Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Jessie Wu and Version 3 by Jessie Wu.
Solid oxide electrolysis cells (SOECs) and solid oxide fuel cells (SOFCs) are the leading high-temperature devices to realize the global “Hydrogen Economy”. These devices are inherently multi-material (ceramic and cermets). They have multi-scale, multilayer configurations (a few microns to hundreds of microns) and different morphology (porosity and densification) requirements for each layer. Adjacent layers should exhibit chemical and thermal compatibility and high-temperature mechanical stability.
- SOEC
- SOFC
- hydrogen economy
1. Introduction
Hydrogen, as an important chemical feedstock in the global economy, has growing demands in transportation, steel production, power generation, and load balancing in grid services. Recently, there has been a significant global investment in the “Hydrogen Economy”, which in turn will advance the manufacturing and recycling of clean hydrogen technologies. For example, in the United States, the mission of the Department of Energy’s “Hydrogen Shot” is to reach USD 1 per 1 kg in 1 decade (“1 1 1”) for hydrogen. Sectors such as long-distance transport via heavy- and medium-duty vehicles, high-temperature heat, energy storage, and synthetic fuels for air and marine transport are among the energy-intensive and difficult sectors to decarbonize. Hydrogen has been proposed as a key energy option for the decarbonization of these sectors (Figure 1).
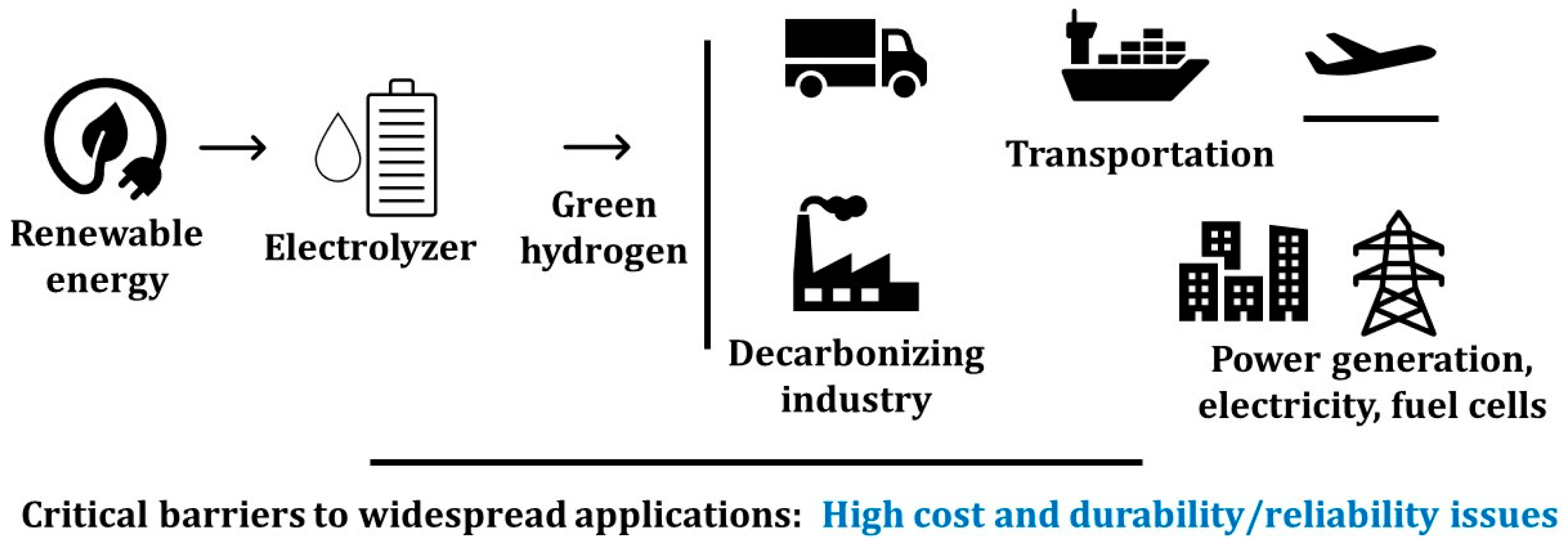
Figure 1. Roles of fuel cells and electrolyzers in the hydrogen economy.
Hydrogen is the simplest element on earth; however, it does not typically exist by itself in nature. It must be produced through chemical reactions from compounds that contain it. Currently, the majority (~95%) of the world’s hydrogen is produced by steam methane reforming (SMR) that releases the greenhouse gas CO2. The electrolytic hydrogen (without any pollution) is more expensive compared to hydrogen produced using the SMR process [1]. Today’s hydrogen market is approximately 10 million metric tons per year (MMT/year) in the U.S. and 65–100 MMT/year globally. However, only approximately 2% of total global hydrogen production is generated via electrolysis. The electrolytic hydrogen market could grow substantially to at least 100 MMT/year by 2050 to meet potential future demands and help difficult-to-decarbonize sectors. In order to meet this market size, the U.S. electrolyzer capacity will likely have to increase from 0.17 gigawatts (GW) today to up to 1000 GW in 2050—or 20% compound annual growth from 2021 to 2050 with an annual manufacturing requirement of over 100 GW/year [2]. In addition, over 50 GW of domestic fuel cell capacity is required in the decarbonization scenario, with an annual manufacturing requirement of over 3 GW/year. Investments in manufacturing and process development and increasing production scale and industrialization will reduce the cost of electrolytic hydrogen.
Solid oxide electrolysis cells (SOECs) and solid oxide fuel cells (SOFCs) are considered among the electrochemical energy storage and conversion devices that are essential for the global rollout of a hydrogen economy. SOECs are energy storage units that produce storable hydrogen from electricity and water (electrolysis of water), electrolyze CO2 to produce CO and oxygen, or even co-electrolyze water and CO2 to produce syngas (CO + H2) and oxygen [3]. High-temperature steam electrolyzers use both electricity (preferably renewable) and heat (preferably waste heat or a low-cost thermal energy generator such as a nuclear reactor) because they operate with steam. SOFCs convert the chemical energy stored in a fuel (H2, CO, CH4, etc.) to electricity directly through an electrochemical reaction (by oxidizing a fuel). SOFCs are often composed of approximately 40–60 individual cells that produce nearly 25 W per cell, interconnected into a single module [4].
The key barriers to the existing technologies are fabrication time and cost, quality assurance and quality control, as well as stack durability. Despite their high efficiency, the global market rollout of these devices is currently short of economies of scale. The benefits of the hydrogen economy will be best played out when it is deployed at scale and across multiple applications. However, the high cost of these devices compared to alternative energy systems is the single most important factor hindering their widespread applications.
SOFCs and SOECs and their stacks are geometrically complex, inherently multi-material, and multilayer devices. The cells are made of thin active elements (~10–50 µm electrolyte and ~50–300 µm anode and cathode) with different compositions and microstructures (porous anode and cathode and dense electrolyte). More than a hundred steps could be involved in the traditional manufacturing process of a complete stack, including tape-casting, screen printing, slip-casting, slurry spraying, spray pyrolysis, dip-coating, thin film deposition, chemical infiltration and ex-solution for catalysts, and laser cutting of the fabricated tapes, punching, laminating, stacking, and firing/sintering [5][6][7]. Many steps, with most requiring manual inputs and multiple joints and seals, result in low reliability, durability and reproducibility, high cost, and a long time to market (Figure 2). For the global-scale adaptation of these devices, manufacturing technologies are needed that reduce the number of cell components in a stack, lower processing temperature, reduce the number of processing steps, and shorten the overall processing time. These improvements may result in an increase in throughput and lower-cost production at scale [2].
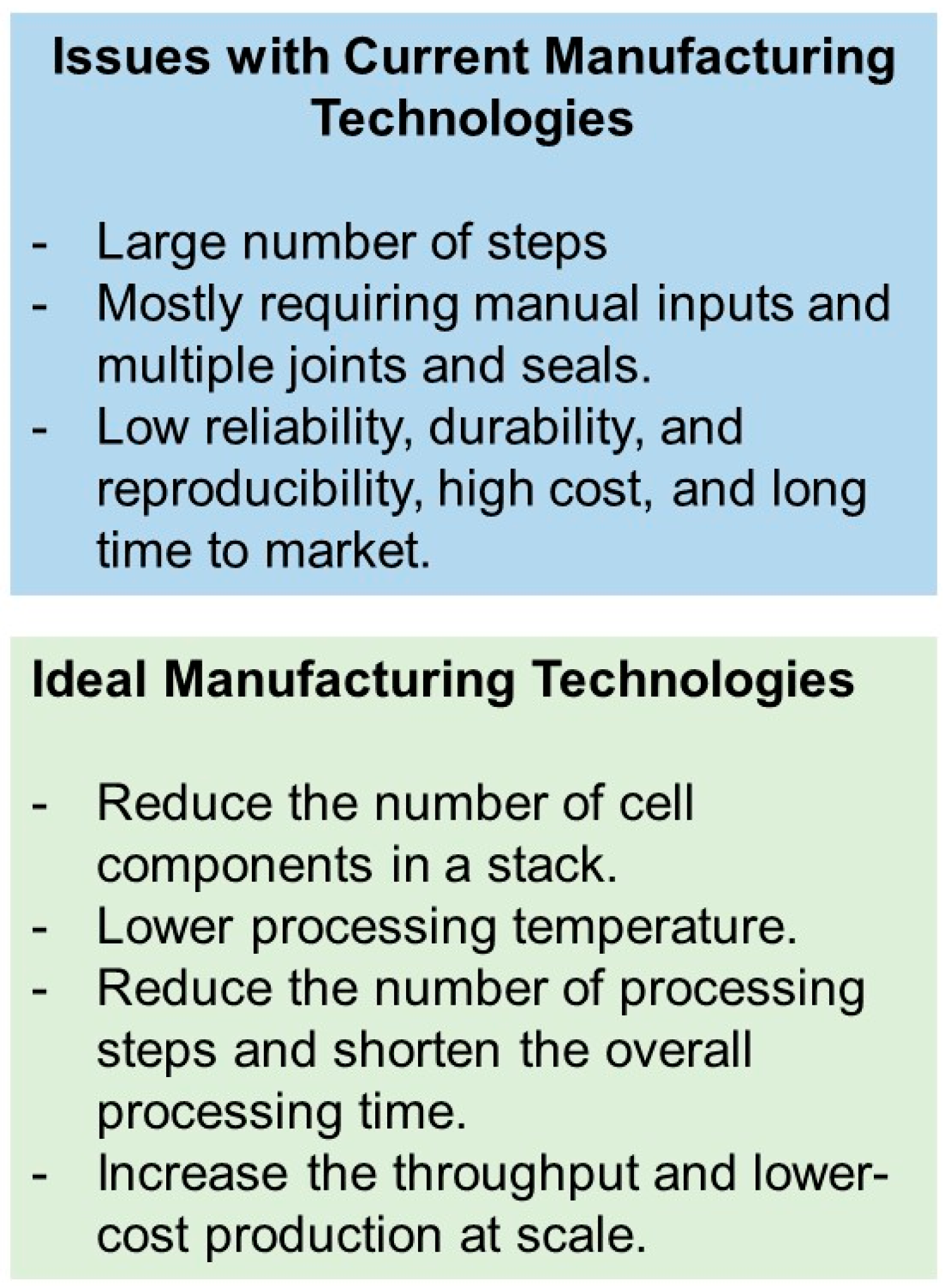
Figure 2.
Issues with current manufacturing technologies for production SOFCs and SOECs and attributes of ideal manufacturing technologies for these devices.
Development or application of suitable additive manufacturing (AM) technologies has the potential to lower the manufacturing cost, decrease waste of often expensive raw materials, provide use of more environmentally friendly materials and processing methods, and use fewer solvents. AM technologies may reduce the number of steps and result in more durable and reliable devices. Another advantage of AM technologies may be the augmentation of the design space for more efficient devices, such as enabling complex geometries beyond planar and tubular ones or enhancing surface area for electrochemical reaction sites and enhancing specific power [8]. Thermomechanical modeling of 3D manufactured electrodes for SOFCs, and rational design of 3D manufactured composite electrodes point to the benefits of 3D printing for performance improvement if certain design criteria are considered [9][10].
Given the largely nascent nature of the SOEC and SOFC industries, there are limited data on supply chain needs and constraints [1]. High-volume production of these energy devices requires building multi-industry supply chains to support components, materials, and equipment [2]. Some cell materials and components, such as interconnects, may face supply chain issues, considering that interconnects are more prone to degradation (cracking, delamination, and coating pinholes). AM allows for distributed manufacturing that can elevate some of the concerns in the supply chain.
2. Solid oxide electrolysis cells (SOECs) and Solid Oxide Fuel Cells (SOFCs) Components and Requirements
At the basic level, these electrochemical devices are made of an electrolyte and two electrodes (anode and cathode). Interconnects and sealing materials are also required for complete cells and stacks. The electrolyte and the electrodes should have a proper thickness to reduce electric and diffusion resistance. The microstructure and, to some extent, the thickness of the functional materials in these devices primarily govern the device’s performance [11]. The electrolyte is a pure ceramic, while the anode and cathode are ceramic-metal composites (cermet). A dense, thin electrolyte is required to separate oxidation gases from fuel gases. When the cell is electrode-supported, the thickness of the electrolyte can be substantially reduced (to a few microns), which results in a significant reduction in the overall ohmic resistance of the cell. Thinner electrolyte, however, limits the number of 3D printing technologies that are applicable. Cathode and anode are a mixture of electrolyte and electrode materials, which is preferred for reduced polarization and expansion of the triple phase boundaries (TPBs).
ZrO2 doped by Yttrium (Y) or Scandia (Sc) are conductors of oxygen ions above 800 °C. Currently, yttria-stabilized zirconia (YSZ) is the state-of-the art electrolyte material for SOFCs and SOECs. YSZ can be generally sintered in the temperature range of 1300–1500 °C [12]. Sc-stabilized zirconia (ScSZ) and gadolinium-doped ceria (GDC) have also been used as electrolytes [13]. The electrolyte must be sufficiently dense to avoid leakage of the fuel/oxidant gases to the electrodes and reduce the resistance to oxygen ion diffusion in the electrolyte. The electronic conductivity of the electrolyte should be low to prevent losses due to leakage current. The density of the electrolyte, which is related to porosity, plays an important role in its electrical conductivity. Flaws, pinholes, and other defects in the electrolyte can drastically reduce the electrochemical performance of the cell. The sintering step of the electrolyte ceramic is, therefore, vital.
Nickel-YSZ (Ni-YSZ) cermet is used as the anode in SOFC and the cathode in SOEC (considered fuel electrode in both devices). YSZ ceramic in this cermet provides ionic conductivity and structural support, while Ni functions as the catalyst and electronic conductor [14]. The cathode in SOFC and anode in SOEC (or the oxygen electrode) can be made of mixed conductors such as lanthanum-strontium cobalt ferrite (LSCF) or lanthanum-strontium cobaltite (LSC). LSCF is a mixed ion-electronic conductor capable of fast oxygen ion and electron conduction. It promotes oxygen reduction reaction as a highly active catalyst. Strontium (Sr)-doped LaMnO3 (LSM) in a cermet with YSZ may be used for less demanding applications. LSM has good compatibility and low chemical reactivity with YSZ and a similar coefficient of thermal expansion (CTE) to YSZ. In this case, LSM provides electronic conduction and catalytic function, while YSZ is the structural component and provides ionic conduction. In some designs, a buffer layer of gadolinium doped ceria (GDC) is used between the electrolyte and the LSCF cathode. In order to prevent a reaction between the oxygen electrode materials and YSZ, a thin (0.1 to 5 µm) layer of GDC may also be utilized [3].
In terms of recycling and circular economy in SOFCs and SOECs, Ni and Lanthanum elements are considered among the materials with environmental burdens. These burdens can be remediated (estimated ~70%) with recycling and considerations of the circular economy approach [15].
Cathode and anode are porous, electrically conductive, and should possess high catalytic activities for fuel oxidation and oxygen reduction, which requires a high density of reactive electrochemical sites, or triple phase boundaries, TPBs. An electrochemical reaction occurs at the TPBs, where electrons, ions, and reactants meet. Porosity is required to provide pathways for mass transport, i.e., diffusion of gaseous fuels and byproducts. The polarization in each electrode includes ohmic, activation, and concentration polarizations, which should be optimized for the overall minimization of the cell polarization [16]. Ohmic, activation, and concentration polarizations are related to electrical conductivity, triple phase boundary, and porosity, respectively. The volume percentage (vol%) of pores is an important parameter. Additionally, factors such as proper connectivity (open/close) pores, pore size and size distribution, and pore tortuosity play dominant roles in impacting polarization characteristics.
The porosity is often provided by pore-formers (such as graphitic carbon, short carbon fibers, polymer spheres, flour, rice, starch, etc.) in addition to the pore generated by NiO to Ni reduction [16]. In general, larger pore-formers (~20 µm) are more effective than small ones (a few microns) [16]. A certain vol% of pore-formers is necessary to generate a network of open percolated pores, which is often ~30 vol%. It has also been suggested that composite pore-formers containing two or more pore-formers with different size ranges can be used to augment the pore network connectivity and tailor the shrinkage kinetics [16]. Other methods, such as freeze-casting, can also be used to generate pores. In freeze-casting, pores are generated as a result of ice sublimation in aqueous slurries [17]. Figure 3 shows side-view schematic of the multilayer structure in a SOEC/SOFC. The corresponding materials, morphology, and other attributes of each layer are given in the right column.
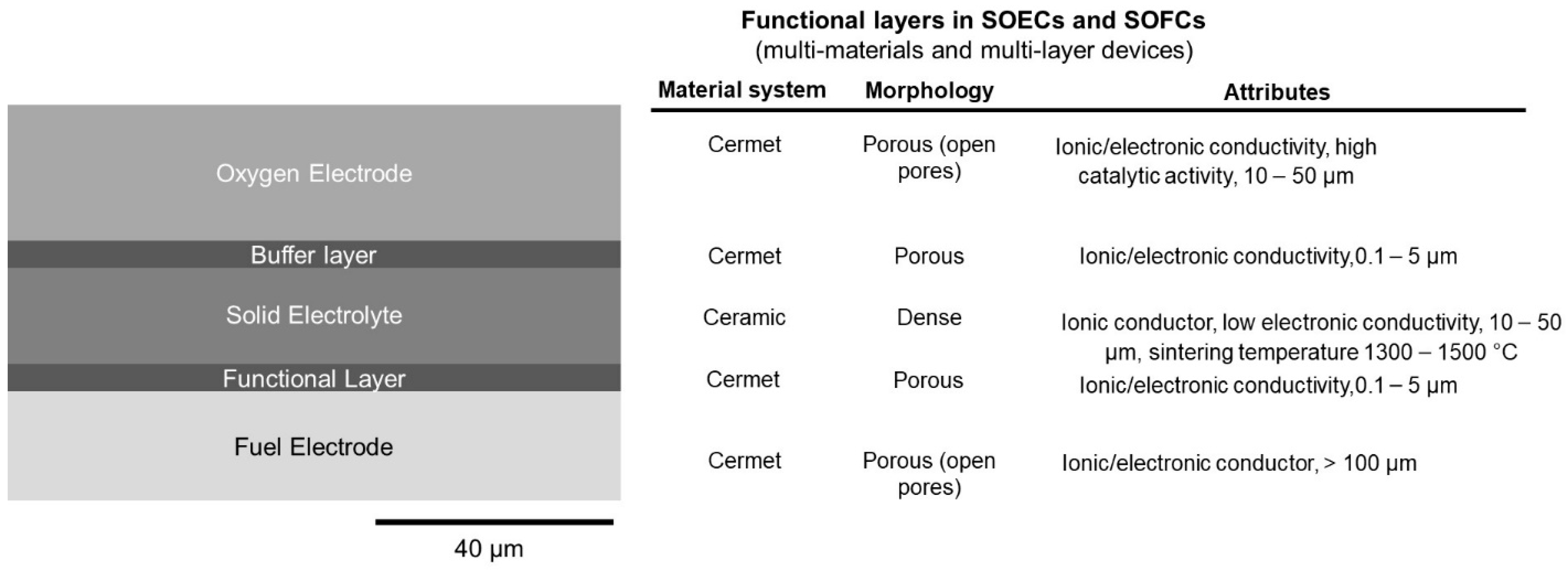
Figure 3.
Side-view schematic of the multilayer structure in a SOEC/SOFC (
left
). The corresponding materials, morphology, and other attributes of each layer (
right
).
The interconnect is a layer that sits between each individual cell and connects them in series. Interconnects are exposed to both oxidizing and reducing sides of the cell at high temperatures and, therefore, have the most demanding material requirements among other cell components in terms of stability. Generally, in these devices, two types of interconnects are used, metallic and ceramic oxides [18]. Ceramics are more stable (particularly for long-term stability) under oxidizing environments; however, they have lower electrical conductivity compared to metals and are expensive. Metallic interconnects have a lower cost and higher electrical conductivity; however, they have less stability than ceramics at high temperatures. One approach to increase the stability of metallic interconnects is coating them with protective ceramic layers, including oxides, perovskites, and spinel. The most common ceramics for interconnect applications include lanthanum and yttrium chromites (YCrO3 and LaCrO3) and perovskite p-type semiconductors [18]. AM processes on these particular ceramics are very limited. The main challenge with these materials has been difficulty in sintering Cr-containing oxides due to the vaporization of Cr-O species that complicates the sintering process.
Ferritic stainless steels (FSSs) are good candidates among metals, given their low cost, favorable CTE, ease of manufacturing, and formation of high electrical conductivity oxides on their surface. Chromium (Cr) evaporation under high operation temperature, however, has been a major limiting factor. Formation of native chromium oxide, which increases the ohmic resistance, and chromium poisoning of the SOFC cathode are two major degradation mechanisms in these devices [18]. Metal-ceramic composites (cermets) are also under consideration for interconnects, given their thermal stability at high temperatures and good electrical conductivity.
Sealant is another important component in these devices, for which no AM process has been yet reported. Often, the maximum working temperature of these devices is determined by the glass transition temperature of the sealant. Gas-tight (hermetic) sealants provide electrical insulation (prevent short-circuiting) and prevent mixing of the fuel and the oxidant. Glass-ceramic sealants are low-cost and have acceptable performance and stability (in both reducing and oxidizing environments) [19]. Thermal attributes of sealants, including CTE, glass transition temperature, crystallization temperature, and melting point, are the defining parameters for choosing a sealant. Glass-ceramic sealants form chemical bonding with the adjoining components and hence do not require external load during operation. These sealants provide low cost and reasonable stability, as well as flexible design by varying the composition. Partial crystallization by sintering above the device’s operating temperature can be achieved, which results in hermetic sealings. Currently, glass ceramics are fabricated by rolling, casting, pressing, and spin casting, among other methods. Both sealants and interconnects can be made of ceramic materials. As such, it is possible to develop AM processes based on full ceramics and cermets.
References
- Achieving American Leadership in the Hydrogen Supply Chain; U.S. Department of Energy: Washington, DC, USA, 2022.
- High Temperature Electrolysis Manufacturing Workshop Summary Report, Hydrogen and Fuel Cell Technologies Office; U.S. Department of Energy: Washington, DC, USA, 2022.
- Hauch, A.; Küngas, R.; Blennow, P.; Hansen, A.B.; Hansen, J.B.; Mathiesen, B.V.; Mogensen, M.B. Recent advances in solid oxide cell technology for electrolysis. Science 2020, 370, eaba6118.
- Water Electrolyzers and Fuel Cells Supply Chain: Supply Chain Deep Dive Assessment U.S. Department of Energy Response to Executive Order 14017, “America’s Supply Chains”; U.S. Department of Energy: Washington, DC, USA, 2022.
- Cramer, C.L.; Ionescu, E.; Graczyk-Zajac, M.; Nelson, A.T.; Katoh, Y.; Haslam, J.J.; Wondraczek, L.; Aguirre, T.G.; LeBlanc, S.; Wang, H.; et al. Additive manufacturing of ceramic materials for energy applications: Road map and opportunities. J. Eur. Ceram. Soc. 2022, 42, 3049–3088.
- Ruiz-Morales, J.C.; Tarancón, A.; Canales-Vázquez, J.; Méndez-Ramos, J.; Hernández-Afonso, L.; Acosta-Mora, P.; Marín Rueda, J.R.; Fernández-González, R. Three dimensional printing of components and functional devices for energy and environmental applications. Energy Environ. Sci. 2017, 10, 846–859.
- Weimar, M.R.; Chick, L.A.; Gotthold, D.W.; Whyatt, G.A. Cost Study for Manufacturing of Solid Oxide Fuel Cell Power Systems; Contract DE-AC05-76RL01830; Pacific Northwest National Laboratory: Richland, WA, USA, 2013.
- Pesce, A.; Hornés, A.; Núñez, M.; Morata, A.; Torrell, M.; Tarancón, A. 3D printing the next generation of enhanced solid oxide fuel and electrolysis cells. J. Mater. Chem. A 2020, 8, 16926–16932.
- Bertei, A.; Tariq, F.; Yufit, V.; Ruiz-Trejo, E.; Brandon, N.P. Guidelines for the Rational Design and Engineering of 3D Manufactured Solid Oxide Fuel Cell Composite Electrodes. J. Electrochem. Soc. 2016, 164, F89–F98.
- Chueh, C.-C.; Bertei, A. Thermo-mechanical analysis of 3D manufactured electrodes for solid oxide fuel cells. J. Eur. Ceram. Soc. 2021, 41, 497–508.
- Connor, P.A.; Yue, X.; Savaniu, C.D.; Price, R.; Triantafyllou, G.; Cassidy, M.; Kerherve, G.; Payne, D.J.; Maher, R.C.; Cohen, L.F.; et al. Tailoring SOFC Electrode Microstructures for Improved Performance. Adv. Energy Mater. 2018, 8, 1800120.
- Xing, B.; Cao, C.; Zhao, W.; Shen, M.; Wang, C.; Zhao, Z. Dense 8 mol% yttria-stabilized zirconia electrolyte by DLP stereolithography. J. Eur. Ceram. Soc. 2020, 40, 1418–1423.
- Shi, Y.; Cai, N.; Cao, T.; Zhang, J. High-Temperature Electrochemical Energy Conversion and Storage: Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2018.
- Liu, Y.; Shao, Z.; Mori, T.; Jiang, S.P. Development of nickel based cermet anode materials in solid oxide fuel cells—Now and future. Mater. Rep. Energy 2021, 1, 100003.
- Ferreira, V.J.; Wolff, D.; Hornés, A.; Morata, A.; Torrell, M.; Tarancón, A.; Corchero, C. 5 kW SOFC stack via 3D printing manufacturing: An evaluation of potential environmental benefits. Appl. Energy 2021, 291, 116803.
- Shri Prakash, B.; Senthil Kumar, S.; Aruna, S.T. Properties and development of Ni/YSZ as an anode material in solid oxide fuel cell: A review. Renew. Sustain. Energy Rev. 2014, 36, 149–179.
- Riyad, M.F.; Mahmoudi, M.; Minary-Jolandan, M. Manufacturing and Thermal Shock Characterization of Porous Yttria Stabilized Zirconia for Hydrogen Energy Systems. Ceramics 2022, 5, 472–483.
- Brouzgou, A.; Demin, A.; Tsiakaras, P. Interconnects for Solid Oxide Fuel Cells. In Advances in Medium and High Temperature Solid Oxide Fuel Cell Technology; Boaro, M., Salvatore, A.A., Eds.; Springer International Publishing: Cham, Switzerland, 2017.
- Sulistyo, G.; Iwan, S. Progress in Glass-Ceramic Seal for Solid Oxide Fuel Cell Technology. J. Adv. Res. Fluid Mech. Therm. Sci. 2021, 82, 39–50.
More
