Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 1 by Maša Čater and Version 2 by Vivi Li.
Diabetes is among the most prevalent diseases of the modern world and is strongly linked to an increased risk of numerous neurodegenerative disorders, although the exact pathophysiological mechanisms are not clear yet. Insulin resistance is a serious pathological condition, connecting type 2 diabetes, metabolic syndrome, and obesity. IRecently, insulin resistance has been proven to be connected also to cognitive decline and dementias, including the most prevalent form, Alzheimer’s disease. The relationship between diabetes and Alzheimer’s disease regarding pathophysiology is so significant that it has been proposed that some presentations of the condition could be termed type 3 diabetes.
- diabetes
- insulin sensitivity
- Alzheimer’s disease
- brain
- cognition
- depression
1. Introduction
The incidence of age-related metabolic and neurodegenerative diseases is increasing as the world population is aging [1][2][1,2]. Many studies confirmed that patients with impaired glucose tolerance and diabetes have an increased risk of developing Alzheimer’s disease (AD) compared to healthy individuals [3][4][5][3,4,5]. The association between diabetes and its effects on the development of retinopathy, neuropathy, nephropathy, and cardiovascular diseases is well established [6]. However, the underlying processes of how diabetes impacts the brain, cognition, and mental health are not yet fully understood. Therefore, in this rentry, researchersview, we gathered up-to-date literature about the effects of impaired glucose metabolism on the brain, cognition, and mental health, and about the intersection of symptoms of diabetes and AD.
AD, the most common cause of dementia, is a progressive brain disorder that causes memory deficits and destroys cognitive functioning. A genetic mutation is proposed to be the cause for early-onset AD, while a sum of genetic, environmental and lifestyle factors are considered to trigger the late-onset AD [7]. Changes in the brain, that occur in the early stages of the disorder, include an abnormal buildup of proteins that form amyloid plaques and tau tangles. This results in neuron malfunctioning and a loss of connections between neurons, and eventually, in neuron death [8]. The damage in the brain is initiated in the hippocampus and the entorhinal cortex, both essential in memory formation. Therefore, the first signs of AD include memory problems and mild cognitive impairment. When damage spreads to other parts of the brain, it causes shrinkage of the brain. Thus, in the mild stage of AD, greater memory loss occurs with additional personality and behavior changes. Moreover, in moderate AD, language, reasoning, and sensory processing are affected by the damage in the brain. Hallucinations, delusions, and paranoia can occur at this stage as well. When AD progresses to a severe stage, with plaques and tangles that are wide-spread throughout the brain, communication and independent self-care become greatly affected [9].
AD and diabetes share many different pathologies, such as hyperglycemia, glucose intolerance, hyperinsulinemia, insulin resistance, adiposity, hypertension, atherosclerosis, and cognitive impairment [10][11][12][10,11,12]. The main bases for their development are proposed to be the malfunctioning of insulin signaling [13] with glucose metabolism disorders, deficits in mitochondrial activity, and cholesterol-associated pathologies—these are some of the many additional causes that have been observed by diabetes researchers and neuroscientists in recent decades [6][14][15][16][17][6,14,15,16,17]. In the following sections, reswearchers will look at each of the proposed factors causing diabetes and/or AD and find their correlations. Moreover, researcherswe will discuss the most frequently used animal models in diabetes and AD studies, which enabled the understanding of the mechanism of action of compromised insulin activity-related pathologies. This entryreview is based on a PubMed search using combinations of the terms, «diabetes», «Alzheimer’s disease», «animal model», «mouse», «rat», «insulin» and «brain». Up-to-date references were selected by the date of publication (last 10–15 years, with exceptions) and by their main discovery conclusions so they fitted to the scope of this entryarticle in at least one of the aspects (diabetes or AD).
2. The Importance of Brain Insulin
The brain is the most energy-demanding organ that relies on glucose for fuel. Most of its energy expenditure is needed for maintaining the potential difference across the membranes of nerve cells—for dendritic and axonal transport, and tissue repair. The brain uses several pathways for gathering glucose. Glucose enters the brain by insulin-insensitive facilitated diffusion across the blood–brain barrier (BBB), followed by entering the brain cells using insulin-independent glucose transporters [18][19][18,19] as well as insulin-regulated glucose transporters [20]. Thus, insulin, a highly active hormone and a crucial polypeptide in the body, plays an important role in the brain (Figure 1). Like glucose, insulin also crosses the BBB, and when in the brain, insulin binds to insulin receptors on neurons and glial cells; there, its main function is to modulate glucose transfer into different brain cells for maintaining the normal functioning of the brain [21]. Additionally, insulin in the brain contributes to the control of nutrient homeostasis, reproduction, cognition, and memory, as well as to neurotrophic, neuromodulatory [22], and neuroprotective effects.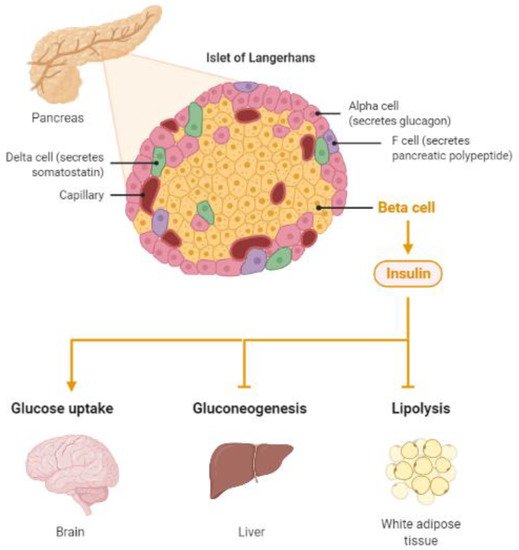
Figure 1. Insulin, a protein secreted by the pancreas, plays a major role in energy homeostasis of the body. Insulin pathologies are directly involved in the development of diabetes and Alzheimer’s disease (Created by BioRender.com, accessed on 23 August 2021).
3. Involvement of Diabetes in the Development of Alzheimer’s Disease
3.1. Abnormal Protein Processing
The main feature of AD is abnormal protein processing in the brain, through which amyloid-β plaques and neurofibrillary tangles are formed, causing morphological pathologies in the brain tissue due to disintegrated microtubules, synaptic impairment, and neuronal apoptosis, thus, resulting in cognitive impairments and several psychopathologies (Figure 2).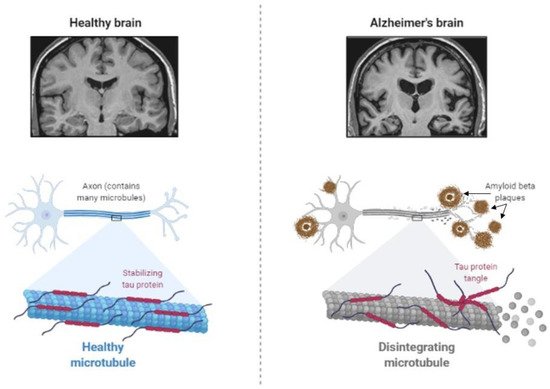
Figure 2. Abnormal protein processing occurs in the brain of patients with Alzheimer’s disease, which leads to the disintegration of microtubules and formation of tau aggregates and amyloid-β plaques, causing morphological pathologies, several cognitive impairments, and behavioral changes (Created by BioRender.com, accessed on 23 August 2021).
3.2. Deficient Insulin Signaling
Dysfunctional insulin receptor signaling is known to affect the expression and metabolism of amyloid-β and tau protein [26][47] and their clearance [35][56]. Insulin receptors are proposed to regulate synaptic activity as well; therefore, the malfunctioning of the receptors could cause neurodegeneration [36][57]. Moreover, insulin resistance in connection with hyperinsulinemia induces the accumulation of amyloid-β due to the lack of available insulin-degrading enzymes (IDEs). Normally, insulin and amyloid-β are both degraded by IDE. When insulin levels are increased, such as in type 2 diabetes, insulin uses the majority of IDE, and undegraded amyloid-β starts to accumulate in neurons [37][58]. As a possible treatment, insulin sensitizers have been tested in rodents [38][59] and early AD patients [39][60], with positive results in improving cognitive performances. Further studies are still needed to confirm the exact potential of insulin sensitizers for AD treatment. Apart from increased amyloid-β accumulation in neurons, hyperinsulinemia causes tau hyperphosphorylation in primary cortical neurons and hippocampal neurons [40][41][42][61,62,63], provoking their degeneration. Alterations in insulin receptor signaling in type 2 diabetes and AD develop due to changes in both major signaling pathways. The mitogen-activated protein kinase (MAPK) pathway, required for cell proliferation, differentiation, and apoptosis [43][64], is accelerated in the brain of patients with AD [44][65]. The expression of MAPK co-localizes with aggregated tau in the hippocampus and cortical regions in AD brains, indicating that MAPK signaling is also involved in tau phosphorylation, synaptic plasticity, and neuroinflammation [45][46][66,67]. With respect to diabetes, Dusp8, which codes for a dual-specificity phosphatase involved in MAPK signaling and is predominantly expressed in the brain, was implicated by genome-wide association studies as a type 2 diabetes risk gene. There is evidence that Dusp8 can have sex-specific effects in mice and men on hypothalamic insulin resistance, hippocampal size, and cognitive, emotional, and hedonic behaviors [47][48][49][68,69,70]. The second insulin receptor signaling pathway, the Akt pathway—which is responsible for cell growth and survival, protein synthesis, and inhibition of the glycogen synthase kinase-3β (GSK-3β) enzyme [50][51][52][53][54][71,72,73,74,75]—is also affected by both AD and type 2 diabetes [55][76]. GSK-3β in the hippocampus and cortex is important for glycogenesis and glucose clearance. In normal conditions, its activity is inhibited by phosphorylation by insulin signaling via an insulin receptor. However, in type 2 diabetes, elevated activity of GSK-3β is proposed to trigger the reduction in glucose clearance by developing insulin resistance [54][75]. Moreover, increased GSK-3β activity is thought to result in increased amyloid-β production and tau phosphorylation [53][56][74,77]. Experiments in AD animal models and cell cultures have shown that GSK-3β is a good target for treatment development, as inhibiting GSK-3β successfully slowed down neurodegeneration [56][57][77,78].3.3. The Cholinergic Hypothesis
Acetylcholine is a neurotransmitter that is involved in cholinergic neurotransmission. It is used by cholinergic neurons and has an important role in the peripheral and central nervous system, as cholinergic neurons are critical for cognition and memory. AD patients present with a continuous decline of cholinergic neurotransmission in their brain [58][79]. This occurs due to a decrease in the production of acetylcholine as well as the hydrolysis of acetylcholine. A mechanism of action has been proposed with a cholinergic hypothesis, which suggests that insulin plays an important role in acetylcholine production [59][80]. In case of hypoinsulinemia, less acetylcholine is produced due to a reduced expression of choline acetyltransferase (Figure 3). This direct effect of insulin on acetylcholine production has additionally strengthened the link between AD development, insulin malfunction, and diabetes; thus, AD has recently been considered a neuroendocrine disease and has been referred to as type 3 diabetes, possessing characteristics of type I and type II diabetes [59][60][61][62][80,81,82,83].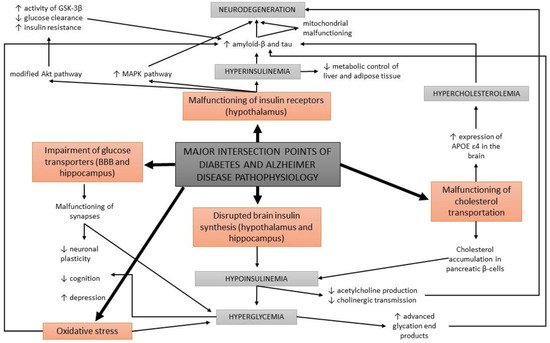
Figure 3.
A schematic overview of the major intersection points of diabetes and Alzheimer’s disease pathophysiology.
3.4. Glucose Metabolism Disorders
Apart from the impairments in brain insulin production and signaling, additional conditions are found at the intersection of diabetes and AD, such as oxidative stress and the formation of advanced glycation end products. Abnormal glucose metabolism and oxidative stress trigger the formation of advanced glycation end products which cause brain damage [14]. Advanced glycation end products are formed by the glycation of proteins or lipids in case of chronic hyperglycemia, and present a useful biomarker for degenerative diseases such as diabetes and AD. In normal aging, the formation of these molecules occurs in low levels, while it is greatly accelerated in patients with diabetes and AD [63][64][84,85]. Moreover, advanced glycation end products were found to induce the glycation of amyloid-β and tau, resulting in their formation and aggregation [65][86] (Figure 3). An increased expression of receptors for advanced glycation end products in neurons was determined in diabetic mice with impaired cognition [66][87], as well as at a clinical level in patients with AD and diabetes compared to non-diabetic AD patients [67][88].3.5. Oxidative Stress
Impaired glucose metabolism has other effects in the body as well. It causes an accelerated production of free radicals, which results in oxidative stress in cells. Oxidative stress is well known to contribute to the development of diabetes and its neuropathies [68][69][89,90]. It has been indicated that oxidative cell damage occurs early in the development of AD [15], as increased concentrations of oxidized proteins in the hippocampus and in the frontal and parietal lobes were determined in patients with only a mild cognitive impairment. Oxidative stress is strongly linked to amyloid-β accumulation. Preclinical research in mice showed that antioxidant capacity decreases first, followed by an increase in lipid peroxidation, and finally, results in AD development [70][71][91,92]. Moreover, oxidative stress triggers local inflammations in the brain [72][93]. Some studies report that the use of anti-inflammatory drugs can decrease the risk of AD development, while others report no beneficial effects of taking these agents for treating AD [73][74][75][94,95,96].3.6. Deficits in Mitochondrial Activity
Another factor correlating diabetes to AD is mitochondrial malfunctioning [6][16][55][6,16,76]. Mitochondrial activity is essential for normal neuronal functioning regarding ATP synthesis and for controlling calcium homeostasis [76][97]. While the efficiency of calcium homeostasis regulation decreases in the brain with normal aging, an increased calcium uptake by mitochondria has been observed among AD pathologies [77][78][98,99], as well as a decrease in mitochondrial mass and an increase in mitochondrial DNA in the cytoplasm [79][100]. Excessive calcium uptake triggers an increase in the level of reactive oxygen species and inhibition of ATP synthesis, resulting in neuronal degeneration and apoptosis. The exact mechanism of action regarding how mitochondrial malfunctioning is correlated to AD is not yet fully understood. However, it is proposed that mitochondrial electron transport is negatively affected by amyloid-β [80][101], which further on leads to mitochondrial malfunction and dysregulation of calcium homeostasis. Increased levels of intracellular calcium have been found to co-localize with neurofibrillary tangles and amyloid-β aggregates [78][81][82][99,102,103]. Interestingly, as excessive calcium uptake by mitochondria causes damage in neurons in AD, it initiates similar damage also in pancreatic cells, causing insulin malfunctions that lead to diabetes pathologies [83][104]. High levels of calcium in pancreatic β-cells are thought to trigger the malfunctioning of insulin secretion [84][105]. Insulin deficiency and oxidative stress due to a lower antioxidant capacity of neuronal mitochondria have been observed in type 1 diabetic rats [85][106], and these defects in mitochondrial DNA are determined to be inheritable. Moreover, in type 2 diabetes, a similar low antioxidant activity of mitochondria has been observed. However, in this type of diabetes, obesity is usually present, which is also known to be associated with smaller mitochondria and reduced energetic capacity [86][107].3.7. Cholesterol-Associated Pathologies
The malfunctioning of cholesterol transportation within the circulatory system has been observed in diabetes and AD, yet the details of the underlying processes are unclear. In the case of diabetes, cholesterol has been found to be accumulated within pancreatic β-cells, causing a decrease in insulin secretion [87][108]. In AD mouse models, cholesterol has been determined at the same locations as amyloid-β plaques and tau proteins [88][109]. Therefore, it has been proposed that cholesterol is directly involved in the formation of protein abnormalities in AD (Figure 3). Normal blood lipid levels are maintained by apolipoprotein E, which is expressed mainly in the brain and liver. Some alleles (APOE ε4) of the gene for apolipoprotein E result in the development of hypercholesterolemia and have been found in 40% of AD patients [55][76]. Additionally, the risk for AD development associated with APOE ε4 is doubled by diabetes [89][110]. Apolipoprotein E, synthesized from APOE ε4, is linked to abnormal protein processing, which is present in AD patients; in addition, apolipoprotein E is able to cooperate with amyloid-β aggregates and it promotes the phosphorylation of tau in neurons, inducing neurodegeneration [17][90][17,111].4. Insulin Effects on Cognition and Mental Health
4.1. Cognitive Changes
Cognitive changes in people with dementia are well documented and studied. Recently, diabetes has received significant attention in psychiatry due to its high mutual frequency with mood disorders and cognitive impairment. Several symptoms of AD, such as cognitive dysfunctions regarding attention, memory, vocabulary, information processing, motor strength and speed, visual-motor and spatial skills as well as impaired general intelligence, were observed in patients with type 1 and type 2 diabetes [55][91][76,112]. Deficits in spatial learning and long-term potentiation in the hippocampus, which are important for memory formation, have been observed in patients with type 1 diabetes. Moreover, the most prevalent form of diabetes, type 2 diabetes, has been determined to trigger early cognitive and mental changes in a similar way as type 1 diabetes. While in type 1 diabetes the major problem causing physiological and cognitive deficits is insulin deficiency, in type 2 diabetes it is the malfunctioning of insulin receptor activity that has enormous consequences on the brain. Hence, insulin resistance, hyperinsulinemia, and impaired insulin signaling have been determined to cause many cognitive pathologies (Figure 4) [50][55][71,76].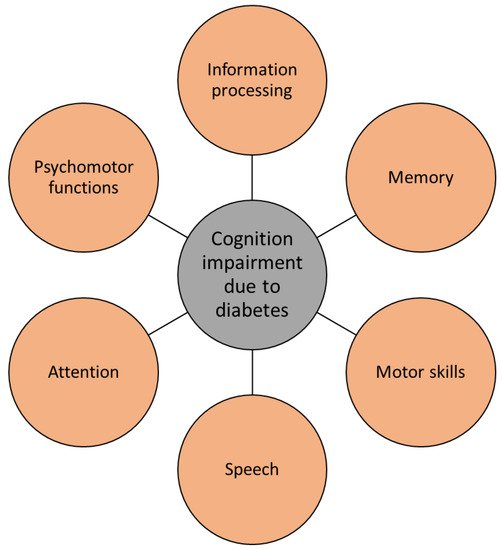
Figure 4.
Diabetes causes several cognitive impairments, typical for Alzheimer’s disease.
