Lignin, being a natural aromatic polymer rich in functional hydroxyl groups, has been drawing the interest of academia and industry for its valorization, especially for the development of polymeric materials. Among the different types of polymers that can be derived from lignin, polyurethanes (PUs) are amid the most important ones, especially due to their wide range of applications. Lignin, being a natural aromatic polymer rich in functional hydroxyl groups, has been drawing the interest of academia and industry for its valorization, especially for the development of polymeric materials. Among the different types of polymers that can be derived from lignin, polyurethanes (PUs) are amid the most important ones, especially due to their wide range of applications.
- lignin
- polyols
- polyurethanes
1. Introduction
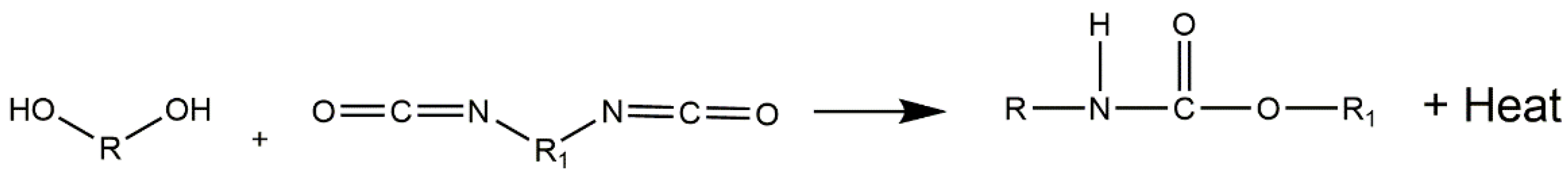
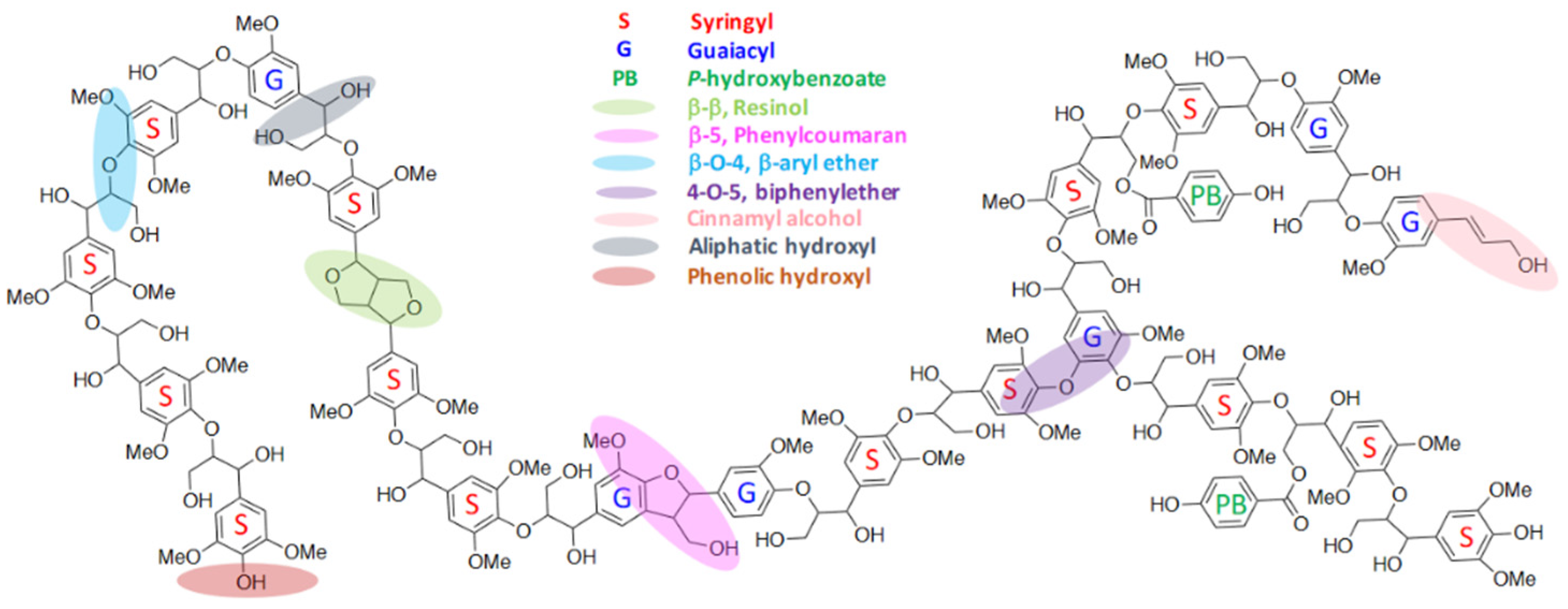
2. Lignin and Its Structural Features
3. Technical Lignins
Lignin can be isolated from different plants, but also from chemical pulping streams, which results in a technical lignin. The major production of industrial lignin comes from the pulp and paper industry, where most of the side streams are in the form of black liquor (annually, around 50–70 million tons are produced) which is mainly burned to supply internal energy and pulping reagents [16]. Commercially available technical lignins are often kraft, soda, and lignosulfonates associated with the corresponding industrial processes for wood delignification and cellulosic pulp production, e.g., kraft (using NaOH and Na2S), soda (using NaOH), and sulfite (using aqueous sulfur dioxide), respectively. Another technical lignin is organosolv lignin, obtained by the delignification of wood using a mixture of water and organic solvents (mainly ethanol) with or without catalysts. The native lignin structure changes minimally during the organosolv process and the organosolv lignin is a good option for further functionalization/modification. Yet, this process is not in industrial use, because of technical concerns, extensive corrosion of the equipment, high energy consumption due to the solvents recovery process, and the lower quality of the pulp produced when compared to the kraft process [17][18]. Amid industrial processes, the kraft process is the most widely used (more than 90% of mills). After the kraft pulping, the black liquor is obtained (14–18% of solids), which contains mainly lignin as well as other chemical species, such as carboxylic acids and inorganics salts [19]. The black liquor is usually concentrated to 70–80% in a series of evaporation effects and burned in a recovery boiler to recover inorganic reagents and to produce steam and electricity. However, in most cases, the amount of black liquor exceeds the design limits of the recovery boiler, which represents the “bottleneck” of the process. A convenient way that can minimize this “bottleneck” is the acidification of black liquor. This leads to the precipitation of lignin from the black liquor which can subsequently be used for higher-value-added applications. Regarding the technologies available to separate the kraft lignin from black liquor by acidification, the techniques are based on changing the solubility of lignin or fractioning [19][20][21]. The MeadWestvaco Corporation Company was one of the first to carry out the precipitation of kraft lignin using sulfuric acid at pH 2–3; this technical lignin is marketed as Indulin™. Kraft lignin obtained by acidification using mineral acids has noticeable amounts of ash, sulfur, and sugars, and the yield can be affected by high filtration resistance [19]. To avoid these constraints, Inventia company and Chalmers Technical University jointly developed a lignin extraction process based on the precipitation with CO2 and acid, named the LignoBoost® process (process owned by Metso corporation). First, the black liquor is acidified with CO2 at pH 9.0–10.5, precipitating the lignin. After flocculation, diluted sulfuric acid is added. In the last step, the lignin is isolated by filtration [21]. Presently, the kraft lignin isolated by the LignoBoost® process is commercialized by the Domtar company and marketed as BioChoice lignin. Recently, Stora Enso company is also producing LignoBoost lignin at 50,000 tons per year [22]. Although the LignoBoost® process provides lignin with high purity and solves the problem of filtration resistance that occurs with the acidification using strong acids, concerns about sulfur compounds (hydrogen sulfide, methyl mercaptan, dimethyl sulfide), that are malodorous and are hazardous to the health of humans, remain. FPInnovations group developed, in collaboration with NORAM Engineering company, the LignoForceTM process [23]. In this approach, before the acidification of black liquor with CO2, the liquor is oxidized with O2 until the sulfide concentration is reduced to a specific level and then follows the acidification and filtration steps, as illustrated in Figure 4 [23].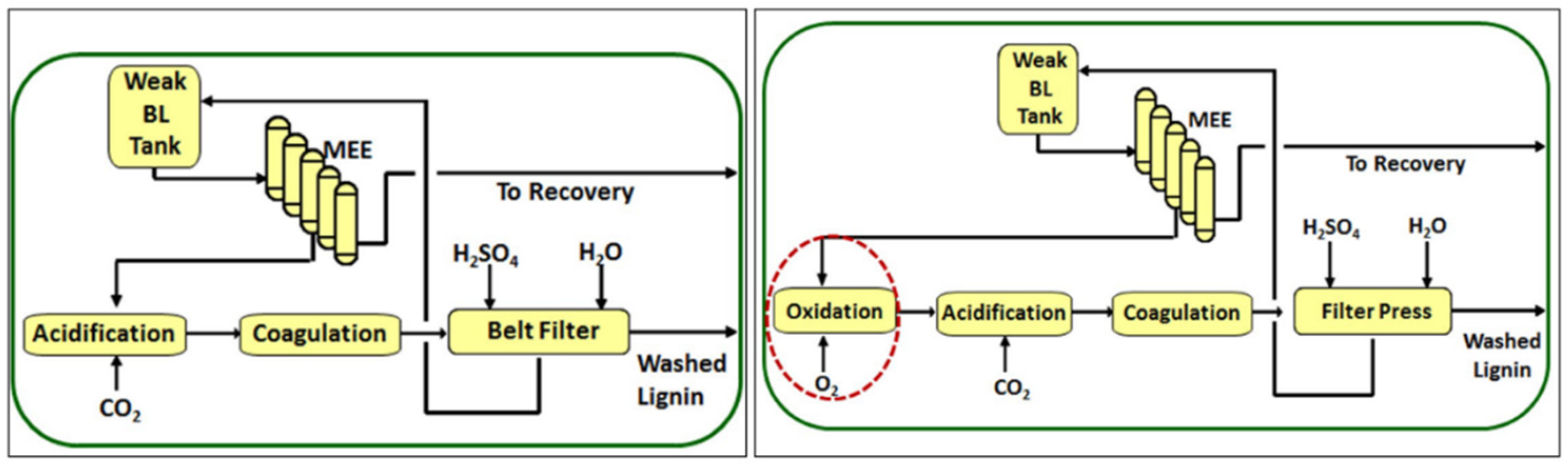
4. Synthesis of Lignin-Based Polyether Polyols
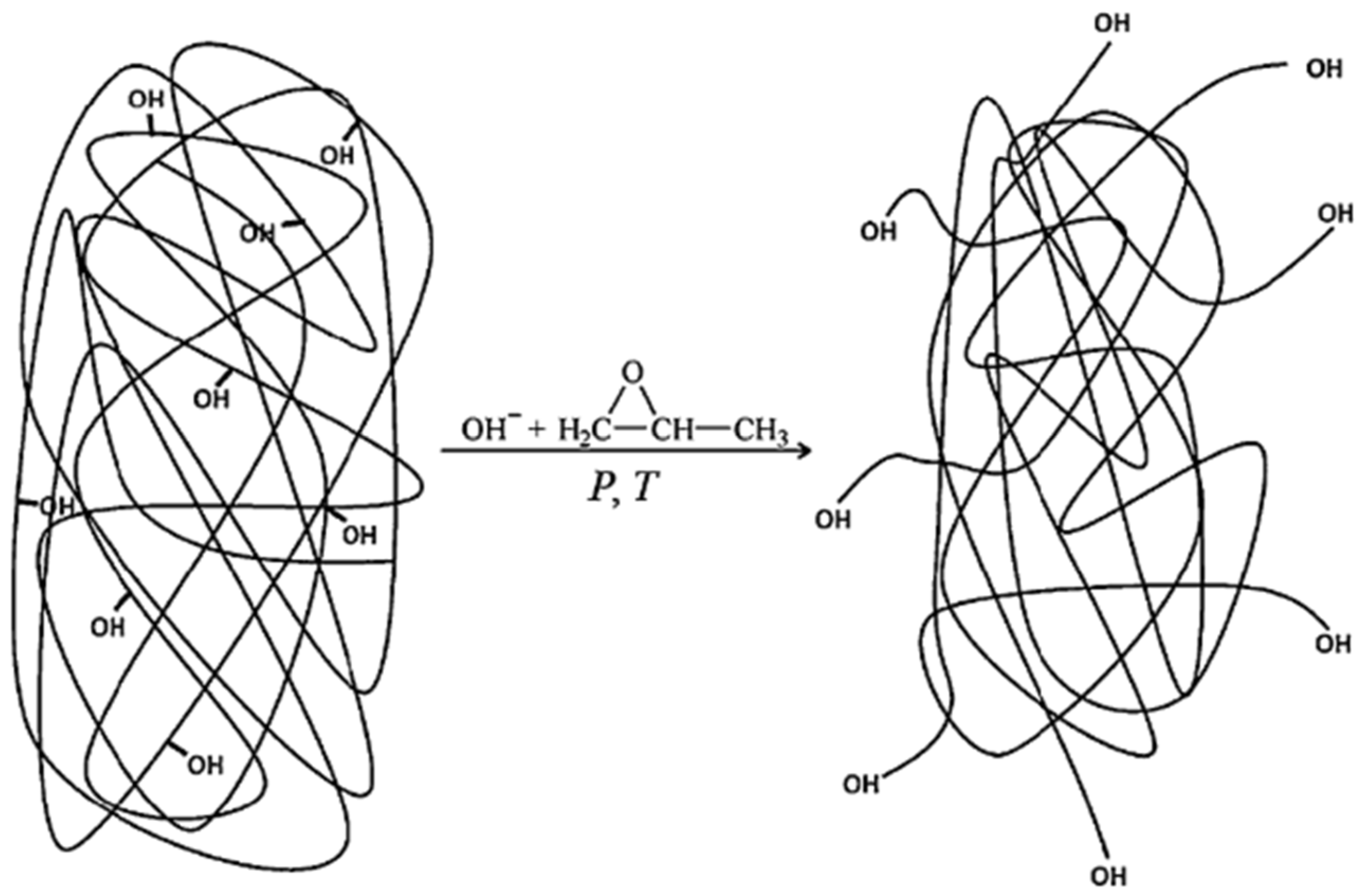
4.1. Lignin-Based Polyol via Oxyalkylation Reaction
4.2. Lignin-Based Polyol via Liquefaction with a Polyhydric Alcohol
4.3. Quality of Lignin-Based Polyol and Its Characterization
For petroleum-derived polyols used in the preparation of polyurethanes, the main characteristics that are normally quantified are the content of hydroxyl groups (hydroxyl number, IOH), the acid number of polyol, water content, viscosity, and molecular weight as they have a direct impact on the formulations. The required values depend on the type of PU to be produced, i.e., foams, films, or adhesives [3]. Figure 6 schematically illustrates these main characteristics.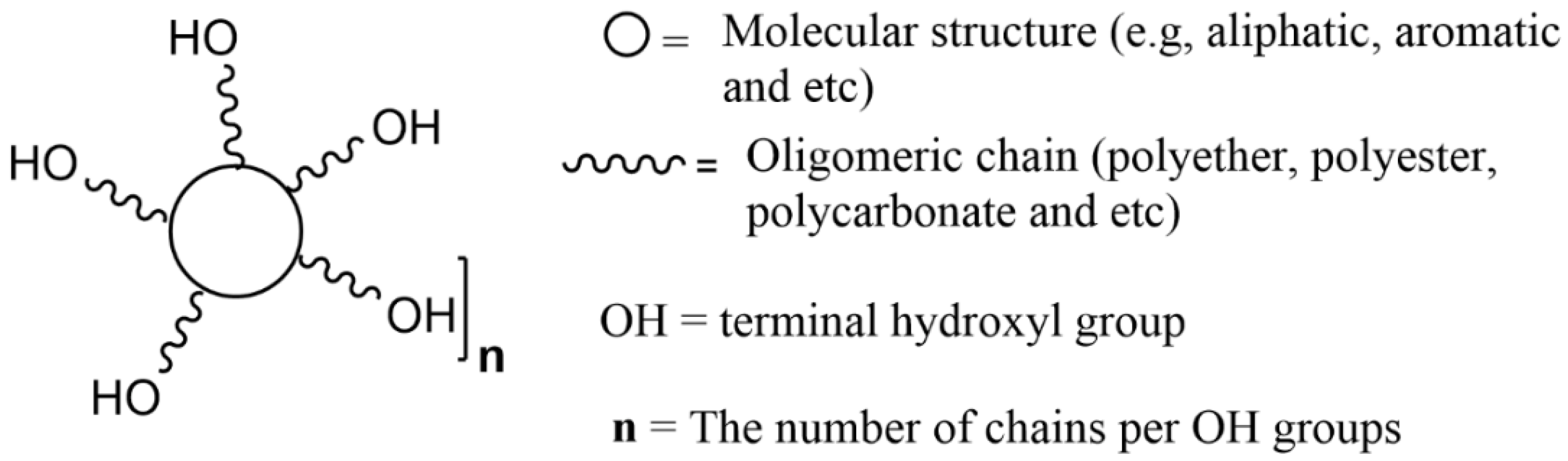
5. Lignin as a Building Block to Synthesize Polyurethanes
The use of lignin in the production of PUs can be carried out in different ways: unmodified, being directly incorporated into polyol formulations, after fractionation, or after chemical modification (in order to make it more reactive), alone, or in combination with other polyols [24][25][55][56][57][58][59][60]. Depending on its interaction with the isocyanate, lignin can act as filler or as reagent, i.e., as polyol, also referred to as cross-linker. Although the use of lignin without any treatment is widely reported, if its OH groups do not react with the isocyanate and become chemically bonded to the PU network, it should not be described as a building block. The direct exploitation of lignin, as a polyol on its own or blended with industrial polyols, is energetically and environmentally advantageous [61], and the ensuing biomass-based PUs are more biodegradable than those derived from petroleum-based polyols. Even though the direct use of lignin as the only polyol can be very appealing, generally, lignin macromonomers have low reactivity towards isocyanate groups yielding products without the desirable performance, and end up acting essentially as fillers [24][62][63].
Recent advances to circumvent the drawbacks of direct incorporation of lignin as polyol in the production of PUs include (1) the use of diols and glycerol as compatibilizer and cross-linker, and (2) lowering the lignin molecular weight using solvent fractionation. Since kraft lignin is the most commonly produced technical lignin, most of the studies are focused on this type of lignin. For example, Haridevan et al. [64] recently evaluated the dispersion and solubility of kraft lignin in different types of polyols at room temperature for the production of polyurethanes based on microscopic, gravimetric, and rheological analyses. The study demonstrated that kraft lignin has different degrees of dispersion in various polyols, depending on the structural characteristics such as solubility parameter, molecular weight, and monomeric unit type. In fact, it was observed that a higher degree of dispersion of kraft lignin was achieved in the lower-molecular-weight diethylene glycol (106.1 g/mol) than in polyethylene glycol (400 g/mol). Although the low dispersibility of lignin in polyols has not yet been solved, systematic studies such as this one are important contributions to increase lignin dispersion. In addition, the performance of PUs can be improved by heating the polyol/lignin dispersions at 120 °C prior to the reaction, which enhances the disaggregation of lignin microparticles, yielding a better lignin dispersion within the polyol system [65][66]. On the other hand, reducing the kraft lignin molecular weight by solvent fractionation can enhance its miscibility and dispersion in the PU matrix, and consequently can improve some properties of the resulting PU such as the mechanical properties [67]. The use of binary organic solvent mixtures such as acetone–methanol [68], aqueous two-phase systems (ATPSs) composed of (NH4)2SO4 and ethanol [69], and the use of ionic liquids [70] are examples of strategies used to reduce the molecular weight of lignin as well as its heterogeneity. However, it is important to take into account that commercializing low-molecular-weight lignin has been economically unfeasible until now.
In addition to PUF, different types of lignins and LBPs have also been used to prepare other forms of Pus, such as adhesives [71][72][73], elastomers [74][75][76], coatings [77][78], and films [79]. Elastomeric PUs are a class of PU materials that have the characteristics of rubber, and their application has increased in recent years due to the high demand for advanced applications such as in biomedicine, shape memory materials, self-healing materials, and gel materials. Mechanical properties such as toughness, tensile strength, and high elongation at break are highly desirable for the production of PU elastomers, and the use of modified or unmodified lignin in the synthesis of PU elastomers has shown that it plays an important role in improving these properties, where a percentage of lignin up to 40 wt.% (based on mass of polyol) did not jeopardize their mechanical properties [80][81][82].
In conclusion, the properties of lignin have a profound impact on the resulting PU product performance, e.g., foams, elastomers, coatings, and films, regardless of the lignin source and isolation process [77][78][80]. Nevertheless, with the exception of a few examples, the exact role of the structural features of lignin during the reaction with isocyanates is hardly discussed. Instead, the mixture of unmodified lignin with other polyols or of LBPs is treated globally as the polyol component of the PU. Yet, with the advancement of fractionation methodologies, a better understanding of the role of structural features of this renewable OH-rich aromatic material will certainly become clearer and bring further insights.
6. Lignin as a Building Block to Synthesize Non-Isocyanate Polyurethane (NIPU)
The industrial synthesis of isocyanates involves the reaction between primary amines and phosgene at high temperatures (100–200 °C). The latter is a highly toxic gas, produced by the reaction between carbon monoxide and chlorine gas. In turn, diisocyanates cause acute adverse health effects, such as irritation of the respiratory tract, eyes, and skin, being a major cause of occupational asthma in workers employed by the polyurethane industry [83][84][85][86][87]. Moreover, the two most widely used isocyanates in the PU industry, MDI (methylene diphenyl diisocyanate) and TDI (toluene diisocyanate), are highly reactive chemicals that bind to DNA and are probably genotoxic [88]. Furthermore, various household PU products, such as mattresses, pillows, cushion packaging, and insulating materials in building construction, among others, exhibit detrimental environmental impact on aquatic life, soil health, plants, and humans due to the presence of toxic components such as isocyanates, flame retardants, and amine-based catalysts [89]. Additionally, some compounds, such as carbon dioxide, carbon monoxide, hydrogen cyanide, acetaldehyde, and methanol, are released when PU products are burned and/or landfilled at their end-life, contributing to the greenhouse effect and having toxic effects on human health [90][91]. All these reported issues led the European Union to adopt the regulation where it was proposed to reduce the content of isocyanate, with the main goal being to ban its use in the future [92].
There are a few routes described in the literature to synthetize NIPU, but the main ones are the rearrangement of acyl azide, the ring-opening polymerization of cyclic carbamates, the polycondensation (or transurethanization) between polycarbamate and polyol, and the polyaddition between bis-cyclic carbonate and diamines. Recently, some works [90][93][94][95] dedicated to exploring the synthesis of NIPU were published, which identified that the most promising routes to produce NIPU are polycondensation and polyaddition (Figure 7).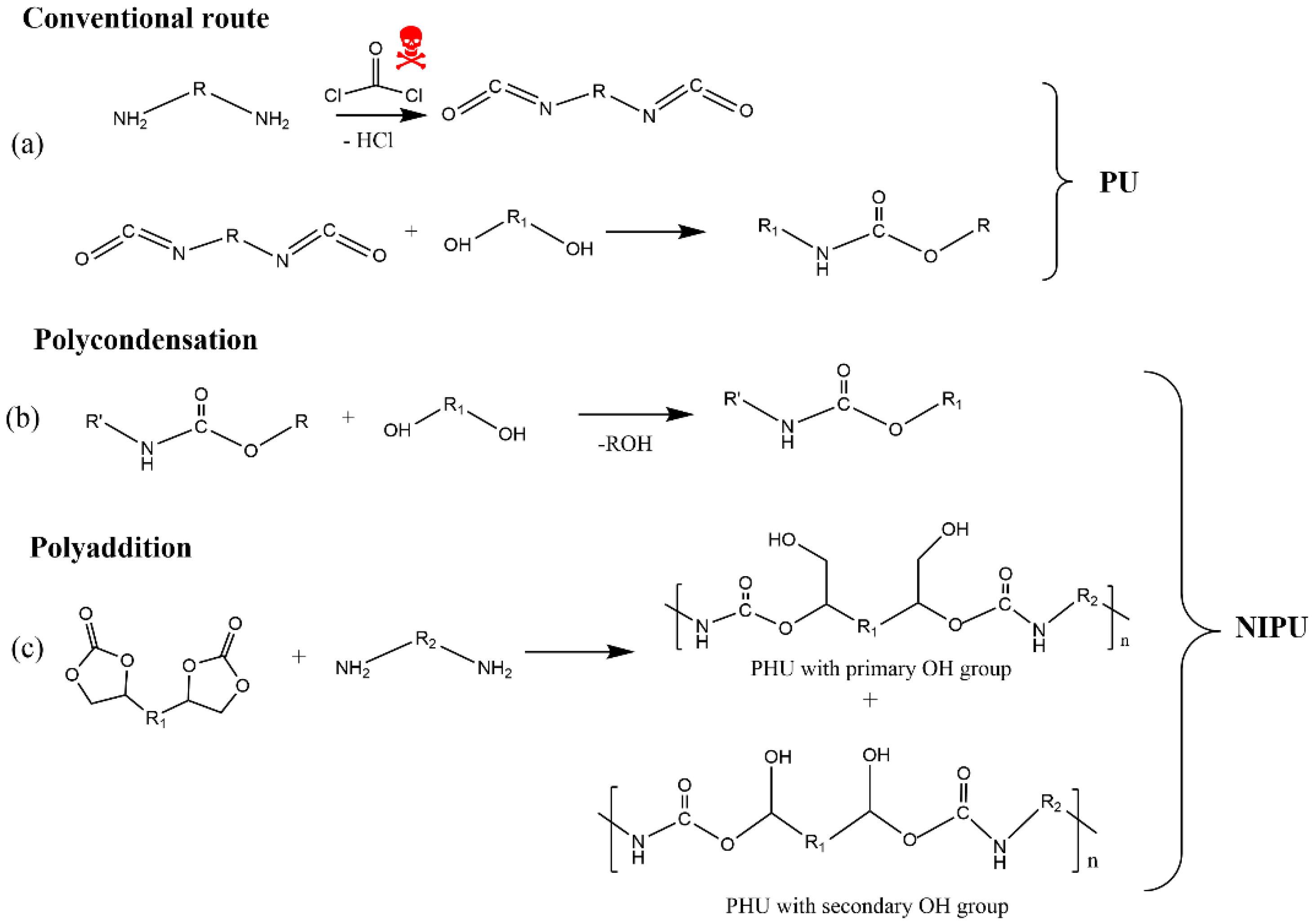
7. Concludsion
On the one hand, technical lignin is a byproduct of the pulp and paper industry that consists of natural aromatic polymers with high carbon content and is a valuable energy source. For example, in the kraft pulp industry, lignin is used entirely for energy recovery. On the other hand, technical lignin has great potential to replace, at least partially, petroleum-based polyols used in the formulation of PU products. Yet, at the moment, to obtain an LBP that is competitive with petroleum-based polyols in terms of characteristics and price is still a challenge. Indeed, there is an old saying among the lignin experts: “you can make anything out of lignin, except money”. This is due to the difficulties involved in obtaining technical lignin with homogenous reproducible characteristics, the cost to convert the solid lignin in a liquid polyol or other chemicals, and the poor compatibility of lignin with many synthetic polymers. However, the pulp and paper industry, in cooperation with the chemical industry, have shown that it is possible to earn money from lignin, otherwise companies would not be investing in it. Although LBPs are not yet on the market, polyurethane foams with added lignin (20–25 wt.%) in the formulations are already a reality in the market. Furthermore, developments regarding the depolymerization and fractionation of lignin into well-defined oligomonomers are required to avoid the need for chemical modification. Indeed, these developments will also contribute to a better understanding of the relevance of the structural features of lignin during the reaction with isocyanates and/or other chemicals, as well as their exact impact on the properties of the ensuing PUs. Likewise, in-depth studies regarding the dissolution of lignin into polyols, especially those obtained from renewable resources, show that this is also a challenge, and an opportunity exists to make the use of this rich and versatile material economically and environmentally viable. As final recommendations on the development of lignin-based polyurethanes, researchers and industry should pay attention to LCA studies and technical–economic assessments. These studies are of paramount importance to find solutions regarding current challenges, such as technology scaling up for lignins and LBPs, high process and product cost, and different environmental and social impacts, as well as regulatory and policy implications. Beyond the development of bio-based polyols to produce polyurethanes, significant efforts have also been made to replace the toxic isocyanate, developing non-isocyanate polyurethane. The main chemical route employed is the reaction between cyclic carbonates and diamines. Moreover, in recent years, lignin has been studied as a building block in the synthesis of cyclic carbonates for the development of new isocyanate-free polyurethanes. It is evident that there are still many challenges to be overcome, but what is expected is that in the future, technical lignin will be used as a raw material for the production of polymeric products such as polyurethanes, and not just burnt for energy, thus contributing to a more sustainable society.References
- Kausar, A. Polyurethane Composite Foams in High-Performance Applications: A Review. Polym.—Plast. Technol. Eng. 2018, 57, 346–369.
- Szycher, M. Szycher’s Handbook of Polyurethanes, 2nd ed.; CRC Press: New York, NY, USA, 2006.
- Ionescu, M. The General Characteristics of Oligo-Polyols. In Chemistry and Technology of Polyols for Polyurethanes; Rapra Technology: Shawbury, UK, 2005; Volume 14, pp. 31–49. ISBN 978-1-84735-035-0.
- Kreye, O.; Mutlu, H.; Meier, M.A.R. Sustainable routes to polyurethane precursors. Green Chem. 2013, 15, 1431–1455.
- Hirvonen, J.; Jokisalo, J.; Heljo, J.; Kosonen, R. Towards the EU emission targets of 2050: Cost-effective emission reduction in Finnish detached houses. Energies 2019, 12, 4395.
- Polyurethane Market Size. Available online: https://www.fortunebusinessinsights.com/industry-reports/polyurethane-pu-market-101801 (accessed on 14 June 2022).
- Desroches, M.; Escouvois, M.; Auvergne, R.; Caillol, S.; Boutevin, B. From vegetable oils to polyurethanes: Synthetic routes to polyols and main industrial products. Polym. Rev. 2012, 52, 38–79.
- Fernando, S.; Adhikari, S.; Chandrapal, C.; Murali, N. Biorefineries: Current status, challenges, and future direction. Energy Fuels 2006, 20, 1727–1737.
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E.; Belgacem, M.N. Optimization study of lignin oxypropylation in view of the preparation of polyurethane rigid foams. Ind. Eng. Chem. Res. 2009, 48, 2583–2589.
- Nadji, H.; Bruzzèse, C.; Belgacem, M.N.; Benaboura, A.; Gandini, A. Oxypropylation of lignins and preparation of rigid polyurethane foams from the ensuing polyols. Macromol. Mater. Eng. 2005, 290, 1009–1016.
- Boerjan, W.; Ralph, J.; Baucher, M. Lignin biosynthesis. Annu. Rev. Plant Biol. 2003, 54, 519–546.
- Briones, R.; Serrano, L.; Labidi, J. Valorization of some lignocellulosic agro-industrial residues to obtain biopolyols. J. Chem. Technol. BioTechnol. 2012, 87, 244–249.
- Meng, X.; Crestini, C.; Ben, H.; Hao, N.; Pu, Y.; Ragauskas, A.J.; Argyropoulos, D.S. Determination of hydroxyl groups in biorefinery resources via quantitative 31P NMR spectroscopy. Nat. Protoc. 2019, 14, 2627–2647.
- Sarkanen, K.V.; Hergert, H.L. Classification and distribution. In Lignins—Occurrence, Formation, Structure and Reactions; Sarkanen, K.V., Ludwig, C.H., Eds.; Wiley-Interscience: New York, NY, USA, 1971.
- Dence, C.W.; Lin, Y.S. Methods in Lignin Chemistry; Springer: New York, NY, USA, 1992; pp. 1–568.
- Tribot, A.; Amer, G.; Abdou Alio, M.; de Baynast, H.; Delattre, C.; Pons, A.; Mathias, J.-D.; Callois, J.-M.; Vial, C.; Michaud, P.; et al. Wood-lignin: Supply, extraction processes and use as bio-based material. Eur. Polym. J 2019, 112, 228–240.
- Zhao, X.; Cheng, K.; Liu, D. Organosolv pretreatment of lignocellulosic biomass for enzymatic hydrolysis. Appl. Microbiol. BioTechnol. 2009, 82, 815–827.
- Rodrigues Gurgel da Silva, A.; Errico, M.; Rong, B.G. Techno-economic analysis of organosolv pretreatment process from lignocellulosic biomass. Clean Technol. Environ. Policy 2018, 20, 1401–1412.
- Zhu, W. Precipitation of Kraft Lignin Yield and Equilibrium. Ph.D. Thesis, Chalmers University of Technology, Gothenburg, Sweden, 2015.
- Hu, Z.; Du, X.; Liu, J.; Chang, H.; Jameel, H. Structural Characterization of Pine Kraft Lignin: BioChoice Lignin vs Indulin AT. J. Wood Chem. Technol. 2016, 36, 432–446.
- Tomani, P. The LignoBoost process. Cellul. Chem. Technol. 2010, 44, 53–58.
- Li, T.; Takkellapati, S. The current and emerging sources of technical lignins and their applications. Biofuels Bioprod. Biorefining 2018, 12, 756–778.
- Kouisni, L.; Holt-Hindle, P.; Maki, K.; Paleologou, M. The LignoForce SystemTM: A new process for the production of high-quality lignin from black liquor. Pulp. Pap. Can. 2014, 115, 18–22.
- Alinejad, M.; Henry, C.; Nikafshar, S.; Gondaliya, A.; Bagheri, S.; Chen, N.; Singh, S.K.; Hodge, D.B.; Nejad, M. Lignin-Based Polyurethanes: Opportunities for Bio-Based Foams, Elastomers, Coatings and Adhesives. Polymers 2019, 11, 1202.
- Evtuguin, D.V.; Andreolety, J.P.; Gandini, A. Polyurethanes based on oxygen-organosolv lignin. Eur. Polym. J 1998, 34, 1163–1169.
- Aniceto, J.P.S.; Portugal, I.; Silva, C.M. Biomass-based polyols through oxypropylation reaction. ChemSusChem 2012, 5, 1358–1368.
- Ahvazi, B.; Wojciechowicz, O.; Hawari, J. Preparation of Lignopolyols from Wheat Straw Soda Lignin. J. Agric. Food Chem. 2011, 59, 10505–10516.
- Clements, J.H. Reactive applications of cyclic alkylene carbonates. Ind. Eng. Chem. Res. 2003, 42, 663–674.
- Dilling, P. Sulfonation of lignins. US Patent 5049661, 17 September 1991.
- Kühnel, I.; Podschun, J.; Saake, B.; Lehnen, R. Synthesis of lignin polyols via oxyalkylation with propylene carbonate. Holzforschung 2015, 69, 531–538.
- Duval, A.; Avérous, L. Oxyalkylation of Condensed Tannin with Propylene Carbonate as an Alternative to Propylene Oxide. ACS Sustain. Chem. Eng. 2016, 4, 3103–3112.
- Belgacem, M.N.; Gandini, A. Partial or Total Oxypropylation of Natural Polymers and the Use of the Ensuing Materials as Composites or Polyol Macromonomers. In Monomers, Polymers and Composites from Renewable Resources; Belgacem, M.N., Gandini, A., Eds.; Elsevier: Oxford, UK, 2008; ISBN 9780080453163.
- Akil, Y.; Lorenz, D.; Lehnen, R.; Saake, B. Safe and non-toxic hydroxyalkylation of xylan using propylene carbonate. Eur. Polym. J. 2016, 77, 88–97.
- Vieira, F.R.; Barros-timmons, A.; Evtuguin, D.V.; Pinto, P.C.R. Effect of different catalysts on the oxyalkylation of eucalyptus Lignoboost® kraft lignin. Holzforschung 2020, 74, 567–576.
- Zhang, X.; Chen, M.; Liu, C.; Zhang, A.; Sun, R. Ring-opening graft polymerization of propylene carbonate onto xylan in an ionic liquid. Molecules 2015, 20, 6033–6047.
- Duval, A.; Avérous, L. Cyclic Carbonates as Safe and Versatile Etherifying Reagents for the Functionalization of Lignins and Tannins. ACS Sustain. Chem. Eng. 2017, 5, 7334–7343.
- Jin, Y.; Ruan, X.; Cheng, X.; Lü, Q. Liquefaction of lignin by polyethyleneglycol and glycerol. Bioresour Technol. 2011, 102, 3581–3583.
- Hu, S.; Luo, X.; Li, Y. Polyols and polyurethanes from the liquefaction of lignocellulosic biomass. ChemSusChem 2014, 7, 66–72.
- Yip, J.; Chen, M.; Szeto, Y.S.; Yan, S. Bioresource Technology Comparative study of liquefaction process and liquefied products from bamboo using different organic solvents. Bioresour. Technol. 2009, 100, 6674–6678.
- Min, N.; Guang-jie, Z.; Alma, M.H. Polycondensation reaction and its mechanism during lignocellulosic liquefaction by an acid catalyst: A review. For. Stud. China 2011, 13, 71–79.
- Soares, B.; Gama, N.; Freire, C.; Timmons, A.B.; Brandão, I.; Silva, R.; Neto, C.P.; Ferreira, A. Ecopolyol production from industrial cork powder via acid liquefaction using polyhydric alcohols. ACS Sustain. Chem. Eng. 2014, 2, 846–854.
- Helena, S.; Egüés, I.; Labidi, J. Industrial Crops & Products Liquefaction of Kraft lignin using polyhydric alcohols and organic acids as catalysts for sustainable polyols production. Ind. Crop Prod. 2019, 137, 687–693.
- Jasiukaityte-Grojzdek, E.; Kunaver, M.; Crestini, C. Lignin structural changes during liquefaction in acidified ethylene glycol. J. Wood Chem. Technol. 2012, 32, 342–360.
- Kim, C.S. Recent Efforts to Prevent Undesirable Reactions From Fractionation to Depolymerization of Lignin: Toward Maximizing the Value From Lignin. Front. Energy Res. 2018, 6, 1–7.
- Crestini, C.; Jasiukaityte, E. Lignin behaviour during wood liquefaction—Characterization by quantitative 31P, 13C NMR and size-exclusion chromatography. Catal. Today 2010, 156, 23–30.
- Faris, A.H.; Mohamad Ibrahim, M.N.; Abdul Rahim, A.; Hussin, M.H.; Brosse, N. Preparation and Characterization of Lignin Polyols from the Residues of Oil Palm Empty Fruit Bunch. BioResources 2015, 10, 7339–7352.
- Cinelli, P.; Anguillesi, I.; Lazzeri, A. Green synthesis of flexible polyurethane foams from liquefied lignin. Eur. Polym. J 2013, 49, 1174–1184.
- Vieira, F.R.; Barros-timmons, A.; Evtuguin, D.V. Oxyalkylation of Lignoboost TM Kraft Lignin with Propylene Carbonate: Design of Experiments towards Synthesis Optimization. Materials 2022, 15, 1925.
- ASTM D 4662-08; Standard Test Methods for Testing Polyurethane Raw Materials: Determination of Acid and Alkalinity Numbers of Polyols. ASTM International: West Conshohocken, PA, USA, 2011.
- Mohd Noor, M.A.; Sendijarevic, V.; Hoong, S.S.; Sendijarevic, I.; Tuan Ismail, T.N.M.; Hanzah, N.A.; Mohd Noor, N.; Poo Palam, K.D.; Ghazali, R.; Abu Hassan, H. Molecular Weight Determination of Palm Olein Polyols by Gel Permeation Chromatography Using Polyether Polyols Calibration. JAOCS J. Am. Oil Chem. Soc 2016, 93, 721–730.
- Aung, M.M.; Yaakob, Z.; Kamarudin, S.; Abdullah, L.C. Synthesis and characterization of Jatropha (Jatropha curcas L.) oil-based polyurethane wood adhesive. Ind. Crops Prod. 2014, 60, 177–185.
- Carey, M.A.; Wellons, S.L.; Elder, D.K. Rapid method for measuring the hydroxyl content of polyurethane polyols. J. Cell. Plast. 1984, 20, 42–48.
- Cateto, C.A.; Barreiro, M.F.; Brochier-salon, M.C.; Thielemans, W.; Belgacem, M.N. Lignins as Macromonomers for Polyurethane Synthesis: A Comparative Study on Hydroxyl Group Determination. J. Appl. Polym. Sci. 2008, 109, 3008–3017.
- Sulaeva, I.; Zinovyev, G.; Plankeele, J.M.; Sumerskii, I.; Rosenau, T.; Potthast, A. Fast Track to Molar-Mass Distributions of Technical Lignins. ChemSusChem 2017, 10, 629–635.
- Zhang, Q.; Zhang, G.; Xu, J.; Gao, C.; Wu, Y. Recent advances on lignin-derived polyurethane polymers. Rev. Adv. Mater. Sci. 2015, 40, 146–154.
- Cateto, C.A.; Barreiro, M.F.; Rodrigues, A.E. Monitoring of lignin-based polyurethane synthesis by FTIR-ATR. Ind. Crops Prod. 2008, 7, 168–174.
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. BioProd. 2020, 5, 163–179.
- Xu, C.; Ferdosian, F. Lignin-Based Polyurethane (PU) Resins and Foams. In Conversion of Lignin into Bio-Based Chemicals and Materials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 133–156. ISBN 9783662549599.
- Gandini, A.; Belgacem, M.N.; Guo, Z.-X.; Montanari, S. Lignins as macromonomers for polyesters and polyurethanes. In Chemical Modification, Properties, and Usage of Lignin; Hu, T.Q., Ed.; Springer Science + Business Media: New York, NY, USA, 2002; pp. 57–80. ISBN 978-1461351733.
- Gondaliya, A.; Nejad, M. Lignin as a partial polyol replacement in polyurethane flexible foam. Molecules 2021, 26, 2302.
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306.
- Xue, B.L.; Wen, J.L.; Sun, R.C. Lignin-based rigid polyurethane foam reinforced with pulp fiber: Synthesis and characterization. ACS Sustain. Chem. Eng. 2014, 2, 1474–1480.
- García, J.L.; Pans, G.; Phanopoulos, C. Use of lignin in polyurethane-based structural wood adhesives. J. Adhes. 2018, 94, 814–828.
- Haridevan, H.; Evans, D.A.C.; Martin, D.J.; Annamalai, P.K. Rational analysis of dispersion and solubility of Kraft lignin in polyols for polyurethanes. Ind. Crops Prod. 2022, 185, 115129.
- Hayati, A.N.; Evans, D.A.C.; Laycock, B.; Martin, D.J.; Annamalai, P.K. A simple methodology for improving the performance and sustainability of rigid polyurethane foam by incorporating industrial lignin. Ind. Crops Prod. 2018, 117, 149–158.
- Luo, S.; Gao, L.; Guo, W. Effect of incorporation of lignin as bio-polyol on the performance of rigid lightweight wood–polyurethane composite foams. J. Wood Sci. 2020, 66, 1–10.
- Ragauskas, A.J.; Wang, Y.; Meng, X.; Wyman, C.E. On Polyurethanes from Technical Lignin: Recent Advances and Challenges. Univ. J. Renew. Energy 2021, 9, 40–47.
- Wang, Y.Y.; Li, M.; Wyman, C.E.; Cai, C.M.; Ragauskas, A.J. Fast Fractionation of Technical Lignins by Organic Cosolvents. ACS Sustain. Chem. Eng. 2018, 6, 6064–6072.
- Sun, H.; Wang, G.; Ge, J.; Wei, N.; Li, W.; Sui, W.; Parvez, A.M.; Si, C. Reduction of lignin heterogeneity using aqueous two-phase system: A facile and universal “one-step-three-fractions” approach. Int J. Biol. Macromol. 2021, 186, 341–350.
- Dias, R.M.; Petrin, L.C.G.; Sosa, F.H.B.; Da Costa Lopes, A.M.; Coutinho, J.A.P.; Da Costa, M.C. Investigation of Kraft Lignin Solubility in Protic Ionic Liquids and Their Aqueous Solutions. Ind. Eng. Chem. Res. 2020, 59, 18193–18202.
- Tavares, L.B.; Boas, C.V.; Schleder, G.R.; Nacas, A.M.; Rosa, D.S.; Santos, D.J. Bio-based polyurethane prepared from Kraft lignin and modified castor oil. Express Polym. Lett. 2016, 10, 927–940.
- Magina, S.; Gama, N.; Carvalho, L.; Barros-Timmons, A.; & Evtuguin, D.V. Lignosulfonate-Based Polyurethane Adhesives. Materials 2021, 14, 7072.
- Nacas, A.M.; Ito, N.M.; De Sousa JR, R.R.; Spinacé, M.A.; Dos Santos, D.J. Effects of NCO:OH ratio on the mechanical properties and chemical structure of Kraft lignin—Based polyurethane adhesive. J. Adhes. 2017, 93, 18–29.
- Ciobanu, C.; Ungureanu, M.; Ignat, L.; Ungureanu, D.; Popa, V.I. Properties of lignin-polyurethane films prepared by casting method. Ind. Crops Prod. 2004, 20, 231–241.
- da Silva, E.A.B.; Zabkova, M.; Araújo, J.D.; Cateto, C.A.; Barreiro, M.F.; Belgacem, M.N.; Rodrigues, A.E. An integrated process to produce vanillin and lignin-based polyurethanes from Kraft lignin. Chem. Eng. Res. Des. 2009, 87, 1276–1292.
- Li, X.; Chen, X.; Zhang, S.; Yin, Y.; Wang, C. UV-resistant transparent lignin-based polyurethane elastomer with repeatable processing performance. Eur. Polym. J. 2021, 159, 110763.
- Griffini, G.; Passoni, V.; Suriano, R.; Levi, M.; Turri, S. Polyurethane coatings based on chemically unmodified fractionated lignin. ACS Sustain. Chem. Eng. 2015, 3, 1145–1154.
- Cao, Y.; Liu, Z.; Zheng, B.; Ou, R.; Fan, Q.; Li, L.; Guo, C.; Liu, T.; Wang, Q. Synthesis of lignin-based polyols via thiol-ene chemistry for high-performance polyurethane anticorrosive coating. Compos. Part B Eng. 2020, 200, 108295.
- Llovera, L.; Benjelloun-Mlayah, B.; Delmas, M. Organic acid lignin-based polyurethane films: Synthesis parameter optimization. BioResources 2016, 11, 6320–6334.
- Ma, X.; Chen, J.; Zhu, J.; Yan, N. Lignin-Based Polyurethane: Recent Advances and Future Perspectives. Macromol. Rapid. Commun. 2020, 2000492, 1–13.
- Liu, W.; Fang, C.; Wang, S.; Huang, J.; Qiu, X. High-Performance Lignin-Containing Polyurethane Elastomers with Dynamic Covalent Polymer Networks. Macromolecules 2019, 52, 6474–6484.
- Li, H.; Sun, J.T.; Wang, C.; Liu, S.; Yuan, D.; Zhou, X.; Tan, J.; Stubbs, L.; He, C. High Modulus, Strength, and Toughness Polyurethane Elastomer Based on Unmodified Lignin. ACS Sustain. Chem. Eng. 2017, 5, 7942–7949.
- Bernstein, J.A. Overview of diisocyanate occupational asthma. Toxicology 1996, 111, 181–189.
- Baur, X.; Marek, W.; Ammon, J.; Czuppon, A.B.; Marczynski, B.; Raulf-Heimsoth, M.; Roemmelt, H.; Fruhmann, G. Respiratory and other hazards of isocyanates. Int. Arch. Occup. Environ. Health 1994, 66, 141–152.
- Fisseler-Eckhoff, A.; Bartsch, H.; Zinsky, R.; Schirren, J. Environmental isocyanate-induced asthma: Morphologic and pathogenetic aspects of an increasing occupational disease. Int. J. Environ. Res. Public Health 2011, 8, 3672–3687.
- Schaal, N.C.; Brazile, W.J.; Finnie, K.L.; Tiger, J.P. Effects of known determinants on methylene bisphenyl isocyanate (MDI) concentration during spray-on truck bed-lining processes. Ann. Work. Expo. Health 2017, 61, 872–882.
- Bello, D.; Herrick, C.A.; Smith, T.J.; Woskie, S.R.; Streicher, R.P.; Cullen, M.R.; Liu, Y.; Redlich, C.A. Skin exposure to isocyanates: Reasons for concern. Environ. Health Perspect. 2007, 115, 328–335.
- Bolognesi, C.; Baur, X.; Marczynski, B.; Norppa, H.; Sepai, O.; Sabbioni, G. Carcinogenic risk of toluene diisocyanate and 4,4′-methylenediphenyl diisocyanate: Epidemiological and experimental evidence. Crit. Rev. Toxicol. 2001, 31, 737–772.
- Adetunji, C.O.; Olaniyan, O.T.; Anani, O.A.; Inobeme, A.; Mathew, J.T. Environmental Impact of Polyurethane Chemistry. In ACS Symposium Series. Polyurethane Chemistry: Renewable Polyols and Isocyanates; American Chemical Society: Washington, DC, USA, 2021; Volume 1380, pp. 393–411.
- Cornille, A.; Auvergne, R.; Figovsky, O.; Boutevin, B.; Caillol, S. A perspective approach to sustainable routes for non-isocyanate polyurethanes. Eur. Polym. J 2017, 87, 535–552.
- Suryawanshi, Y.; Sanap, P.; Wani, V. Advances in the synthesis of non-isocyanate polyurethanes. Polym. Bull. 2018, 76, 3233–3246.
- REACH Registration, Evaluation, Authorisation and Restriction of Chemicals. Euratom 2006, 2001, 20–30. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1907-20161011 (accessed on 1 September 2022).
- Maisonneuve, L.; Lamarzelle, O.; Rix, E.; Grau, E.; Cramail, H. Isocyanate-Free Routes to Polyurethanes and Poly(hydroxy Urethane)s. Chem. Rev. 2015, 115, 12407–12439.
- Blattmann, H.; Fleischer, M.; Bähr, M.; Mülhaupt, R. Isocyanate- and Phosgene-Free Routes to Polyfunctional Cyclic Carbonates and Green Polyurethanes by Fixation of Carbon Dioxide. Macromol. Rapid. Commun. 2016, 48, 169–190.
- Leykin, A.; Figovsky, O.; Shapovalov, L. Non-isocyanate polyurethanes—yesterday, today and conscious. Int. Sci. J. Altern. Energy Ecol. 2016, 95–108.
- Datta, J.; Włoch, M. Progress in non-isocyanate polyurethanes synthesized from cyclic carbonate intermediates and di- or polyamines in the context of structure–properties relationship and from an environmental point of view. Polym. Bull. 2015, 73, 1459–1496.
- Błażek, K.; Datta, J. Renewable natural resources as green alternative substrates to obtain bio-based non-isocyanate polyurethanes-review. Crit. Rev. Environ. Sci. Technol. 2019, 3389, 1–39.
- Carré, C.; Ecochard, Y.; Caillol, S.; Averous, L. From the Synthesis of Biobased Cyclic Carbonate to Polyhydroxyurethanes: A Promising Route towards Renewable Non-Isocyanate Polyurethanes. ChemSusChem 2019, 12, 3410–3430.
- Ghasemlou, M.; Daver, F.; Ivanova, E.P.; Adhikari, B. Bio-based routes to synthesize cyclic carbonates and polyamines precursors of non-isocyanate polyurethanes: A review. Eur. Polym. J. 2019, 118, 668–684.
- Basso, M.C.; Pizzi, A.; Delmotte, L. Polyurethanes from Kraft Lignin without Using Isocyanates. J. Renew. Mater. 2018, 6, 413–425.
- Arias, A.; Entrena-Barbero, E.; Feijoo, G.; Moreira, M. Sustainable non-isocyanate polyurethanes bio-adhesives for engineered wood panels are revealed as promising candidates to move from formaldehyde-based alternatives. J. Environ. Chem. Eng. 2022, 10, 107053.
