Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Agnieszka Gęgotek and Version 2 by Conner Chen.
Ascorbic acid, commonly known as vitamin C, is one of the basic and best-known compounds necessary for the proper functioning of the human body. Ascorbic acid, as a one of the basic exogenous vitamins, occurs in the body in the form of ascorbate, known for its strong antioxidant and anti-inflammatory properties.
- ascorbic acid
- ROS scavenging
- antioxidant enzymes
- Nrf2
- NFκB
- DNA reparation
1. Suppression of Generation of Free Radicals
Ascorbic acid is one of the basic low-molecular antioxidants functioning in the human body. It takes part in the regulation of the levels of reactive oxygen species (ROS) and the effectiveness of other antioxidants. Ascorbic acid regulates the level of ROS as early as at the stage of their formation. The main sources of ROS are the mitochondrial respiratory chain and specific enzymes, such as NADPH oxidases (NOXs) or xanthine oxidase (XO) [1][25]. Ascorbic acid (100 µM) has been shown to modify both of the above systems (Figure 12). XO is an enzyme that generates ROS through the oxidation of hypoxanthine to xanthine and then to uric acid. Both of these reactions are necessary for the functioning of the organism, but the result of their occurrence are hydrogen peroxide and the generation of the superoxide radical anion [2][26]. It is known that XO can also directly oxidize ascorbate [3][27]; however, ascorbate supplementation significantly protects the organism against XO hyperactivity [4][28]. Moreover, the latest studies show that due to the possibility of continuous supplementation with ascorbate, the resulting inhibition of XO activity in plasma significantly contributes to the improvement in gout treatment [5][6][7][8][29,30,31,32]. On the other hand, ascorbate has no effect on the activity of XO under physiological conditions, as found with skin cells (fibroblasts and keratinocytes). However, in cells under stress caused by, e.g., UV radiation or hydrogen peroxide, treatment with ascorbate (100 µM) inhibited XO hyperactivation [9][10][33,34]. Moreover, such decrease in XO activity induced by ascorbate may be useful in preventing or reducing reperfusion injuries also in stimulated neutrophils (6 µM of ascorbate) [11][35] and delay the progression of hyperuricemic nephropathy (10 mg/kg/day of ascorbate) [8][32].
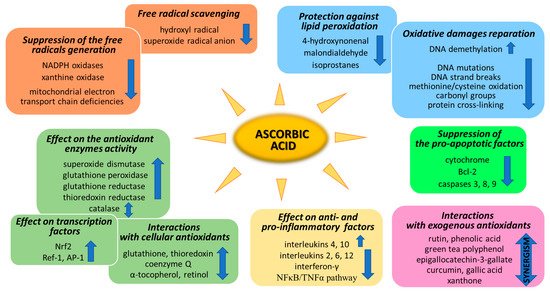
Figure 12.
Pathways of the antioxidant action of ascorbic acid.
NOXs is a group of enzymes widespread in cells transmembrane that, similarly to XO, generate the superoxide radical anion or hydrogen peroxide as the signaling molecules, whose controlled levels enable the proper functioning of cells [12][36]. However, NOX hyperactivity inevitably leads to oxidative stress, which can be prevented by ascorbate [13][37]. As described in the case of XO in skin cells, NOX activity is also not affected by ascorbate (100 µM) in healthy cells. Only strong stress inducers, such as hydrogen peroxide or UVB irradiation, activate NOX strongly enough for ascorbate to start inhibiting the enzyme [9][10][33,34]. Other data show that, due to the fact that ascorbic acid can inhibit NOX in microvascular endothelial cells, the vitamin may reduce the development of sepsis [14][15][38,39]. However, ascorbate (100 µM) also shows the activating properties of NOX in embryonic stem cells, where cardiomyogenesis increases as a result of NOX-induced enhanced levels of ROS [16][40].
Mitochondria also play an important function in the action of ascorbate antioxidant. On the one hand, due to the activity of Complexes II and III, mitochondria regenerate ascorbate from its oxidized form, thus maintaining redox status both in the mitochondrial matrix and in the cytoplasm [17][41]. On the other hand, ascorbate (5 mM) favors sealing the mitochondrial electron transport chain [18][42] and reduces the superoxide radical anion generation, especially in cells with electron transport chain deficiencies [19][43]. However, the described effect raises concerns as to whether the influence of ascorbate on mitochondrial processes does not reduce the elimination of damaged cells by apoptosis, thus promoting carcinogenesis [20][44].
2. ROS Scavenging by Ascorbic Acid
Every living organism constantly generates reactive oxygen and nitrogen species (ROS/RNS) that participate in its physiological activities. However, their level increases significantly in pathological conditions as a result of dysfunction of pro-oxidative systems of cells/biological fluids. In both cases, ROS should be counterbalanced by an effective natural antioxidant system. A disruption of this balance leads to oxidative stress and, as a consequence, the possibility of ROS interactions with the endogenous components of the organism, such as nucleic acids, proteins, lipids, and small molecules, thus causing irreversible oxidative damage to cells and their components. A living cell’s antioxidant system has three lines of defense: (I) free radical scavenging; (II) biosynthesis and activation of antioxidant enzymes; and (III) the repair of oxidative damage. Ascorbate has been shown to be a molecule involved in all these stages (Figure 12). Its antioxidant properties as a scavenger of free radicals are related to its ability to form a stabilized radical (Figure 23). This allows ascorbate to react with more reactive molecules, including the hydroxyl radical or the superoxide radical anion, which prevents their interaction with biomolecules important for proper cell functioning [21][45]. On the other hand, the strong antioxidant properties of ascorbate can induce the transformation of Fe3 + into Fe2 +. However, the ascorbate–Fe2 + chelate may catalyze ROS generation via Fenton’s reaction [21][45]. Hence, ascorbate is an antioxidant; however, the products of its transformation show pro-oxidant properties in the presence of oxygen [22][23][46,47]. Additionally, the oxidation of ascorbate results in the formation of the ascorbate radical (Asc•−) and a high flux of H2O2 [24][48]. Therefore, one should be very careful in formulating hypotheses and pay attention to whether the results obtained in the experiments come directly from ascorbate or possibly from reactive oxygen species generated during cell supplementation.
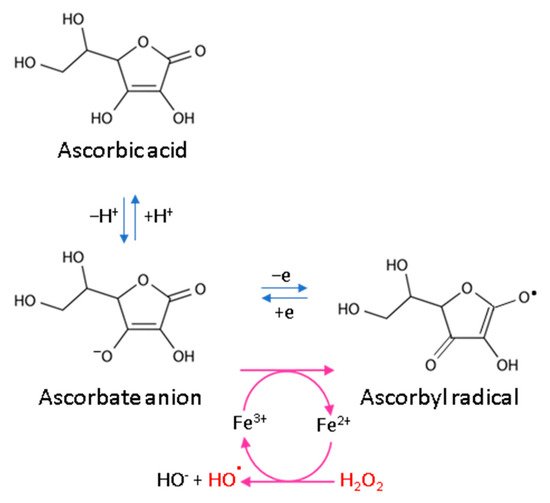
Figure 23.
Scheme of ascorbic acid reaction under oxidative conditions.
3. Ascorbic Acid Interaction with the Cellular Antioxidant System
To prevent oxidative stress or reduce its destructive effect on cellular compartments, ascorbate not only suppresses the generation of free radicals or directly reduces their amounts, but it also significantly stimulates the cellular antioxidant system at the level of low molecular weight antioxidants, as well as by acting on antioxidant enzymes (Figure 2).
The first line of the natural cell protection against the uncontrolled overproduction of ROS is created by low molecular weight antioxidants, such as glutathione (GSH), thioredoxin (Trx), coenzyme Q, α-tocopherol, and retinol. Ascorbic acid also belongs to this group, but its antioxidant effect is limited to ROS elimination as well as the interaction with the other molecules mentioned earlier. The small molecules in the center of the oxidation–reduction reaction cycle also include tocopherol and GSH/Trx (Figure 34). As some of these compounds (GSH/Trx) are not soluble in lipids, they can act only in cytoplasm and are not capable of protecting the cell membrane against ROS. Furthermore, ascorbate is a hydrophilic molecule; however, it can react with tocopherol and its derivatives over the lipid/water interface. Under oxidative conditions, tocopherol neutralizes free radicals, which attack cell membrane components, and itself becomes an oxidized form (tocopheroxyl radical). Ascorbate from cytoplasm restores the reduced form of tocopherol in the lipid fraction, owing to which it can further protect cell membranes. This reaction leads to the oxidation of ascorbate to the ascorbyl radical, which is reduced in the cytoplasm by the thiol group of GSH or Trx [25][26][27][49,50,51]. In addition, tocopherol reduced by ascorbic acid can interact with lipophilic retinol and control its reduced pool [28][29][52,53], which allows the maintenance of the continuity of the membranes. Another lipophilic compound, i.e., coenzyme Q, which interacts with the tocopheroxyl radical, regenerating its antioxidant form, can also participate in these cycles [30][54]. Moreover, significant data suggest that the antioxidant properties of coenzyme Q not only accompany but also complement the attributes of ascorbic acid [31][32][33][34][55,56,57,58]. However, so far it has not been possible to accurately describe their relationship.
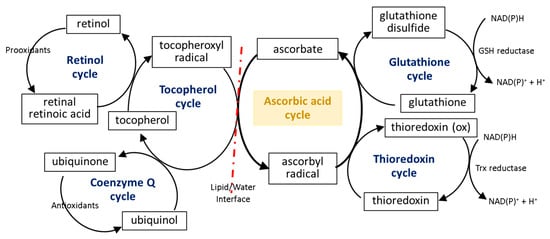
The described reduction/oxidation cycles of low molecular weight antioxidants can also be modified by the effect of ascorbate on the activity of enzymes involved in these reactions. So far, numerous experiments have shown that ascorbic acid can influence the GSH-based action of enzymes, which prevents disturbances in the GSH-based system induced by chemical factors or the development of diseases [35][36][59,60]. However, there are two patterns of action of this compound: (I) ascorbic acid significantly increases the level of GSH without affecting or even reducing the activity of enzymes associated with this molecule (glutathione peroxidase, GSH-Px; glutathione reductase, GSSG-R) and (II) the action of ascorbic acid is primarily based on inducing GSH-Px and GSSG-R activity. It has been found that hepatoprotective and gastroprotective effects of ascorbate are mainly based on the activation of GSH-Px and GSSG-R [37][38][61,62], while in the case of UV-irradiated skin cells, ascorbic acid leads to GSH-Px and GSSG-R down-regulation with a simultaneous increase in the GSH level [9][10][33,34]. On the other hand, the antioxidant effect of ascorbate (14–47 mM) is strong enough to replace the GSH-based system, observed as a decrease in GSH-Px activity and the GSH level in bovine semen [39][63].
Another important element of the maintenance of redox balance in cells is Trx reductase (TrxR) activity. So far it has been widely shown that the oxidized form of ascorbate activates TrxR, resulting in the restoration of the reduced form of ascorbate [40][18]. Additionally, it has been noted that the induction of TrxR activity by ascorbate is accompanied by the stimulation of the GSH-based antioxidant system [41][64]. In skin cells, ascorbic acid (100 µM) is capable of enhancing both the Trx level and TrxR activity under physiologic conditions; it can also prevent UV-induced lowering of these parameters [9][33]. Moreover, ascorbic acid also affects inflammasome functioning through the stimulation of the thioredoxin-interacting protein (TXNIP), thus reducing ROS production and the expression of pro-inflammatory proteins (interleukins 1β, 18 and caspase-1) in human macrophages [42][65]. On the other hand, the effect of ascorbic acid on TrxR activity may be a promising tool in cancer therapies focused on TrxR hyperactivity [43][66].
Ascorbic acid also stimulates the antioxidant system by affecting the activity of other antioxidant enzymes. An example of such an enzyme is superoxide dismutase (Cu, Zn-SOD; SOD), responsible for the conversion of the superoxide radical anion to the less cytotoxic hydrogen peroxide. There are no conclusive data concerning the effect of ascorbic acid on SOD activity in cells under physiologic conditions. However, a series of data points to an essential function of this vitamin on the SOD activity under oxidative stress. In all cases, regardless of whether the oxidative stress was caused by heavy metals, UV radiation, brain damage, or depression, supplementation with ascorbate (0.1–1 mM, 60–200 mg/kg/day) significantly increased the activity of SOD in in vitro cultured cells, as well as in animal plasma [9][10][35][44][45][46][33,34,59,67,68,69]. As a result, not only does this lead to a lowering of the level of the superoxide radical anion but it also protects lipids against oxidation observed as a decreased level of products of lipid peroxidation, including malondialdehyde (MDA) [9][10][45][33,34,68]. On the other hand, ascorbic acid (10–50µM) in SOD-depleted cells also reduces the superoxide radical anion level, thus preventing oxidative stress [47][48][70,71]. However, other studies indicate that oral ascorbic acid supplementation (0.2–1 g/day) does not significantly affect the superoxide dismutase activity in human plasma [49][72].
The hydrogen peroxide formed in the cells as a result of the SOD-catalyzed reaction is decomposed into oxygen and water by another antioxidant enzyme, i.e., catalase (CAT). Ascorbate influences the activity of this enzyme in various ways, depending on the type of cells. In the case of plant cells, high concentrations of ascorbic acid, constituting the signal pointing to a high antioxidant potential of the cell, reduce catalase activity and cause an increase in the level of hydrogen peroxide [50][73], allowing the molecule to perform the signaling function. A similar effect of ascorbate in the case of CAT activity is also observed in rapidly proliferating mammalian cells, especially cancer cells, e.g., gastric cancer cells, glioblastoma, and carcinoma cells [51][52][74,75]. However, in the case of non-neoplastic cells, such as keratinocytes, ascorbic acid (100 µM) significantly enhances CAT activity, thereby increasing the antioxidant potential of these cells, which is important due to the peripheral location of keratinocytes in the skin [9][33]. In the same experiment, it has been shown that following UV-induced oxidative stress, ascorbate affects catalase activity in different skin cells in various ways. In UV-irradiated keratinocytes, ascorbic acid stimulates CAT activity and protects cells against UV-induced hydrogen peroxide overexpression, while this effect is not observed in UV-irradiated skin fibroblasts [9][33].
4. Effect of Ascorbic Acid on Cytoprotective Gene Transcription
Another aspect of the action of ascorbic acid as an antioxidant is its effect on gene expression, resulting in the biosynthesis of antioxidant proteins. Among the most important transcriptional factors involved in cellular antioxidant response are Nrf2 (nuclear factor, erythroid 2-like 2), Ref-1 (redox effector factor 1), and AP-1 (activator protein 1) (Figure 12).
Nrf2 is a protein common in the cytoplasm regardless of the oxidation–reduction conditions. Under physiological conditions, it is attached to its inhibitor, i.e., Keap1, which under oxidative conditions changes conformation and dissociates from Nrf2. The free form of Nrf2 transfers to the nucleus, where it heterodimerizes with sMaf protein. The created complex is capable of binding to the DNA in a sequence-specific manner, i.e., to the antioxidant response element (ARE), and starts the biosynthesis of antioxidant proteins [53][54][55][76,77,78]. Ascorbate (2.9–224.5 mg/kg/day) is known as an activator of the Nrf2 factor, as well as the whole Keap1/Nrf2/ARE pathway, and its deficiency leads to impaired Nrf2 action resulting in inflammation and apoptosis [56][57][79,80]. It is especially pronounced in cells under chemically or physically induced oxidative stress [9][58][33,81]. That is the most important in the case of cells that are constantly exposed to the harmful stressors, such as hepatocytes and keratinocytes [9][59][60][33,82,83]. On the one hand, in keratinocytes, ascorbate (100 µM) reduces the level of Nrf2 inhibitor, i.e., Keap1 protein, and increases free Nrf2 expression, as well as its activators, including p62 and KAP1, on the other [9][60][33,83]. At the same time, ascorbate (1 mM) favors Keap1 conformational changes induced by other antioxidants, such as polyphenols, which additionally stimulates Nrf2 dissociation [61][84]. In hepatocytes, Nrf2 activation by ascorbic acid (1–10 µM) results in the expression of antioxidant enzymes observed as a reduced level of lipid hydroperoxides [59][82]. On the other hand, some data indicate that a high ascorbic acid (1 mM) concentration may lead to a disturbed Keap1/Nrf2/ARE pathway activation [62][85]. However, in accordance with the dual role of Nrf2 in cancers, its activation by ascorbate becomes an ambiguous and possibly a dangerous outcome [63][64][86,87].
The activities of Ref-1 and AP-1 factors are closely related to each other. Ref-1 is a Trx-dependent endonuclease that facilitates AP-1 DNA-binding activity [65][88], while AP-1 is a heterodimer composed of proteins belonging to the c-Fos, c-Jun, ATF, and Maf families and manifests transcriptional activity through the regulation of gene expression in response to a variety of stimuli, including cytokines, growth factors, and oxidative stress [66][89]. Only oxidized ascorbate (1 µM) indirectly affects Ref-1 activity, in accordance with the decrease in Trx levels [41][64]. In the case of AP-1 ascorbic acid, it has been recognized as a molecule that mutes ascorbate action in epidermal keratinocytes, thus preventing these cells from staying alive when their DNA is oxidatively modified, which would pose a threat consisting of the formation of cancer [67][90]. Moreover, in keratinocytes exposed to UV radiation, where AP-1 should be activated, supplementation with ascorbic acid induces reduced levels of the active positive component, i.e., c-Jun, and increases the levels of fra-1 messenger, which is an AP-1 inhibitor [68][91]. On the other hand, the similar silencing of AP-1 activity by ascorbate (200 µM) is observed in respiratory epithelial cells, resulting in reduced levels of signaling molecules, such as pro-inflammatory chemokines [69][92].
