydrogels used for inner ear delivery of therapeutics [59][57]. Poloxamer 407 is an amphiphilic, non-ionic triblock copolymer consisting of a residue of polyoxypropylene (POP) between two units of polyoxyethylene (POE). It is a widely used thermo-sensitive hydrogel due to its non-irritating action on biological membranes and can remain as a gel for several weeks to months [71][58]. Its thermo-sensitivity is due to the hydrophobic interactions between the copolymer chains of Poloxamer 407. As temperature increases, copolymer chains of Poloxamer 407 aggregate to form a micellar structure due to the dehydration of hydrophobic poly(propylene oxide) units [72][59] with a micelle diameter in the 20–100 nm range. The hydrophobic core is the drug loading site, creating space for the encapsulation of drugs through chemical interactions. The properties of the inner and outer shell determine the rate of drug release. Different methods are employed for encapsulating drugs in Poloxamer 407, such as direct dissolution, evaporation, and freeze-drying [73][60]. The gelation of Poloxamer 407 is reversible once gelling conditions, such as temperature, pH, or chemical, are removed [62][48].4.3. Nanoparticulate Injection Systems
Nanoparticulate drug delivery is one of the most advanced technologies in drug design due to its advantages such as surface modification, improved drug solubility, stability, and bioavailability, as well as sustained controlled drug release at the target site. Injecting nanoparticulates at the targeted site leads to lower systemic toxicity, fewer side effects, improved kinetics of the drug, and extended drug bioavailability [77][61]. There are two primary nanoparticulate strategies: passive and self-delivery. In passive delivery, drugs are encapsulated in nanocarriers and are slowly released from the carriers. In self-delivery, drugs are conjugated to the carrier for easy delivery, and the drug dissociates from the carrier quickly at the presumptive targeted site, e.g., in the vicinity of tumors [78,79][62][63]. A large variety of nanoparticles have emerged, including polymeric, liposomes, and lipid-based structures, among others.
4.3.1. Polymeric Nanoparticles
Biodegradable polymeric microparticles or nanoparticles have been developed for a wide range of therapeutic applications and as inner ear drug delivery systems. They are often based on poly (lactic) co-glycolic acid (PLGA) or chitosan [6] and have advantages over other delivery systems due to their biocompatibility, biodegradability, small size, long shelf life, stability during storage, and highly reproducible formulation methods [59][57]. Nanoparticles have a diameter of <1 µm and are usually formulated with diameters of 100–300 nm, and for inner ear delivery, ~200 nm or less [4]. Polymeric nanoparticles can also incorporate visualization agents such as fluorescent dyes and MRI contrast agents [100][64]. Iron oxide nanoparticles have been extensively studied as a contrast agent for MRI. It has a magnetic core and different ligands focus on targeting specific sites or cells. PLGA is one of the more popular polymeric particles that can encapsulate both hydrophobic and hydrophilic drugs for intratympanic delivery and can be transported across the RWM into perilymph via the transcellular pathway [101][65].4.3.2. Solid Lipid Nanoparticles
Solid lipid nanoparticles (SLNs) are a novel class of stable nanoparticles that are particularly suitable for the encapsulation of hydrophobic drugs such as curcumin, resveratrol, or quercetin [102][66]. They can also act as a carrier for hydrophilic drugs when formulated without the use of organic solvents [103][67]. SLNs are composed of a hydrophobic triglyceride core with an amphiphilic surfactant shell [104][68]. SLNs are biodegradable, biocompatible, and are non-ototoxic over a wide dose range. Low doses of hydrocortisone encapsulated in SLNs increase their protective effect and prolong the survival of auditory cells treated with cisplatin in vitro [97][69]. In vivo application of SLNs has shown no interference in hearing threshold or loss of hair cells [105][70].4.3.3. Liposomes
Liposomes are composed of two layers of amphipathic molecules with a hydrophilic external layer and an internal surface composed of a phospholipid bilayer [106][71]. A key advantage of using liposomes for cochlear drug delivery is their ability to control drug release [96][72]. In an in vivo study, the microinjection of cationic liposome-mediated gene transfer into guinea pig cochleas revealed that transgene expression was steady for up to 14 days in the neurosensory epithelia and surrounding tissues without any toxicity [107][73]. Others have successfully demonstrated cell–gene delivery of therapeutic agents to the inner ear using a liposome-mediated delivery method [108][74].4.3.4. Superparamagnetic Iron Oxide Nanoparticles (SPION)
SPIONs are Fe3O4 particles that can be magnetically controlled to focus on the migration of particles into the inner ear after crossing the RWM [60][75]. These particles are encapsulated in a polymeric layer of PLGA [109][76] or chitosan [110][77] to contain the therapeutic agent. Other polymers such as polyacrylic acid and polyvinylpyrrolidone are used to form a coat around iron oxide nanoparticles to increase stability and improve their magnetic properties [111][78]. This technique has been demonstrated in vivo in rat and guinea pig models, as well as in vitro in cell lines and the human temporal bone. Biocompatibility and safety have been demonstrated by various methods, including hair cell survival in organotypic cell cultures [112][79].4.4. Advantages and Disadvantages of the Nanoparticulate Injection System
Nanoparticles are created from a variety of materials with a diameter range of 10 to several 100 nanometers, and customized to encapsulate various therapeutic agents [113][80]. Nanoparticles are widely used for non-invasive application, sustained, or controlled release of drugs, drug stabilization, and surface modification for targeting specific organs [114][81]. Various studies that administered nanoparticles onto the RWM have shown successful delivery of biomaterials into the inner ear [115][82]. Nanoparticles can enter the perilymph and the endolymph [106][71] following intratympanic administration and can be targeted to a specific intracochlear site of interest. Nanoparticles, when combined with hydrogel, improve the bioavailability of the drug at the targeted site and prevent rapid drug release [106][71]. Nanoparticles can also be conjugated to peptides that can penetrate cells, or their surface can be modified to increase their contact with RWM [116][83]. Challenges for nanoparticle delivery into the inner ear include limited access to the inner ear and poor uptake of drugs by inner ear cells [106][71].4.5. Positively-Charged Biomaterials for Local Drug Delivery
The charge of nanoparticles can determine their uptake by inner ear hair cells and their penetration of epithelial membranes. Phospholipid-based nanoparticles with a positive charge of +26 mV were taken up by sensory hair cells at a two-fold higher rate than nanoparticles with a neutral or negative charge. This is likely due to the interaction between positively-charged nanoparticles and negatively-charged lipid plasma membranes [119][84]. The addition of cationic polyethylene glycol (PEG) to phospholipid-based nanoparticles increases their trafficking across the RWM but has enhanced cytotoxicity [120][85]. Nanoparticulates containing cationic-PEG to deliver dexamethasone to the RWM in mice provide an anti-inflammatory effect during combined kanamycin and furosemide treatment and higher cellular uptake within the organ of Corti [99][86]. Positively-charged chitosan nanoparticles enter the inner ear at a faster rate [59][57]. Positively-charged chitosan nanoparticles containing D-glucosamine and N-acetyl-D-glucosamine facilitate penetration of lipid cell membranes in the inner ear [119][84]. Other positively-charged nanoparticles, such as 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP), are distributed widely in the inner ear after RWM application [14][32]. In rodents, positively-charged ferritin readily passes through RWM, while negatively-charged ferritin does not [13].4.6. Negatively-Charged Biomaterials for Local Drug Delivery
Negatively charged polymers have been frequently used for the preparation of nanoparticles due to their biocompatible properties. PLGA, an anionic polymer, is one of the most successful biodegradable systems for inner ear drug delivery [128][87]. PLGA with penetration enhancers, such as cell-penetrating peptides, has been used to investigate their impact on cochlear drug delivery in vivo [129][88]. PLGA nanoparticles coated with Poloxamer-407, with a zeta-potential of −15.90 mV, had a 1.6-fold higher distribution in the cochlea as compared to anionic PLGA nanoparticles coated with chitosan [128][87]. Negatively charged nanoparticles, with a surface charge of −22.1 mV, were administered intratympanically, diffused across the RWM, and distributed in the basal and middle cochlear turns when visualized by transmission electron microscopy [130][89]. Negatively-charged gelatin hydrogels composed of highly biocompatible polymers are used for controlled drug release, such as insulin-like growth factor 1 to the inner ear after noise-induced hearing loss in guinea pigs, resulting in increased outer hair cell survival [131][90]. Zeolitic imidazole nanoparticles have great potential to deliver drugs, proteins, and RNA to the inner ear for the treatment of noise-induced hearing loss. These nanoparticles are anionic carriers with superior cell viability and biocompatibility [132][91].5. Conclusions
Local delivery has the advantage of maximizing targeted effects in the inner ear while minimizing systemic toxicity. Intratympanic injections are a relatively minimally invasive procedure. Future research to enhance permeation through the RWM and methods to increase the release duration of therapeutic agents from biomaterials may provide higher and more sustained concentrations of the drug in the cochlea following intratympanic administration. The use of nanoparticles encapsulating therapeutic agents that can target the sensory hair cells in the inner ear is innovative and exciting. Currently, there are no FDA-approved drugs or licensed therapies on the market for hearing loss due to ototoxicity. As the field of inner ear therapeutics evolves, drug delivery strategies must recognize the relationships between therapeutic agents, formulations, delivery systems, and the disease. Treatment options for hearing loss will undeniably be further refined and optimized in the coming years as new therapeutics become available. Future research is needed to identify new mechanisms of action and delivery that will enable exciting novel treatments for inner ear disorders.References
- Bernabei, R.; Bonuccelli, U.; Maggi, S.; Marengoni, A.; Martini, A.; Memo, M.; Pecorelli, S.; Peracino, A.P.; Quaranta, N.; Stella, R.; et al. Hearing loss and cognitive decline in older adults: Questions and answers. Aging Clin. Exp. Res. 2014, 26, 567–573.
- Daniel, E. Noise and hearing loss: A review. J. Sch. Health 2007, 77, 225–231.
- Lin, F.R.; Yaffe, K.; Xia, J.; Xue, Q.L.; Harris, T.B.; Purchase-Helzner, E.; Satterfield, S.; Ayonayon, H.N.; Ferrucci, L.; Simonsick, E.M. Hearing Loss and Cognitive Decline in Older Adults. JAMA Intern. Med. 2013, 173, 293–299.
- McCall, A.A.; Swan, E.E.L.; Borenstein, J.T.; Sewell, W.F.; Kujawa, S.G.; McKenna, M.J. Drug delivery for treatment of inner ear disease: Current state of knowledge. Ear Hear. 2010, 31, 156–165.
- Kros, C.J.; Steyger, P.S. Aminoglycoside- and Cisplatin-Induced Ototoxicity: Mechanisms and Otoprotective Strategies. Cold Spring Harb. Perspect. Med. 2019, 9, a033548.
- Swan, E.E.L.; Mescher, M.J.; Sewell, W.F.; Tao, S.L.; Borenstein, J.T. Inner ear drug delivery for auditory applications. Adv. Drug Deliv. Rev. 2008, 60, 1583–1599.
- Agrahari, V.; Agrahari, V.; Mitra, A.K. Inner ear targeted drug delivery: What does the future hold? Ther. Deliv. 2017, 8, 179–184.
- Van der Jeught, S.; Dirckx, J.J.J.; Aerts, J.R.M.; Bradu, A.; Podoleanu, A.G.; Buytaert, J.A.N. Full-Field Thickness Distribution of Human Tympanic Membrane Obtained with Optical Coherence Tomography. J. Assoc. Res. Otolaryngol. 2013, 14, 483.
- Szymanski, A.; Toth, J.; Ogorevc, M.; Geiger, Z. Anatomy, Head and Neck, Ear Tympanic Membrane; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK448117/ (accessed on 30 July 2022).
- Gyo, K.; Aritomo, H.; Goode, R.L. Measurement of the ossicular vibration ratio in human temporal bones by use of a video measuring system. Acta Oto Laryngol. 1987, 103, 87–95.
- Zdilla, M.J.; Skrzat, J.; Kozerska, M.; Leszczyński, B.; Tarasiuk, J.; Wroński, S. Oval window size and shape: A micro-CT anatomical study with considerations for stapes surgery. Otol. Neurotol. 2018, 39, 558.
- Mancheño, M.; Aristegui, M.; Sañudo, J.R. Round and Oval Window Anatomic Variability: Its Implication for the Vibroplasty Technique. Otol. Neurotol. 2017, 38, e50–e57.
- Goycoolea, M.V.; Lundman, L. Round window membrane. Structure function and permeability: A review. Microsc. Res. Tech. 1997, 36, 201–211. Available online: https://analyticalsciencejournals.onlinelibrary.wiley.com/doi/10.1002/(SICI)1097-0029(19970201)36:3%3C201::AID-JEMT8%3E3.0.CO;2-R (accessed on 23 September 2021).
- Szeto, B.; Chiang, H.; Valentini, C.; Yu, M.; Kysar, J.W.; Lalwani, A.K. Inner ear delivery: Challenges and opportunities. Laryngoscope Investig. Otolaryngol. 2020, 5, 122.
- Ren, Y.; Landegger, L.D.; Stankovic, K.M. Gene therapy for human sensorineural hearing loss. Front. Cell. Neurosci. 2019, 13, 323.
- Peppi, M.; Marie, A.; Belline, C.; Borenstein, J.T. Intracochlear drug delivery systems: A novel approach whose time has come. Expert Opin. Drug Deliv. 2018, 15, 319–324.
- Nyberg, S.; Joan Abbott, N.; Shi, X.; Steyger, P.S.; Dabdoub, A. Delivery of therapeutics to the inner ear: The challenge of the blood-labyrinth barrier. Sci. Transl. Med. 2019, 11, eaao0935.
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte—Endothelial interactions at the blood–brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53.
- Koo, J.W.; Quintanilla-Dieck, L.; Jiang, M.; Liu, J.; Urdang, Z.D.; Allensworth, J.J.; Cross, C.P.; Li, H.; Steyger, P.S. Endotoxemia-mediated inflammation potentiates aminoglycoside-induced ototoxicity. Sci. Transl. Med. 2015, 7, 298ra118.
- Marcotti, W.; van Netten, S.M.; Kros, C.J. The aminoglycoside antibiotic dihydrostreptomycin rapidly enters mouse outer hair cells through the mechano-electrical transducer channels. J. Physiol. 2005, 567, 505.
- Karasawa, T.; Wang, Q.; Fu, Y.; Cohen, D.M.; Steyger, P.S. TRPV4 enhances the cellular uptake of aminoglycoside antibiotics. J. Cell Sci. 2008, 121, 2871–2879.
- Jiang, M.; Wang, Q.; Karasawa, T.; Koo, J.W.; Li, H.; Steyger, P.S. Sodium-Glucose Transporter-2 (SGLT2; SLC5A2) Enhances Cellular Uptake of Aminoglycosides. PLoS ONE 2014, 9, e108941.
- Hoskison, E.; Daniel, M.; Al-Zahid, S.; Shakesheff, K.M.; Bayston, R.; Birchall, J.P. Drug delivery to the ear. Ther. Deliv. 2013, 4, 115–124.
- Wooltorton, E. Health and Drug Alerts: Ototoxic effects from gentamicin ear drops. Can. Med. Assoc. J. 2002, 167, 56. Available online: https://pmc/articles/PMC116645/ (accessed on 24 April 2022).
- Macfadyen, C.A.; Acuin, J.M.; Gamble, C.L. Topical antibiotics without steroids for chronically discharging ears with underlying eardrum perforations. Cochrane Database Syst. Rev. 2005, 2005, CD004618.
- Liu, H.; Feng, L.; Tolia, G.; Liddell, M.R.; Hao, J.; Li, S.K. Evaluation of intratympanic formulations for inner ear delivery: Methodology and sustained release formulation testing. Drug Dev. Ind. Pharm. 2014, 40, 896.
- Piu, F.; Bishop, K.M. Local drug delivery for the treatment of neurotology disorders. Front. Cell. Neurosci. 2019, 13, 238.
- Li, W.; Hartsock, J.J.; Dai, C.; Salt, A.N. Permeation Enhancers for Intratympanically-Applied Drugs studied using Fluorescent Dexamethasone as a Marker. Otol. Neurotol. 2018, 39, 639.
- Cros, O.; Borga, M.; Pauwels, E.; Dirckx, J.J.J.; Gaihede, M. Micro-channels in the mastoid anatomy. Indications of a separate blood supply of the air cell system mucosa by micro-CT scanning. Hear. Res. 2013, 301, 60–65.
- Gaihede, M. Treatment of Otitis Media with Retroauricular Steroid Injection—Aalborg University’s Research Portal. 2015. Available online: https://vbn.aau.dk/en/publications/treatment-of-otitis-media-with-retroauricular-steroid-injection (accessed on 21 July 2021).
- Fooken Jensen, P.V.; Gaihede, M. Congestion of mastoid mucosa and influence on middle ear pressure—Effect of retroauricular injection of adrenaline. Hear. Res. 2016, 340, 121–126.
- Liu, H.; Hao, J.; Li, K.S. Current strategies for drug delivery to the inner ear. Acta Pharm. Sin. B 2013, 3, 86–96.
- Plontke, S.K.; Hartsock, J.J.; Gill, R.M.; Salt, A.N. Intracochlear Drug Injections through the Round Window Membrane: Measures to Improve Drug Retention. Audiol. Neurotol. 2016, 21, 72–79.
- Manrique-Huarte, R.; de Linera-Alperi, M.A.; Parilli, D.; Rodriguez, J.A.; Borro, D.; Dueck, W.F.; Smyth, D.; Salt, A.; Manrique, M. Inner ear drug delivery through a cochlear implant: Pharmacokinetics in a Macaque experimental model. Hear. Res. 2021, 404, 108228.
- Pararas, E.E.L.; Borkholder, D.A.; Borenstein, J.T. Microsystems Technologies for Drug Delivery to the Inner Ear. Adv. Drug Deliv. Rev. 2012, 64, 1650.
- Shimogori, H.; Yamashita, H. Efficacy of intracochlear administration of betamethasone on peripheral vestibular disorder in the guinea pig. Neurosci. Lett. 2000, 294, 21–24.
- Abaamrane, L.; Raffin, F.; Schmerber, S.; Sendowski, I. Intracochlear perfusion of leupeptin and z-VAD-FMK: Influence of antiapoptotic agents on gunshot-induced hearing loss. Eur. Arch. Oto-Rhino-Laryngol. 2011, 268, 987–993.
- Tandon, V.; Kang, W.S.; Robbins, T.A.; Spencer, A.J.; Kim, E.S.; McKenna, M.J.; Kujawa, S.G.; Fiering, J.; Pararas, E.E.L.; Mescher, M.J.; et al. Microfabricated reciprocating micropump for intracochlear drug delivery with integrated drug/fluid storage and electronically controlled dosing. Lab Chip 2016, 16, 829–846.
- Plontke, S.K.; Götze, G.; Rahne, T.; Liebau, A. Intracochlear drug delivery in combination with cochlear implants: Current aspects. HNO 2017, 65 (Suppl. 1), 19–28.
- Boisvert, I.; Reis, M.; Au, A.; Cowan, R.; Dowell, R.C. Cochlear implantation outcomes in adults: A scoping review. PLoS ONE 2020, 15, e0232421.
- Benet, L.Z.; Hosey, C.M.; Ursu, O.; Oprea, T.I. BDDCS, the Rule of 5 and drugability. Adv. Drug Deliv. Rev. 2016, 101, 89–98.
- Barich, D.H.; Zell, M.T.; Munson, E.J. Physicochemical properties, formulation, and drug delivery. In Drug Delivery: Principles and Applications, 2nd ed.; Wang, B., Siahaan, T.J., Soltero, R., Eds.; John Wiley and Sons: Hoboken, NJ, USA, 2016; pp. 35–48.
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26.
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014, 21, 1115–1142.
- Hao, J.; Li, S.K. Inner ear drug delivery: Recent advances, challenges, and perspective. Eur. J. Pharm. Sci. 2019, 126, 82–92.
- Kanzaki, S. Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance. Molecules 2018, 23, 2507.
- Dumortier, G.; Grossiord, J.L.; Agnely, F.; Chaumeil, J.C. A review of poloxamer 407 pharmaceutical and pharmacological characteristics. Pharm. Res. 2006, 23, 2709–2728.
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels 2017, 3, 6.
- Fariba, G.; Farahani, S.V. Theoretical Description of Hydrogel Swelling: A Review. Iran. Polym. J. 2020, 19, 375–398. Available online: https://www.sid.ir/en/Journal/ViewPaper.aspx?ID=171784 (accessed on 30 July 2022).
- El Kechai, N.; Agnely, F.; Mamelle, E.; Nguyen, Y.; Ferrary, E.; Bochot, A. Recent advances in local drug delivery to the inner ear. Int. J. Pharm. 2015, 494, 83–101.
- Lajud, S.A.; Nagda, D.A.; Qiao, P.; Tanaka, N.; Civantos, A.; Gu, R.; Cheng, Z.; Tsourkas, A.; O’Malley, B.W.; Li, D. A Novel Chitosan-Hydrogel-Based Nanoparticle Delivery System for Local Inner Ear Application. Otol. Neurotol. 2015, 36, 341.
- Hütten, M.; Dhanasingh, A.; Hessler, R.; Stöver, T.; Esser, K.H.; Möller, M.; Lenarz, T.; Jolly, C.; Groll, J.; Scheper, V. In Vitro and In Vivo Evaluation of a Hydrogel Reservoir as a Continuous Drug Delivery System for Inner Ear Treatment. PLoS ONE 2014, 9, e104564.
- Gausterer, J.C.; Saidov, N.; Ahmadi, N.; Zhu, C.; Wirth, M.; Reznicek, G.; Arnoldner, C.; Gabor, F.; Honeder, C. Intratympanic application of poloxamer 407 hydrogels results in sustained N-acetylcysteine delivery to the inner ear. Eur. J. Pharm. Biopharm. 2020, 150, 143–155.
- Borden, R.C.; Saunders, J.E.; Berryhill, W.E.; Krempl, G.A.; Thompson, D.M.; Queimado, L. Hyaluronic Acid Hydrogel Sustains the Delivery of Dexamethasone across the Round Window Membrane. Audiol. Neurotol. 2011, 16, 1–11.
- Yu, D.; Sun, C.; Zheng, Z.; Wang, X.; Chen, D.; Wu, H.; Wang, X.; Shi, F. Inner ear delivery of dexamethasone using injectable silk-polyethylene glycol (PEG) hydrogel. Int. J. Pharm. 2016, 503, 229–237.
- Shibata, S.B.; Cortez, S.R.; Wiler, J.A.; Swiderski, D.L.; Raphael, Y. Hyaluronic Acid Enhances Gene Delivery into the Cochlea. Hum. Gene Ther. 2012, 23, 302.
- Rathnam, C.; Chueng, S.T.D.; Ying YL, M.; Lee, K.B.; Kwan, K. Developments in Bio-Inspired Nanomaterials for Therapeutic Delivery to Treat Hearing Loss. Front. Cell. Neurosci. 2019, 13, 493.
- Giuliano, E.; Paolino, D.; Fresta, M.; Cosco, D. Mucosal Applications of Poloxamer 407-Based Hydrogels: An Overview. Pharmaceutics 2018, 10, 159.
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390.
- Russo, E.; Villa, C. Poloxamer Hydrogels for Biomedical Applications. Pharmaceutics 2019, 11, 671.
- Mirza, A.Z.; Siddiqui, F.A.; Mirza, A.Z.; Siddiqui, F.A. Nanomedicine and drug delivery: A mini review. Int. Nano Lett. 2014, 4, 94.
- Lu, H.; Wang, J.; Wang, T.; Zhong, J.; Bao, Y.; Hao, H. Recent Progress on Nanostructures for Drug Delivery Applications. J. Nanomater. 2016, 2016, 5762431.
- Seymour, L.W.; Ulbrich, K.; Steyger, P.S.; Brereton, M.; Subr, V.; Strohalm, J.; Duncan, R. Tumour tropism and anti-cancer efficacy of polymer-based doxorubicin prodrugs in the treatment of subcutaneous murine B16F10 melanoma. Br. J. Cancer 1994, 70, 636.
- Pyykko, I.; Zou, J.; Zhang, Y.; Zhang, W.; Feng, H.; Kinnunen, P. Nanoparticle based inner ear therapy. World J. Otorhinolaryngol. 2013, 3, 114–133.
- Zhang, L.; Xu, Y.; Cao, W.; Xie, S.; Wen, L.; Chen, G. Understanding the translocation mechanism of PLGA nanoparticles across round window membrane into the inner ear: A guideline for inner ear drug delivery based on nanomedicine. Int. J. Nanomed. 2018, 13, 479.
- Scioli Montoto, S.; Muraca, G.; Ruiz, M.E. Solid Lipid Nanoparticles for Drug Delivery: Pharmacological and Biopharmaceutical Aspects. Front. Mol. Biosci. 2020, 7, 587997.
- Huynh, N.T.; Passirani, C.; Saulnier, P.; Benoit, J.P. Lipid nanocapsules: A new platform for nanomedicine. Int. J. Pharm. 2009, 379, 201–209.
- Li, L.; Chao, T.; Brant, J.; O’Malley, B.; Tsourkas, A.; Li, D. Advances in Nano-based Inner Ear Delivery Systems for the Treatment of Sensorineural Hearing Loss. Adv. Drug Deliv. Rev. 2017, 108, 2.
- Cervantes, B.; Arana, L.; Murillo-Cuesta, S.; Bruno, M.; Alkorta, I.; Varela-Nieto, I. Solid Lipid Nanoparticles Loaded with Glucocorticoids Protect Auditory Cells from Cisplatin-Induced Ototoxicity. J. Clin. Med. 2019, 8, 1464.
- Scheper, V.; Wolf, M.; Scholl, M.; Kadlecova, Z.; Perrier, T.; Klok, H.A.; Saulnier, P.; Lenarz, T.; Stöver, T. Potential novel drug carriers for inner ear treatment: Hyperbranched polylysine and lipid nanocapsules. Nanomedicine 2009, 4, 623–635.
- Mittal, R.; Pena, S.A.; Zhu, A.; Eshraghi, N.; Fesharaki, A.; Horesh, E.J.; Mittal, J.; Eshraghi, A.A. Nanoparticle-based drug delivery in the inner ear: Current challenges, limitations and opportunities. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1312–1320.
- Gao, W.; Zhang, Y.; Zhang, Q.; Zhang, L. Nanoparticle-Hydrogel: A Hybrid Biomaterial System for Localized Drug Delivery. Ann. Biomed. Eng. 2016, 44, 2049.
- Wareing, M.; Mhatre, A.N.; Pettis, R.; Han, J.J.; Haut, T.; Pfister, M.H.F.; Hong, K.; Zheng, W.W.; Lalwani, A.K. Cationic liposome mediated transgene expression in the guinea pig cochlea. Hear. Res. 1999, 128, 61–69.
- Okano, T.; Nakagawa, T.; Kita, T.; Endo, T.; Ito, J. Cell-gene delivery of brain-derived neurotrophic factor to the mouse inner ear. Mol. Ther. 2006, 14, 866–871.
- Patel, J.; Szczupak, M.; Rajguru, S.; Balaban, C.; Hoffer, M.E. Inner ear therapeutics: An overview of middle ear delivery. Front. Cell. Neurosci. 2019, 13, 261.
- Ge, X.; Jackson, R.L.; Liu, J.; Harper, E.A.; Hoffer, M.E.; Wassel, R.A.; Dormer, K.J.; Kopke, R.D.; Balough, B.J. Distribution of PLGA nanoparticles in chinchilla cochleae. Otolaryngol. Head Neck Surg. 2007, 137, 619–623.
- Shimoji, M.; Ramaswamy, B.; Shukoor, M.I.; Benhal, P.; Broda, A.; Kulkarni, S.; Malik, P.; McCaffrey, B.; Lafond, J.F.; Nacev, A.; et al. (2019). Toxicology study for magnetic injection of prednisolone into the rat cochlea. Eur. J. Pharm. Sci. 2019, 126, 33–48.
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 40.
- Kopke, R.D.; Wassel, R.A.; Mondalek, F.; Grady, B.; Chen, K.; Liu, J.; Gibson, D.; Dormer, K.J. Magnetic Nanoparticles: Inner Ear Targeted Molecule Delivery and Middle Ear Implant. Audiol. Neurotol. 2006, 11, 123–133.
- Chen, G.; Zhang, X.; Yang, F.; Mu, L. Disposition of nanoparticle-based delivery system via inner ear administration. Curr. Drug Metab. 2010, 11, 886–897.
- Buckiová, D.; Ranjan, S.; Newman, T.A.; Johnston, A.H.; Sood, R.; Kinnunen, P.K.J.; Popelář, J.; Chumak, T.; Syka, J. Minimally invasive drug delivery to the cochlea through application of nanoparticles to the round window membrane. Nanomedicine 2012, 7, 1339–1354.
- Zou, J.; Saulnier, P.; Perrier, T.; Zhang, Y.; Manninen, T.; Toppila, E.; Pyykkö, I. Distribution of lipid nanocapsules in different cochlear cell populations after round window membrane permeation. J. Biomed. Mater. Res. Part B Appl. Biomater. 2008, 87, 10–18.
- Wen, X.; Ding, S.; Cai, H.; Wang, J.; Wen, L.; Yang, F.; Chen, G. Nanomedicine strategy for optimizing delivery to outer hair cells by surface-modified poly(lactic/glycolic acid) nanoparticles with hydrophilic molecules. Int. J. Nanomed. 2016, 11, 5959–5969.
- Lee, J.J.; Jang, J.H.; Choo, O.S.; Lim, H.J.; Choung, Y.H. Steroid intracochlear distribution differs by administration method: Systemic versus intratympanic injection. Laryngoscope 2018, 128, 189–194.
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591.
- Yang, K.J.; Son, J.; Jung, S.Y.; Yi, G.; Yoo, J.; Kim, D.K.; Koo, H. Optimized phospholipid-based nanoparticles for inner ear drug delivery and therapy. Biomaterials 2018, 171, 133–143.
- Cai, H.; Liang, Z.; Huang, W.; Wen, L.; Chen, G. Engineering PLGA nano-based systems through understanding the influence of nanoparticle properties and cell-penetrating peptides for cochlear drug delivery. Int. J. Pharm. 2017, 532, 55–65.
- Dash-Wagh, S.; Jacob, S.; Lindberg, S.; Fridberger, A.; Langel, Ü.; Ulfendahl, M. Intracellular Delivery of Short Interfering RNA in Rat Organ of Corti Using a Cell-penetrating Peptide PepFect6. Mol. Ther. Nucleic Acids 2012, 1, e61.
- Youm, I.; Musazzi, U.M.; Gratton, M.A.; Murowchick, J.B.; Youan, B.B.C. Label-Free Ferrocene-Loaded Nanocarrier Engineering for In Vivo Cochlear Drug Delivery and Imaging. J. Pharm. Sci. 2016, 105, 3162–3171.
- Iwai, K.; Nakagawa, T.; Endo, T.; Matsuoka, Y.; Kita, T.; Kim, T.S.; Tabata, Y.; Ito, J. Cochlear protection by local insulin-like growth factor-1 application using biodegradable hydrogel. Laryngoscope 2006, 116, 529–533.
- Xu, X.; Lin, K.; Wang, Y.; Xu, K.; Sun, Y.; Yang, X.; Yang, M.; He, Z.; Zhang, Y.; Zheng, H.; et al. A metal–Organic framework based inner ear delivery system for the treatment of noise-induced hearing loss. Nanoscale 2020, 12, 16359–16365.
