The growing emergence of antimicrobial resistance represents a global problem that not only influences healthcare systems but also has grave implications for political and economic processes. As the discovery of novel antimicrobial agents is lagging, one of the solutions is innovative therapeutic options that would expand our armamentarium against this hazard. Compounds of interest in many such studies are antimicrobial peptides (AMPs), which actually represent the host’s first line of defense against pathogens and are involved in innate immunity. They have a broad range of antimicrobial activity against Gram-negative and Gram-positive bacteria, fungi, and viruses, with specific mechanisms of action utilized by different AMPs.
- antimicrobial peptides
- antimicrobial resistance
- antimicrobial effects
- antibacterial activity
1. Introduction
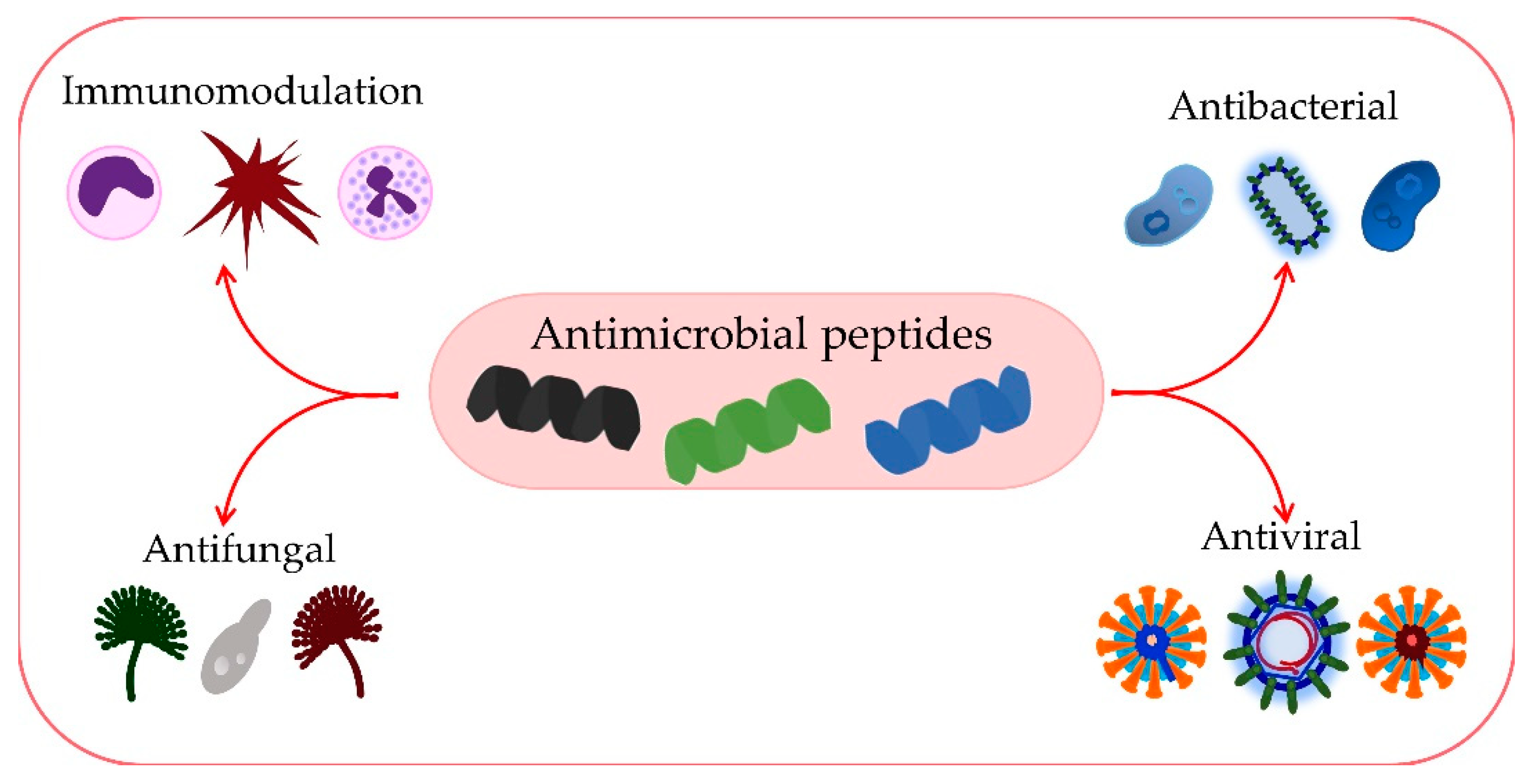
2. Antimicrobial Effects of AMPs
2.1. Antibacterial Activity of AMPs
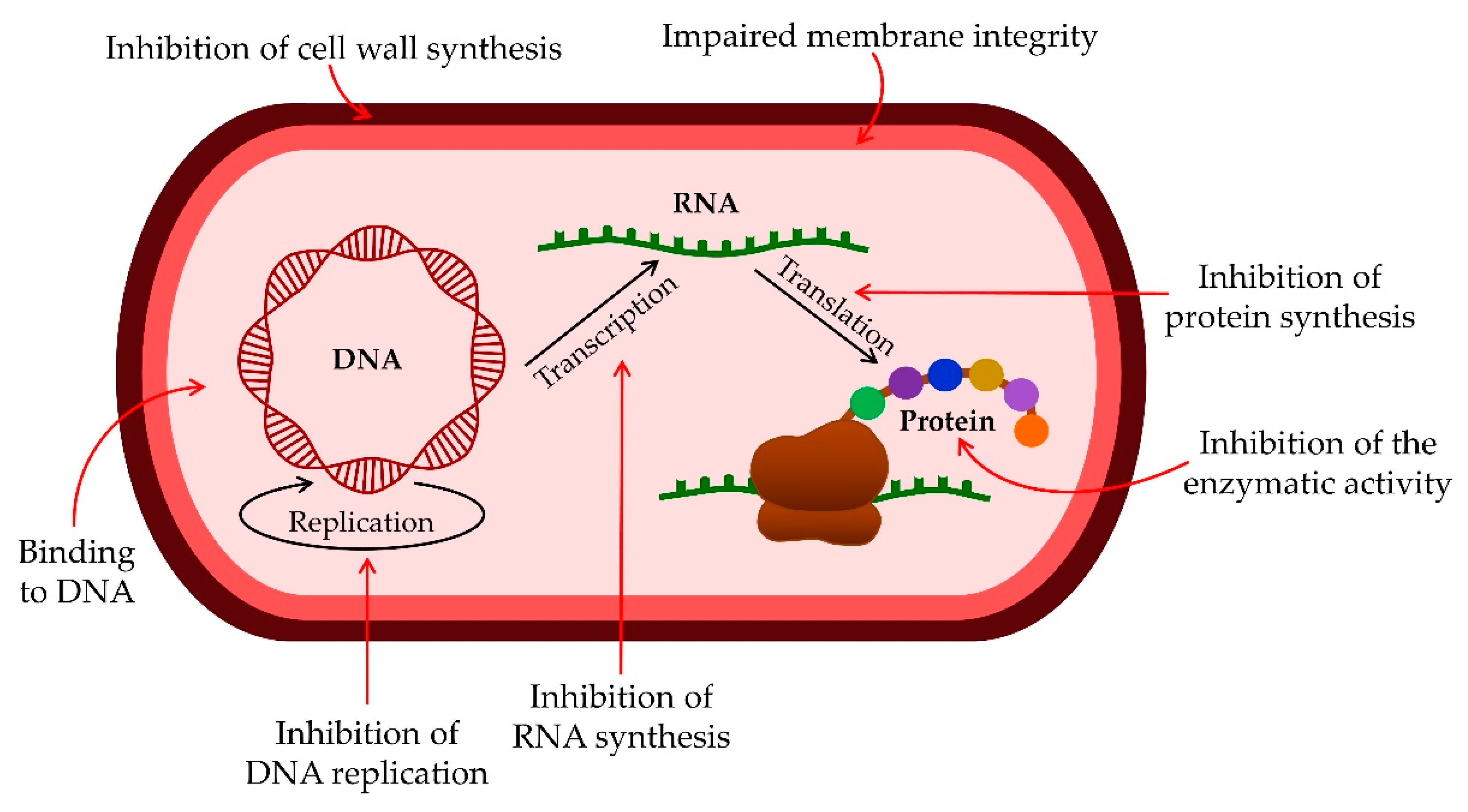
2.2. Antiviral Activity of AMPs
2.3. Antifungal Activity of AMPs
2.4. Immunomodulatory Activity of AMPs
2.4.1. Defenses
2.4.2. Histatins
2.4.3. Cathelicidins
References
- Lei, J.; Sun, L.C.; Huang, S.; Zhu, C.; Li, P.; He, J.; Mackey, V.; Coy, D.H.; He, Q.Y. The antimicrobial peptides and their potential clinical applications. Am. J. Transl. Res. 2019, 11, 3919–3931.
- Talapko, J.; Škrlec, I. The Principles, Mechanisms, and Benefits of Unconventional Agents in the Treatment of Biofilm Infection. Pharmaceuticals 2020, 13, 299.
- Burnham, J.P. Climate change and antibiotic resistance: A deadly combination. Ther. Adv. Infect. Dis. 2021, 8, 2049936121991374.
- Giacomini, E.; Perrone, V.; Alessandrini, D.; Paoli, D.; Nappi, C.; Esposti, L.D. Evidence of Antibiotic Resistance from Population-Based Studies: A Narrative Review. Infect. Drug Resist. 2021, 14, 849–858.
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Robles Aguilar, G.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655.
- Bhattacharjya, S.; Mohid, S.A.; Bhunia, A. Atomic-Resolution Structures and Mode of Action of Clinically Relevant Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 4558.
- Shaka, M.; Arias-Rojas, A.; Hrdina, A.; Frahm, D.; Iatsenko, I. Lipopolysaccharide-mediated resistance to host antimicrobial peptides and hemocyte-derived reactive-oxygen species are the major Providencia alcalifaciens virulence factors in Drosophila melanogaster. PLoS Pathog. 2022, 18, e1010825.
- Bhattacharjya, S.; Straus, S.K. Design, Engineering and Discovery of Novel α-Helical and β-Boomerang Antimicrobial Peptides against Drug Resistant Bacteria. Int. J. Mol. Sci. 2020, 21, 5773.
- Ghimire, J.; Guha, S.; Nelson, B.J.; Morici, L.A.; Wimley, W.C. The Remarkable Innate Resistance of Burkholderia bacteria to Cationic Antimicrobial Peptides: Insights into the Mechanism of AMP Resistance. J. Membr. Biol. 2022; in press.
- Gan, B.H.; Gaynord, J.; Rowe, S.M.; Deingruber, T.; Spring, D.R. The multifaceted nature of antimicrobial peptides: Current synthetic chemistry approaches and future directions. Chem. Soc. Rev. 2021, 50, 7820–7880.
- Marshall, J.S.; Warrington, R.; Watson, W.; Kim, H.L. An introduction to immunology and immunopathology. Allergy Asthma. Clin. Immunol. 2018, 14, 49.
- Reddy, K.V.R.; Yedery, R.D.; Aranha, C. Antimicrobial peptides: Premises and promises. Int. J. Antimicrob. Agents 2004, 24, 536–547.
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779.
- León-Buitimea, A.; Garza-Cárdenas, C.R.; Garza-Cervantes, J.A.; Lerma-Escalera, J.A.; Morones-Ramírez, J.R. The Demand for New Antibiotics: Antimicrobial Peptides, Nanoparticles, and Combinatorial Therapies as Future Strategies in Antibacterial Agent Design. Front. Microbiol. 2020, 11, 1669.
- Larsson, D.G.J.; Flach, C.F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269.
- Pollard, A.J.; Bijker, E.M. A guide to vaccinology: From basic principles to new developments. Nat. Rev. Immunol. 2021, 21, 83–100.
- Moravej, H.; Moravej, Z.; Yazdanparast, M.; Heiat, M.; Mirhosseini, A.; Moosazadeh Moghaddam, M.; Mirnejad, R. Antimicrobial Peptides: Features, Action, and Their Resistance Mechanisms in Bacteria. Microb. Drug Resist. 2018, 24, 747–767.
- Boman, H.G. Antibacterial peptides: Basic facts and emerging concepts. J. Intern. Med. 2003, 254, 197–215.
- Fjell, C.D.; Hiss, J.A.; Hancock, R.E.W.; Schneider, G. Designing antimicrobial peptides: Form follows function. Nat. Rev. Drug Discov. 2011, 11, 37–51.
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401.
- Koch, A.L. Bacterial wall as target for attack: Past, present, and future research. Clin. Microbiol. Rev. 2003, 16, 673–687.
- Münch, D.; Sahl, H.G. Structural variations of the cell wall precursor lipid II in Gram-positive bacteria—Impact on binding and efficacy of antimicrobial peptides. Biochim. Biophys. Acta 2015, 1848, 3062–3071.
- Wenzel, M.; Chiriac, A.I.; Otto, A.; Zweytick, D.; May, C.; Schumacher, C.; Gust, R.; Albada, H.B.; Penkova, M.; Krämer, U.; et al. Small cationic antimicrobial peptides delocalize peripheral membrane proteins. Proc. Natl. Acad. Sci. USA 2014, 111, E1409–E1418.
- Barreto-Santamaría, A.; Curtidor, H.; Arévalo-Pinzón, G.; Herrera, C.; Suárez, D.; Pérez, W.H.; Patarroyo, M.E. A New Synthetic Peptide Having Two Target of Antibacterial Action in E. coli ML35. Front. Microbiol. 2016, 7, 2006.
- Haney, E.F.; Mansour, S.C.; Hancock, R.E.W. Antimicrobial Peptides: An Introduction. Methods Mol. Biol. 2017, 1548, 3–22.
- Di Somma, A.; Moretta, A.; Canè, C.; Cirillo, A.; Duilio, A. Antimicrobial and Antibiofilm Peptides. Biomolecules 2020, 10, 652.
- Yeaman, M.R.; Yount, N.Y. Mechanisms of antimicrobial peptide action and resistance. Pharmacol. Rev. 2003, 55, 27–55.
- Ciumac, D.; Gong, H.; Hu, X.; Lu, J.R. Membrane targeting cationic antimicrobial peptides. J. Colloid Interface Sci. 2019, 537, 163–185.
- Milletti, F. Cell-penetrating peptides: Classes, origin, and current landscape. Drug Discov. Today 2012, 17, 850–860.
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250.
- Neundorf, I. Antimicrobial and Cell-Penetrating Peptides: How to Understand Two Distinct Functions Despite Similar Physicochemical Properties. Adv. Exp. Med. Biol. 2019, 1117, 93–109.
- Yan, J.; Wang, K.; Dang, W.; Chen, R.; Xie, J.; Zhang, B.; Song, J.; Wang, R. Two hits are better than one: Membrane-active and DNA binding-related double-action mechanism of NK-18, a novel antimicrobial peptide derived from mammalian NK-lysin. Antimicrob. Agents Chemother. 2013, 57, 220–228.
- Ko, S.J.; Kang, N.H.; Kim, M.K.; Park, J.; Park, E.; Park, G.H.; Kang, T.W.; Na, D.E.; Park, J.B.; Yi, Y.E.; et al. Antibacterial and anti-biofilm activity, and mechanism of action of pleurocidin against drug resistant Staphylococcus aureus. Microb. Pathog. 2019, 127, 70–78.
- Wolz, C.; Geiger, T.; Goerke, C. The synthesis and function of the alarmone (p)ppGpp in firmicutes. Int. J. Med. Microbiol. 2010, 300, 142–147.
- Otto, M. Bacterial evasion of antimicrobial peptides by biofilm formation. Curr. Top. Microbiol. Immunol. 2006, 306, 251–258.
- Maiti, B.K. Potential Role of Peptide-Based Antiviral Therapy against SARS-CoV-2 Infection. ACS Pharmacol. Transl. Sci. 2020, 3, 783–785.
- Li, X.; Zuo, S.; Wang, B.; Zhang, K.; Wang, Y. Antimicrobial Mechanisms and Clinical Application Prospects of Antimicrobial Peptides. Molecules 2022, 27, 2675.
- Ahmed, A.; Siman-Tov, G.; Hall, G.; Bhalla, N.; Narayanan, A. Human Antimicrobial Peptides as Therapeutics for Viral Infections. Viruses 2019, 11, 704.
- Vilas Boas, L.C.P.; Campos, M.L.; Berlanda, R.L.A.; de Carvalho Neves, N.; Franco, O.L. Antiviral peptides as promising therapeutic drugs. Cell. Mol. Life Sci. 2019, 76, 3525–3542.
- Skalickova, S.; Heger, Z.; Krejcova, L.; Pekarik, V.; Bastl, K.; Janda, J.; Kostolansky, F.; Vareckova, E.; Zitka, O.; Adam, V.; et al. Perspective of Use of Antiviral Peptides against Influenza Virus. Viruses 2015, 7, 5428–5442.
- Lee, H.; Lee, Y.; Kim, J.; An, J.; Lee, S.; Kong, H.; Song, Y.; Lee, C.K.; Kim, K. Modulation of the gut microbiota by metformin improves metabolic profiles in aged obese mice. Gut Microbes 2018, 9, 155–165.
- Barlow, P.G.; Svoboda, P.; Mackellar, A.; Nash, A.A.; York, I.A.; Pohl, J.; Davidson, D.J.; Donis, R.O. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS ONE 2011, 6, e25333.
- Holani, R.; Babbar, A.; Blyth, G.A.D.; Lopes, F.; Jijon, H.; McKay, D.M.; Hollenberg, M.D.; Cobo, E.R. Cathelicidin-mediated lipopolysaccharide signaling via intracellular TLR4 in colonic epithelial cells evokes CXCL8 production. Gut Microbes 2020, 12, 1785802.
- Hoffmann, J.; Schneider, C.; Heinbockel, L.; Brandenburg, K.; Reimer, R.; Gabriel, G. A new class of synthetic anti-lipopolysaccharide peptides inhibits influenza A virus replication by blocking cellular attachment. Antiviral Res. 2014, 104, 23–33.
- Horne, W.S.; Wiethoff, C.M.; Cui, C.; Wilcoxen, K.M.; Amorin, M.; Ghadiri, M.R.; Nemerow, G.R. Antiviral cyclic D,L-alpha-peptides: Targeting a general biochemical pathway in virus infections. Bioorg. Med. Chem. 2005, 13, 5145–5153.
- Mulder, K.C.L.; Lima, L.A.; Miranda, V.J.; Dias, S.C.; Franco, O.L. Current scenario of peptide-based drugs: The key roles of cationic antitumor and antiviral peptides. Front. Microbiol. 2013, 4, 321.
- Albiol Matanic, V.C.; Castilla, V. Antiviral activity of antimicrobial cationic peptides against Junin virus and herpes simplex virus. Int. J. Antimicrob. Agents 2004, 23, 382–389.
- He, M.; Zhang, H.; Li, Y.; Wang, G.; Tang, B.; Zhao, J.; Huang, Y.; Zheng, J. Cathelicidin-derived antimicrobial peptides inhibit Zika virus through direct inactivation and interferon pathway. Front. Immunol. 2018, 9, 722.
- Bakovic, A.; Risner, K.; Bhalla, N.; Alem, F.; Chang, T.L.; Weston, W.; Harness, J.A.; Narayanan, A. Brilacidin Demonstrates Inhibition of SARS-CoV-2 in Cell Culture. Viruses 2021, 13, 271.
- Bhattacharya, R.; Gupta, A.M.; Mitra, S.; Mandal, S.; Biswas, S.R. A natural food preservative peptide nisin can interact with the SARS-CoV-2 spike protein receptor human ACE2. Virology 2021, 552, 107–111.
- Liscano, Y.; Oñate-Garzón, J.; Ocampo-Ibáñez, I.D. In Silico Discovery of Antimicrobial Peptides as an Alternative to Control SARS-CoV-2. Molecules 2020, 25, 5535.
- Zhang, R.; Jiang, X.; Qiao, J.; Wang, Z.; Tong, A.; Yang, J.; Yang, S.; Yang, L. Antimicrobial peptide DP7 with potential activity against SARS coronavirus infections. Signal Transduct. Target. Ther. 2021, 6, 140.
- De Cesare, G.B.; Cristy, S.A.; Garsin, D.A.; Lorenz, M.C. Antimicrobial peptides: A new frontier in antifungal therapy. mBio 2020, 11, e02123-20.
- Oshiro, K.G.N.; Rodrigues, G.; Monges, B.E.D.; Cardoso, M.H.; Franco, O.L. Bioactive Peptides against Fungal Biofilms. Front. Microbiol. 2019, 10, 2169.
- Vallabhaneni, S.; Chiller, T.M. Fungal Infections and New Biologic Therapies. Curr. Rheumatol. Rep. 2016, 18, 29.
- Chowdhary, A.; Sharma, C.; Meis, J.F. Azole-resistant aspergillosis: Epidemiology, molecular mechanisms, and treatment. J. Infect. Dis. 2017, 216, S436–S444.
- Fisher, M.C.; Hawkins, N.J.; Sanglard, D.; Gurr, S.J. Health and Food Security—TCLocal. Science 2018, 742, 739–742.
- di Luca, M.; Maccari, G.; Nifosí, R. Treatment of microbial biofilms in the post-antibiotic era: Prophylactic and therapeutic use of antimicrobial peptides and their design by bioinformatics tools. Pathog. Dis. 2014, 70, 257–270.
- Rautenbach, M.; Troskie, A.M.; Vosloo, J.A. Antifungal peptides: To be or not to be membrane active. Biochimie 2016, 130, 132–145.
- Fernández de Ullivarri, M.; Arbulu, S.; Garcia-Gutierrez, E.; Cotter, P.D. Antifungal Peptides as Therapeutic Agents. Front. Cell. Infect. Microbiol. 2020, 10, 105.
- Lucca, A.J. De Expert Opinion on Investigational Drugs Antifungal peptides: Potential candidates for the treatment of fungal infections. Expert Opin. Investig. Drugs 2000, 9, 273–299.
- Bondaryk, M.; Staniszewska, M.; Zielińska, P.; Urbańczyk-Lipkowska, Z. Natural Antimicrobial Peptides as Inspiration for Design of a New Generation Antifungal Compounds. J. Fungi 2017, 3, 46.
- Akkam, Y. A review of antifungal peptides: Basis to new era of antifungal drugs. Jordan J. Pharm. Sci. 2016, 9, 51–75.
- Cools, T.L.; Struyfs, C.; Cammue, B.P.; Thevissen, K. Antifungal plant defensins: Increased insight in their mode of action as a basis for their use to combat fungal infections. Future Microbiol. 2017, 12, 441–454.
- Shafee, T.M.A.; Lay, F.T.; Hulett, M.D.; Anderson, M.A. The Defensins Consist of Two Independent, Convergent Protein Superfamilies. Mol. Biol. Evol. 2016, 33, 2345–2356.
- Parisi, K.; Shafee, T.M.A.; Quimbar, P.; van der Weerden, N.L.; Bleackley, M.R.; Anderson, M.A. The evolution, function and mechanisms of action for plant defensins. Semin. Cell Dev. Biol. 2019, 88, 107–118.
- Chairatana, P.; Chiang, I.L.; Nolan, E.M. Human α-Defensin 6 Self-Assembly Prevents Adhesion and Suppresses Virulence Traits of Candida albicans. Biochemistry 2017, 56, 1033–1041.
- Ballard, E.; Yucel, R.; Melchers, W.J.G.; Brown, A.J.P.; Verweij, P.E.; Warris, A. Antifungal activity of antimicrobial peptides and proteins against Aspergillus fumigatus. J. Fungi 2020, 6, 65.
- Martinez, L.R.; Casadevall, A. Cryptococcus neoformans cells in biofilms are less susceptible than planktonic cells to antimicrobial molecules produced by the innate immune system. Infect. Immun. 2006, 74, 6118–6123.
- Zanetti, M.; Gennaro, R.; Romeo, D. Cathelicidins: A novel protein family with a common proregion and a variable C-terminal antimicrobial domain. FEBS Lett. 1995, 374, 1–5.
- Ridyard, K.E.; Overhage, J. The potential of human peptide ll-37 as an antimicrobial and anti-biofilm agent. Antibiotics 2021, 10, 650.
- Risso, A.; Braidot, E.; Sordano, M.C.; Vianello, A.; Macrì, F.; Skerlavaj, B.; Zanetti, M.; Gennaro, R.; Bernardi, P. BMAP-28, an Antibiotic Peptide of Innate Immunity, Induces Cell Death through Opening of the Mitochondrial Permeability Transition Pore. Mol. Cell. Biol. 2002, 22, 1926–1935.
- Scarsini, M.; Tomasinsig, L.; Arzese, A.; D’Este, F.; Oro, D.; Skerlavaj, B. Antifungal activity of cathelicidin peptides against planktonic and biofilm cultures of Candida species isolated from vaginal infections. Peptides 2015, 71, 211–221.
- Oppenheim, F.G.; Xu, T.; McMillian, F.M.; Levitz, S.M.; Diamond, R.D.; Offner, G.D.; Troxler, R.F. Histatins, a novel family of histidine-rich proteins in human parotid secretion. Isolation, characterization, primary structure, and fungistatic effects on Candida albicans. J. Biol. Chem. 1988, 263, 7472–7477.
- Pusateria, C.R.; Monacoa, E.A.; Edgertona, M. Sensitivity of Candida albicans Biofilm Cells Grown on Denture Acrylic to Antifungal Proteins and Chlorhexidine. Arch. Oral Biol. 2009, 54, 588–594.
- Konopka, K.; Dorocka-Bobkowska, B.; Gebremedhin, S.; Düzgüneş, N. Susceptibility of Candida biofilms to histatin 5 and fluconazole. Antonie van Leeuwenhoek Int. J. Gen. Mol. Microbiol. 2010, 97, 413–417.
- van der Does, A.M.; Hiemstra, P.S.; Mookherjee, N. Antimicrobial Host Defence Peptides: Immunomodulatory Functions and Translational Prospects. Adv. Exp. Med. Biol. 2019, 1117, 149–171.
- Kang, H.K.; Lee, H.H.; Seo, C.H.; Park, Y. Antimicrobial and Immunomodulatory Properties and Applications of Marine-Derived Proteins and Peptides. Mar. Drugs 2019, 17, 350.
- Kumar, P.; Kizhakkedathu, J.N.; Straus, S.K. Antimicrobial Peptides: Diversity, Mechanism of Action and Strategies to Improve the Activity and Biocompatibility In Vivo. Biomolecules 2018, 8, 4.
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48.
- Ehmann, D.; Koeninger, L.; Wendler, J.; Malek, N.P.; Stange, E.F.; Wehkamp, J.; Jensen, B.A.H. Fragmentation of Human Neutrophil α-Defensin 4 to Combat Multidrug Resistant Bacteria. Front. Microbiol. 2020, 11, 1147.
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499.
- Ouellette, A.J. Paneth cell α-defensins in enteric innate immunity. Cell. Mol. Life Sci. 2011, 68, 2215–2229.
- Pahar, B.; Madonna, S.; Das, A.; Albanesi, C.; Girolomoni, G. Immunomodulatory Role of the Antimicrobial LL-37 Peptide in Autoimmune Diseases and Viral Infections. Vaccines 2020, 8, 517.
- Meade, K.G.; O’Farrelly, C. β-Defensins: Farming the Microbiome for Homeostasis and Health. Front. Immunol. 2019, 9, 2072.
- Semple, F.; Dorin, J.R. β-Defensins: Multifunctional modulators of infection, inflammation and more? J. Innate Immun. 2012, 4, 337–348.
- Ghosh, S.K.; Feng, Z.; Fujioka, H.; Lux, R.; McCormick, T.S.; Weinberg, A. Conceptual Perspectives: Bacterial Antimicrobial Peptide Induction as a Novel Strategy for Symbiosis with the Human Host. Front. Microbiol. 2018, 9, 302.
- Machado, L.R.; Ottolini, B. An evolutionary history of defensins: A role for copy number variation in maximizing host innate and adaptive immune responses. Front. Immunol. 2015, 6, 115.
- Candela, M.E.; Allsop, D.J.P.; Carter, R.N.; Semple, F.; Kilanowski, F.; Webb, S.; Taggart, D.; Mullan, H.J.; McHugh, B.J.; Dockrell, D.H.; et al. Classical macrophage polarisation is limited by human β-defensin-3 via an autocrine IL-4 dependent process. bioRxiv 2021. Preprint.
- Xu, D.; Lu, W. Defensins: A Double-Edged Sword in Host Immunity. Front. Immunol. 2020, 11, 764.
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506.
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell. Infect. Microbiol. 2021, 11, 668632.
- Gera, S.; Kankuri, E.; Kogermann, K. Antimicrobial peptides—Unleashing their therapeutic potential using nanotechnology. Pharmacol. Ther. 2021, 232, 107990.
- Lachowicz, J.I.; Szczepski, K.; Scano, A.; Casu, C.; Fais, S.; Orrù, G.; Pisano, B.; Piras, M.; Jaremko, M. The Best Peptidomimetic Strategies to Undercover Antibacterial Peptides. Int. J. Mol. Sci. 2020, 21, 7349.
- Komatsu, T.; Watanabe, K.; Hamada, N.; Helmerhorst, E.; Oppenheim, F.; Lee, M.C. Il Association between Antimicrobial Peptide Histatin 5 Levels and Prevalence of Candida in Saliva of Patients with Down Syndrome. Antibiotics 2021, 10, 494.
- Sharma, P.; Chaudhary, M.; Khanna, G.; Rishi, P.; Kaur, I.P. Envisaging Antifungal Potential of Histatin 5: A Physiological Salivary Peptide. J. Fungi 2021, 7, 1070.
- Bastos, P.; Trindade, F.; da Costa, J.; Ferreira, R.; Vitorino, R. Human Antimicrobial Peptides in Bodily Fluids: Current Knowledge and Therapeutic Perspectives in the Postantibiotic Era. Med. Res. Rev. 2018, 38, 101–146.
- Norris, H.L.; Kumar, R.; Ong, C.Y.; Xu, D.; Edgerton, M. Zinc Binding by Histatin 5 Promotes Fungicidal Membrane Disruption in C. albicans and C. glabrata. J. Fungi 2020, 6, 124.
- Lee, S.M.; Son, K.N.; Shah, D.; Ali, M.; Balasubramaniam, A.; Shukla, D.; Aakalu, V.K. Histatin-1 Attenuates LPS-Induced Inflammatory Signaling in RAW264.7 Macrophages. Int. J. Mol. Sci. 2021, 22, 7856.
- van Harten, R.M.; van Woudenbergh, E.; van Dijk, A.; Haagsman, H.P. Cathelicidins: Immunomodulatory Antimicrobials. Vaccines 2018, 6, 63.
- Zhang, L.; Wu, W.K.K.; Gallo, R.L.; Fang, E.F.; Hu, W.; Ling, T.K.W.; Shen, J.; Chan, R.L.Y.; Lu, L.; Luo, X.M.; et al. Critical Role of Antimicrobial Peptide Cathelicidin for Controlling Helicobacter pylori Survival and Infection. J. Immunol. 2016, 196, 1799–1809.
- Benfield, A.H.; Henriques, S.T. Mode-of-Action of Antimicrobial Peptides: Membrane Disruption vs. Intracellular Mechanisms. Front. Med. Technol. 2020, 2, 610997.
- Lin, L.; Chi, J.; Yan, Y.; Luo, R.; Feng, X.; Zheng, Y.; Xian, D.; Li, X.; Quan, G.; Liu, D.; et al. Membrane-disruptive peptides/peptidomimetics-based therapeutics: Promising systems to combat bacteria and cancer in the drug-resistant era. Acta Pharm. Sin. B 2021, 11, 2609–2644.
- Barańska-Rybak, W.; Sonesson, A.; Nowicki, R.; Schmidtchen, A. Glycosaminoglycans inhibit the antibacterial activity of LL-37 in biological fluids. J. Antimicrob. Chemother. 2006, 57, 260–265.
- Alford, M.A.; Baquir, B.; Santana, F.L.; Haney, E.F.; Hancock, R.E.W. Cathelicidin Host Defense Peptides and Inflammatory Signaling: Striking a Balance. Front. Microbiol. 2020, 11, 1902.
- Choi, K.Y.G.; Mookherjee, N. Multiple immune-modulatory functions of cathelicidin host defense peptides. Front. Immunol. 2012, 3, 149.
