Spectro-Fluor technology is an unique, innovative as well as a versatile and green spectroscopic method that allows to detect very sensitively, specifically and rapidly C-F bonds in any solutions, gas, materials, organisms (e.g. human/animal cells, bacteria, virus, fungi, body tissues) and molecules (e.g. drugs, polymers, nano and smaller molecules).
Spectro-Fluor technology is an unique, innovative as well as a versatile and green spectroscopic method that allows to detect very sensitively, specifically and rapidly C-F bonds in any solutions, gas, materials, organisms (e.g. human/animal cells, bacteria, virus, fungi, body tissues) and molecules (e.g. drugs, polymers, nano and smaller molecules). We offer licensing, services, training and consulting.
- Spectro-Fluor Technology
- C-F bonds
- Sensing
- Biomarkers
- Imaging
- Integrative and Green Medicine
- Personalized medicine
- Translational medicine
- Spectroscopy
- Raman
Please note: Below is an entry draft based on your previous paper, which is wrirren tightly around the entry title. Since it may not be very comprehensive, we kindly invite you to modify it (both title and content can be replaced) according to your extensive expertise. We believe this entry would be beneficial to generate more views for your work. In addition, no worry about the entry format, we will correct it and add references after the entry is online (you can also send a word file to us, and we will help you with submitting).
Definition
Quantitative and qualitative characterization of fluorinated molecules represents an important task. Fluorine-based medicinal chemistry is a fast-growing research area due to the positive impact of fluorine in drug discovery, and clinical and molecular imaging (e.g., magnetic resonance imaging, positron emission tomography). Common detection methods include fluorinated-based labelling using radioactive isotopes or fluorescent dyes. Nevertheless, these molecular imaging methods can be harmful for health due to the potential instability of fluorochromes and cytoxicity of radioisotopes. we have developed, validated and patented carbon-fluorine spectroscopy (CFS™), recently renamed Spectro-Fluor™ technology, which among a non-competitive family of in-house made devices called PLIRFA™ (Pulsed Laser Isochronic Raman and Fluorescence Apparatus™), allows reliable detection of Carbon-Fluorine (C-F) bonds. C-F bonds are known to be stable and safe labels once incorporated to any type of molecules, cells, compounds or (nano-) materials. In this pioneered research study, we used Spectro-Fluor™ to assess biomarkers. As a proof-of-principle experiment, we have established a three-step protocol intended to rapid protein detection, which simply consisted of: (i) incorporating a sufficient concentration of an aromatic amino-acid (fluorinated versus non-fluorinated) into cultured cells; (ii) simultaneously isolating the fluorinated protein of interest and the non-fluorinated form of the protein (control) by immune-precipitation; (iii) comparatively analyzing the respective spectrum obtained for the two protein forms by Spectro-Fluor™. Thereby, we were able to differentiate, from colon cancer cells HCT-116, the fluorinated and non-fluorinated forms of p21, a key transcriptional factor and downstream target of p53, the so-called “guardian of the genome”. Taken together, our data again demonstrates the beneficial alternative use of Spectro-Fluor™, which once combined with an innovative methodology permits one to quickly, reliably, safely and cost-effectively detect physiological or pathological proteins in cells.
1. Introduction
The concept of Spectro-Fluor™ technology is mainly based on the discovery of characteristic C-F fingerprint(s) in the spectral area of 500–900 cm−1 allowing detection, characterization, imaging, monitoring, screening and measurement of fluoro-nanomaterials, fluoro-compounds, fluoro-molecules, fluoroorganic impurities or fluoro-degradation products [1][2][3][1,2,3]. Spectro-Fluor™ aka Carbon-Fluorine Spectroscopy™ (CFS™) is one of the key tools of our in-house technological platform called PLIRFA™ (Pulsed Laser Isochronic Raman and Fluorescence Apparatus™), which provides alternative solutions and applications across the biomedical (e.g., molecular/cell/tissue imaging), life (e.g., unravelling of molecular interactions, structures, conformations), pharmaceutical (e.g., drug design, delivery, tracing, discovery), environmental (e.g., characterization of pesticides, other contaminants), and nano(bio)technological (e.g., characterization of nano-molecules/materials) fields [1][2][1,2]. Thereby, Spectro-Fluor™ represents an innovative, disruptive, green, affordable, cost-effective, reliable and flexible analytical tool of high resolution, sensitivity and specificity (Figure 1) [1][2][3][1,2,3]. Interestingly, this technology constitutes a major advance over conventional spectroscopy technologies (e.g., conventional Raman tools) because of its capability to: (i) highly specific and ultrasensitive detect (ppm-ppb level or <10 cells) C-F bonds (–C-F, =CF2, –CF3); (ii) detect and characterize any halo-organic bonds or unlabeled molecules, regardless their physical state (solid, liquid or gas), via glass and polymer containers, quartz vials; (iii) acquire data in real-time (0.1 second per data point from 1000 average pulses); (iv) quantitatively determine any fluorinated substance since the emitted C-F bond(s) signal is directly proportional to the analyte concentration; (v) unravel chemical structures (e.g., molecular length determination, molecular analogs differentiation with as low as one carbon resolution); (vi) preserve the sample integrity (i.e., non-destructive and non-invasive technology) allowing re-using of the sample; (vii) analyze any unprepared sample in a solvent-less/green fashion; (viii) easily provide data interpretation thanks to its interconnection with powerful software and in-house developed databases; (ix) be used with low maintenance/calibration requirements; (x) operate using adequate time-gating and time-delays with a single laser source functioning in three modes: continuous detection, pulsed detection and two photons excitation/time resolved fluorescence; (xi) be conveniently employed anywhere due to new generation of portable/compact devices incorporating fiber optics; (xii) be versatile, flexible, progressive and hyphenated opening possibilities to be coupled/hybridized to diverse technology platforms such as high-throughput screening (HTS) (e.g., arrays, multiplex assays systems), microscopy and other imaging systems (e.g., confocal laser scanning microscopy, atomic force microscopy (AFM), positron emission tomography (PET), magnetic resonance imaging (MRI), computed tomography (CT)), chromatography (e.g., high-performance liquid chromatography (HPLC), mass spectrometry (MS)), sorting instruments (e.g., fluorescence-activated cell sorting (FACS) or magnetic-activated cell sorting (MACS)).
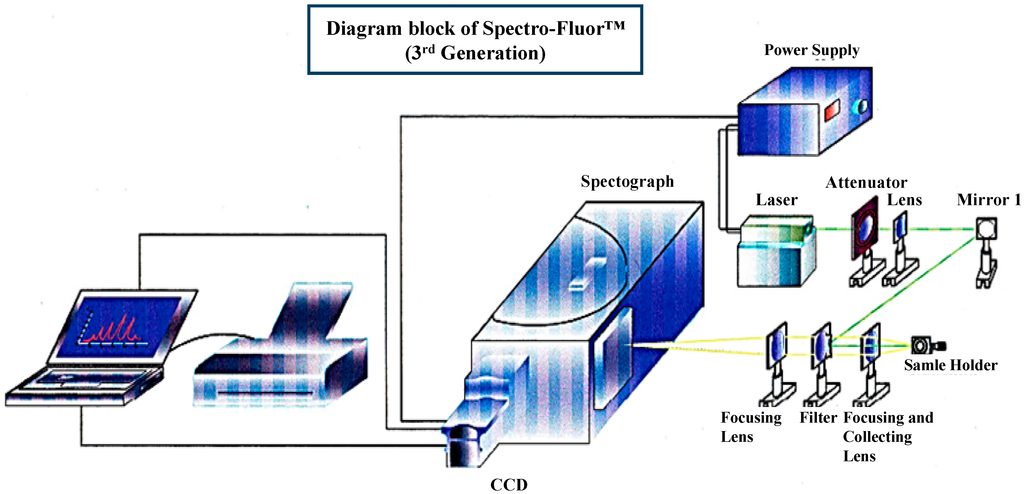
Figure 1.
Besides, the specific features of fluorine in the formation of fluorocarbons make it attractive in the design of non-viscous but polar organic compounds, with a polarity limited to influencing the intra-molecular nature and inter-molecular interactions with the microenvironment [4][5][4,5]. Interestingly, carbon-fluorination displays the additional following properties [6][7][6,7]: (i) enhancement of thermal stability (107 Kcal/mol); (ii) increase of lipophilicity, hydrophobicity and solubility; (iii) improvement of the molecular bioavailability (e.g., diminution of the basicity of neighboring amines); and (iv) size comparable to H (i.e., 1.47 Å versus 1.20 Å) capable of mimicking enzyme substrates. Importantly, C-F bonds are [1][2][3][4][1,2,3,4]: (i) unique in nature; (ii) smaller and more stable than fluorescent dyes due to their covalent interactions; (iii) much less toxic than radioisotopes avoiding handling and logistic problems; (iv) less harmful at long-term exposure than radio-waves; and (v) inexpensively incorporated into molecules, compounds, materials or cells. Therefore, C-F bond(s) can be used in a number of industrial and academic areas including pharmacy, medicine and nanotechnology to characterize them, monitor them or confer some enhanced physicochemical features [1][2][3][4][1,2,3,4]. In terms of applications, a C-F bond can be employed as [1][2][3][4][8][9][10][11][1,2,3,4,8,9,10,11]: (i) a pharmaceutical security label to enhance the drug safety, thereby preventing and banning illegitimate drugs (i.e., counterfeited or sub-standard drugs); (ii) a nano-label to enable drug trace during the whole pharmaceutical development process (i.e., discovery, synthesis and production cycles, clinical and post-approval stages), subsequently bringing the product faster to the market; (iii) an ex vivo, in situ or in vivo label for biologics (e.g., peptides, proteins including antibodies, nucleic acids) and cells/tissues (e.g., stem cells, tumor cells); (iv) a functional chemical group for nanomaterials (e.g., nanoparticles, nanocarbon tubes, nanogels, nanoemulsions, nanoporous silica glasses).
2. Results
We have developed an innovative photonic biotool called Spectro-Fluor™ Technology (Fluorotronics, Inc.) as well as a new in vitro method based on ex vivo incorporation of fluorinated amino acids into cells in order to detect lowly-expressed molecules such as biomarkers of disease. (Figure 1 and Figure 2). Indeed, the advantages of Spectro-Fluor™ Technology (aka Carbon-Fluorine spectroscopy) over routinely used laboratory methods (e.g., Western blots, ELISA, protein dot blots) are represented by the rapid, safe, reliable, highly sensitive and specifique detection of C-F bond(s) formed into molecules [1].
We have established a valuable ex vivo protein labeling protocol that consisted of three steps (Figure 2): (i) addition in excess (e.g., in this study, 10 mg/mL as non-limiting concentration) of a selected aromatic fluoro-aminoacid (e.g., in this study, 4-fluoro-l-phenylalanine) into an adequate medium-containing cells (e.g., in this study, human colon carcinoma HCT-116 cells). In parallel, the corresponding non-modified amino-acid (e.g., in this study, l-phenylalanine) was used as control; (ii) immediately after the amino-acid addition (control) and after a convenient period of time (e.g., in this study, 24 h), simultaneous isolation of both F-p21 and p21 (control) was performed by immune-precipitation (IP). IP is a procedure by which proteins or peptides that react specifically with an antibody are removed from solution and examined for quantity or physical characteristics. The expression of the two p21 protein forms (i.e., F-p21 and p21) was routinely checked by Western blot, a widely used biochemical technique albeit long and expensive (data not shown due to low basal expression detectable with Western blot); (iii) comparative examination of the respective spectra obtained by Spectro-Fluor™ technology obtained in same experimental conditions.
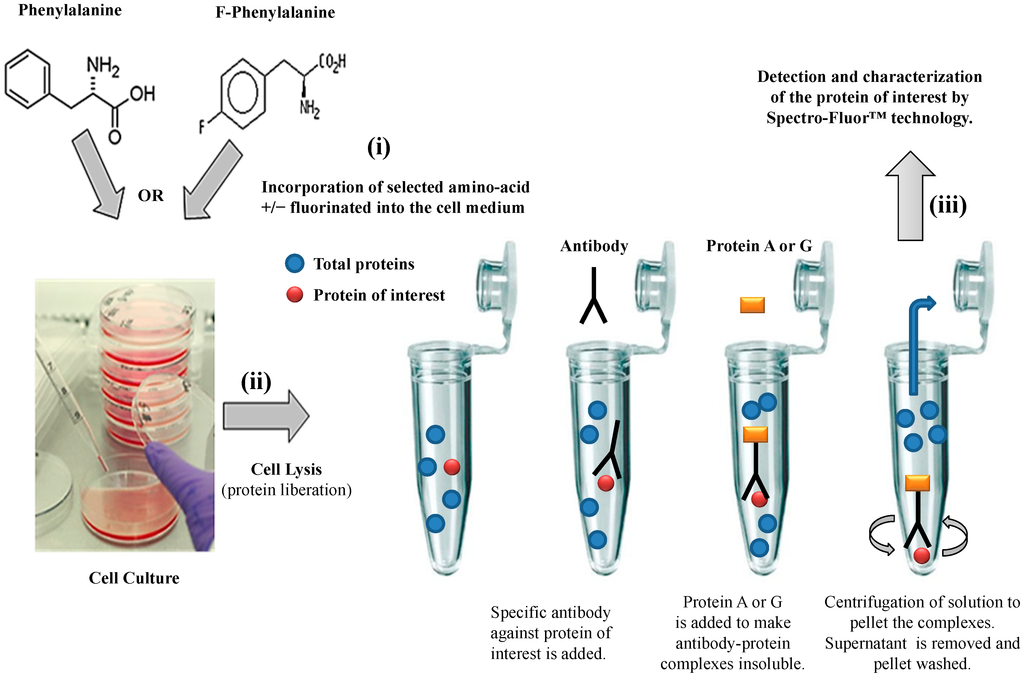
Figure 2. Flow chart of the experimental methodology. The three-step process is resumed by: (i) Ex-vivo protein labeling by incorporation of either 4-fluoro-l-phenylalanine. l-phenylalanine is used as a control; (ii) immune-precipitation by using only one antibody against the antigen/epitope of the protein of interest; (iii) detection and characterization of the protein of interest by Spectro-Fluor™ technology.
3. Conclusions
Fluorinated molecules, widely used in medicinal chemistry (e.g., drug design), emerge in the biomedical field (e.g., fluorinated bio-probes and theranostics™ such as F-nucleic acids, F-peptides) because of their special features (e.g., enhanced bioavailability and stability). Pharmacological studies suggest that fluorine modification may significantly reduce undesirable drug side-effects meantime offering superior efficacy. Therefore, we postulated that fluorinated proteins (e.g., biomarkers of disease, antibodies) should also be beneficial. Within the PLIRFA™ technology platform, which includes progressive analytical devices, we developed a becoming gold-standard Spectro-Fluor™ technology as well as a panel of green methods, enabling rapid, cost-effective, sensitive and specific detection and characterization of F-molecules, F-compounds, F-cells, F- tissues or F-(nano-)biomaterials. In this study, we successively show the possibility to detect a fluorinated protein (i.e., p53-downstream target p21) by Spectro-Fluor™ technology. This strongly suggests that this innovative biotechnology could significantly offer alternative solutions for a wide number of applications (e.g., molecular design, molecular imaging, development of F-antibodies) and technologies routinely used in laboratories and hospitals (e.g., western-blot, enzyme-linked immune sorbent assay, mass-spectrometry, MRI, PET). Eventually, we report that Spectro-Fluor™ technology is able to reduce the troublesome detection associated with low-expressed molecules (e.g., p21).
