Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 1 by Sabine Groeger.
Orthodontically induced inflammatory root resorption (OIIRR) is considered an undesired and inevitable complication induced by orthodontic forces. This inflammatory mechanism is regulated by immune cells that precede orthodontic tooth movement (OTM) and can influence the severity of OIIRR. OIIRR occurs on almost all tooth roots exposed to orthodontic forces.
- orthodontic tooth movement
- orthodontic force
- OIIRR
- PD-L1
- immunorthodontics
1. Pathophysiology of OIIRR
In 2002, Brezniak et al. proposed the more descriptive and accurate term of orthodontic force-induced root resorption in light of the actual histologic process and termed it OIIRR [4][1]. The pathogenesis of OIIRR has received a lot of attention in the past decade, especially in the view of the immune system responses [27][2]. The apical root third (1/3) is covered mainly with cellular cementum, which consists of cementocytes in lacunae and living cementoblasts with the supporting vasculature, thus forming a non-mineralized cementoid layer [28][3] and making this area vulnerable to forces and cell-injury-related reactions [29][4]. On the other hand, blood vessels occupy approximately 47% of the space within the PDL in the apical region, in comparison with 4% at the cervical area of the root [30][5], which makes the blood supply of this apical third of the PDL better than in the rest of the root surface. Therefore, when forces damage the protective layer, it leads to exposure of dentine and, affected by resorption, the corresponding apical 1/3 parts of the root are more easily “digested” and the newly available space is filled with bone components [26][6]. However, in the cervical area, the root is covered by acellular cementum and a resorption-resistant layer made of predentine, dentine, and mineralized repair tissue [31][7], forming a protective local lining of the cervical area on the root surface. Moreover, osteoclasts are not capable of binding to a non-mineralized surface [32][8], indicating that resorption cervically is rare. Radiographically, this results in tooth root shortening. Common sites of OIIRR are found close to the hyalinized zone on the pressure area of the tooth root [33][9] when strong forces are subjected for a sustained period of time. Based on mice and rat studies, Brudvik et al. (1993) observed a consistent pattern of OIIRR. This started with the rapid development of an ischemic necrosis (hyalinization) of the compressed PDL. Root resorption then started in the circumference of this necrotic (hyalinized) area. Only after several days did resorption occur in the central parts of the hyalinized zone [33][9]. The authors suggested that OIIRR is an elimination process of the hyaline zone. These resorptive phenomena are followed by compromised blood vessels and a low oxygen supply [34][10], with PDL cells dying as a result of hypoxia in the nearby hyaline zone and the resulting cell-free area developing a glassy histological appearance. During removal of the hyaline tissue, the surrounding outer surface of the root, which is composed of the cementoblast layer covering the cementoid, can be further destroyed [35][11], thereby exposing the dense mineralized cementum beneath to the forces. Odontoclasts/osteoclasts are then activated and attach to the mineral matrix, forming a sealing zone and adopting a polarized morphology that initiates mineral resorption of cementum and dentine [36][12]. This resorption process continues until there is no hyalinized tissue present and/or the level of orthodontic force application diminishes. Then, the hyalinized tissue is resorbed by an influx of phagocytic cells and osteoclasts, which are recruited to the necrotic tissues (indirect resorption). As a result of these resorptive cells’ indiscriminate action against both necrotic and root hard tissue, cementum and occasionally dentine are resorbed by the recruited macrophages, odontoclasts, and osteoclasts [4][1]. Resorption eventually developed across the entire root surface as time progressed. In certain tooth roots, resorptive activity even continued after the force removal [37][13].
The cause and risks of OIIRR are complex, but it is believed that sterile inflammatory processes include different components: mechanical forces (magnitudes), types of mechanical forces (continuous, interrupted, or intermitted), direction and duration of force, tooth root morphology, distance of root apical movement, surrounding matrix, PDL, cementum, and certain biological messengers [26,38][6][14]. The severity of OIIRR is classified into three levels: (1) cementum or surface resorption with remodeling; (2) dentine resorption with repair (deep resorption); and (3) circumferential apical root resorption [4][1]. In humans, the OIIRR-associated risk factors such as exact force magnitude may be dependent on the individual variations in humans (Harris et al., 2006), and thus have not been determined until now. It has been widely known that the heavy rotational forces produced significantly more root resorption than light rotational forces and the compression area shows significantly higher root resorption than other areas in the human study [39][15].
2. Resorptive and Immune Cells in OIIRR
The key resorptive cells involved in OIIRR of cementum include mono-nucleated macrophage-like cells, osteoclasts, dentinoclasts, and odontoclasts, the latter of which are extremely similar in the morphologic and functional aspects to those of osteoclasts, albeit slightly smaller [40][16] (Figure 1).
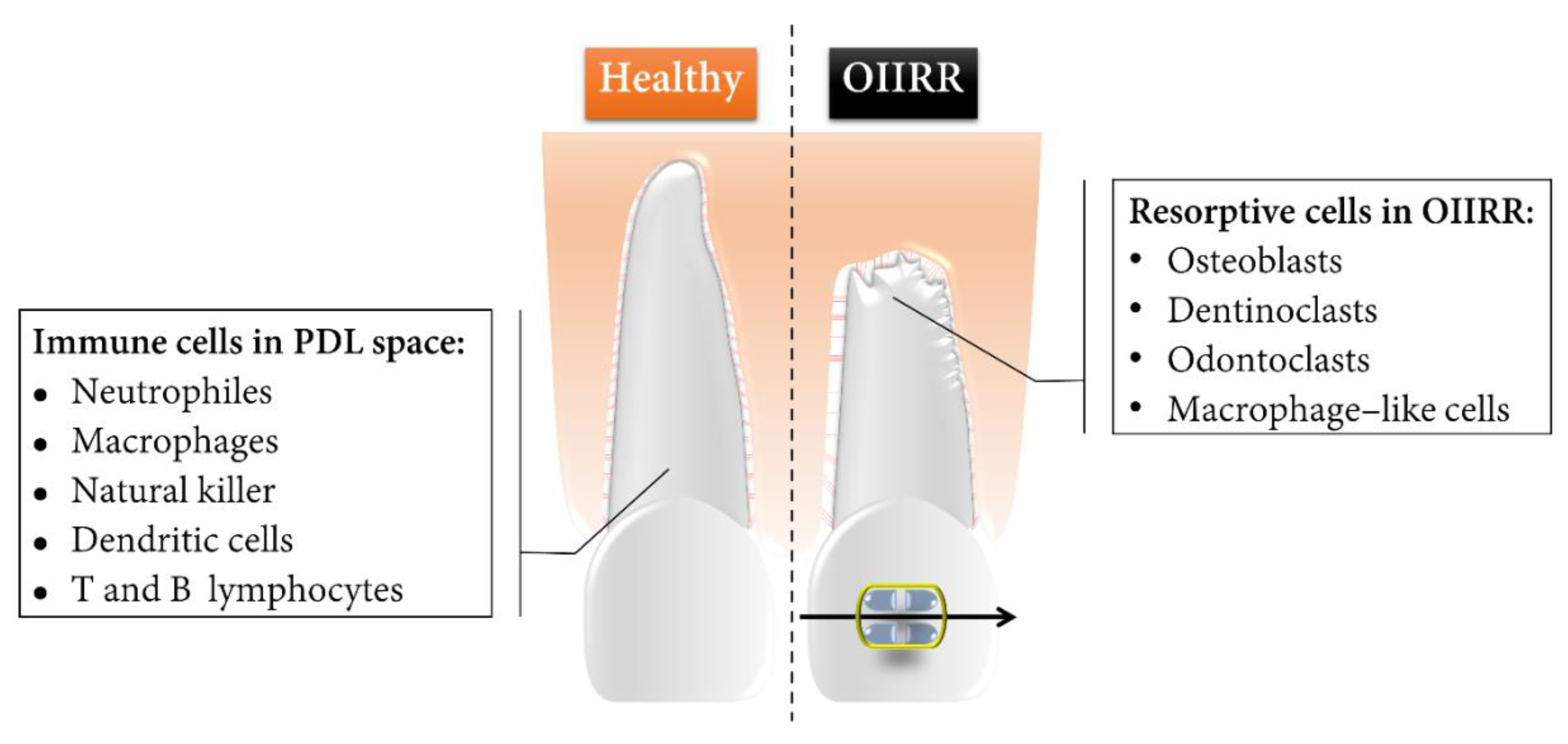
Figure 1. Schematic representation of the healthy tooth root and OIIRR and the core components of the immune and resorptive cells during OTM. Root resorption lacunae were marked mainly in the apical region as well as on the pressure side of the orthodontically moved tooth root.
Endocytotic vesicles containing liberated apatite crystals are found in odontoclasts, implying that demineralization in the resorptive microenvironments is not completely comparable with osteoclasts [41][17]. Activated osteoclasts are derived from the bone-marrow-derived circulating monocyte/macrophage lineage [42][18]. Activated odontoclasts are differentiated from circulating monocytes that express tartarate-resistant acid phosphatase (TRAP) [43][19].
The immunological system response accompanied with inflammatory responses during OTM and OIIRR relies on the cooperative activities between innate and adaptive immunity [27][2]. Monocytes are precursors of DCs, osteoclasts, and macrophages [44][20]. Monocytes would be recruited to the site of irritation by locally produced pro-inflammatory cytokines, and they afterwards differentiate into macrophages or dendritic cells [45][21]. Odontoclasts and dentinoclasts share a common origin, but the latter specifically resorb dentine [46][22]. Macrophages are scavenger cells whose function is to eliminate necrotic tissues [4][1] depending on the phenotypes of the macrophages that could be activated by endotoxins and cytokines from T cells. Activated macrophages can eliminate pathogens, produce pro-inflammatory cytokines, and present the antigens to T cells [47][23]. T lymphocyte cells are a crucial mediator of an adaptive immune response for OTM to initiate cellular immunity [27][2].
In addition, multinucleated TRAP-positive giant cells participate in hyalinized tissue removal and the adjacent root structure resorption [33][9]. TRAP-positive cells are able to differentiate into fully developed odontoclasts or osteoclasts in response to a mechanical stimulus in a matter of hours [48][24].
3. Cementum Repair in OIIRR
Decompression alters the resorption process and repair of the cementum process is initiated once the applied orthodontic forces have been discontinued [49,50][25][26]. Physiologically speaking, cementoblasts begin the biological process of repairing these resorptive defects within a few days. The resorption lacunae are initially covered in a layer of acellular cementum deposition. However, over several weeks or months, these lacunae are mostly replenished with cellular cementum [51][27]. Jaeger et al. (2008) [51][27] showed that, in the in vitro rodent model, the reparation processes of cementum did not initiate until the orthodontic force was released. It is also notable that the cervical and apical portions of the root appear to usually lead to cellular cementum repair. Nearly half of the resorption lesions were being repaired after five weeks without the orthodontic forces, and almost 90% after 10 weeks. Similar results were also reported in human studies.
This process usually takes at least 6–8 weeks to become radiographically visible [29][4]. However, this process is only superficial and can thus not replace a resorbed apical part of a root. Apical root resorptions of less than one-third of the root length usually do not have clinical consequences. Irreparable damage to the root surface and loss of more than 1/3 of the original root length occur in 1–5% of clinical cases [52][28]. This kind of severe root resorption can manifest itself clinically as increased tooth mobility and even occasional tooth loss.
References
- Brezniak, N.; Wasserstein, A. Orthodontically induced inflammatory root resorption. Part I: The basic science aspects. Angle Orthod. 2002, 72, 175–179.
- Al-Ghurabi, B.; Al-Hindawi, S.; Mohammed, I. Physiological Role of Immune System Elements in Orthodontic Treatment. Med.-Leg. Update 2020, 20, 6767–6772.
- Hammarstrom, L.; Lindskog, S. General morphological aspects of resorption of teeth and alveolar bone. Int. Endod. J. 1985, 18, 93–108.
- Krishnan, V. Root Resorption with Orthodontic Mechanics: Pertinent Areas Revisited. Aust. Dent. J. 2017, 62 (Suppl. S1), 71–77.
- Blaushild, N.; Michaeli, Y.; Steigman, S. Histomorphometric study of the periodontal vasculature of the rat incisor. J. Dent. Res. 1992, 71, 1908–1912.
- Yamaguchi, M.; Fukasawa, S. Is Inflammation a Friend or Foe for Orthodontic Treatment?: Inflammation in Orthodontically Induced Inflammatory Root Resorption and Accelerating Tooth Movement. Int. J. Mol. Sci. 2021, 22, 2388.
- Mavridou, A.M.; Pyka, G.; Kerckhofs, G.; Wevers, M.; Bergmans, L.; Gunst, V.; Huybrechts, B.; Schepers, E.; Hauben, E.; Lambrechts, P. A novel multimodular methodology to investigate external cervical tooth resorption. Int. Endod. J. 2016, 49, 287–300.
- Galler, K.M.; Gratz, E.M.; Widbiller, M.; Buchalla, W.; Knuttel, H. Pathophysiological mechanisms of root resorption after dental trauma: A systematic scoping review. BMC Oral. Health 2021, 21, 163.
- Brudvik, P.; Rygh, P. The initial phase of orthodontic root resorption incident to local compression of the periodontal ligament. Eur. J. Orthod. 1993, 15, 249–263.
- Goz, G.R.; Rahn, B.A.; Schulte-Monting, J. The effects of horizontal tooth loading on the circulation and width of the periodontal ligament—An experimental study on beagle dogs. Eur. J. Orthod. 1992, 14, 21–25.
- Hellsing, E.; Hammarstrom, L. The hyaline zone and associated root surface changes in experimental orthodontics in rats: A light and scanning electron microscope study. Eur. J. Orthod. 1996, 18, 11–18.
- Georgess, D.; Machuca-Gayet, I.; Blangy, A.; Jurdic, P. Podosome organization drives osteoclast-mediated bone resorption. Cell Adhes. Migr. 2014, 8, 191–204.
- Winter, B.U.; Stenvik, A.; Vandevska-Radunovic, V. Dynamics of orthodontic root resorption and repair in human premolars: A light microscopy study. Eur. J. Orthod. 2009, 31, 346–351.
- Brezniak, N.; Wasserstein, A. Orthodontically induced inflammatory root resorption. Part II: The clinical aspects. Ang. Orthod. 2002, 72, 180–184.
- Wu, A.T.; Turk, T.; Colak, C.; Elekdag-Turk, S.; Jones, A.S.; Petocz, P.; Darendeliler, M.A. Physical properties of root cementum: Part 18. The extent of root resorption after the application of light and heavy controlled rotational orthodontic forces for 4 weeks: A microcomputed tomography study. Am. J. Orthod. Dentofac. Orthop. 2011, 139, e495–e503.
- Wang, Z.; McCauley, L.K. Osteoclasts and odontoclasts: Signaling pathways to development and disease. Oral. Dis. 2011, 17, 129–142.
- Limeback, H. Molecular mechanisms in dental hard tissue mineralization. Curr. Opin. Dent. 1991, 1, 826–835.
- Kamat, M.; Puranik, R.; Vanaki, S.; Kamat, S. An insight into the regulatory mechanisms of cells involved in resorption of dental hard tissues. J. Oral. Maxillofac. Pathol. 2013, 17, 228–233.
- Kumar, G. Orban’s Oral Histology & Embryology-E-BOOK; Elsevier Health Sciences: Philadelphia, PA, USA, 2015.
- Chaushu, S.; Klein, Y.; Mandelboim, O.; Barenholz, Y.; Fleissig, O. Immune Changes Induced by Orthodontic Forces: A Critical Review. J. Dent. Res. 2022, 101, 11–20.
- Ne, R.F.; Witherspoon, D.E.; Gutmann, J.L. Tooth resorption. Quintessence Int. 1999, 30, 9–25.
- Nanci, A. Physiologic tooth movement: Eruption and shedding. In Ten Cate’s Oral Histology—Development, Structure, and Function; Elsevier: Philadelphia, PA, USA, 2007; pp. 268–289.
- Hidalgo, M. Study About the Immunogenic Potential of Dentin: A Contribution to the Etiopathogeny of Root Resorption. Ph.D. Thesis, Faculdade de Odontologia de Bauru, Universidade de São Paulo, Bauru, Brazil, 2001.
- Sismanidou, C.; Hilliges, M.; Lindskog, S. Healing of the root surface-associated periodontium: An immunohistochemical study of orthodontic root resorption in man. Eur. J. Orthod. 1996, 18, 435–444.
- Brudvik, P.; Rygh, P. Transition and determinants of orthodontic root resorption-repair sequence. Eur. J. Orthod. 1995, 17, 177–188.
- Brudvik, P.; Rygh, P. The repair of orthodontic root resorption: An ultrastructural study. Eur. J. Orthod. 1995, 17, 189–198.
- Jager, A.; Kunert, D.; Friesen, T.; Zhang, D.; Lossdorfer, S.; Gotz, W. Cellular and extracellular factors in early root resorption repair in the rat. Eur. J. Orthod. 2008, 30, 336–345.
- Weltman, B.; Vig, K.W.; Fields, H.W.; Shanker, S.; Kaizar, E.E. Root resorption associated with orthodontic tooth movement: A systematic review. Am. J. Orthod. Dentofac. Orthop. 2010, 137, 462–476, discussion 12A.
More
