Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Dean Liu and Version 1 by Bartłomiej Barczyński.
The eubiotic vaginal microbiota of reproductive-aged women is composed mostly of various Lactobacillus species (spp.), which exert protective effects via the production of lactic acid, bacteriocins, polysaccharides, peptidoglycans, and hydrogen peroxide (H2O2), lowering pH, raising the viscosity of cervicovaginal mucus, and hampering both the adhesion of cells to epithelial tissue and the entry of HPV. The depletion of beneficial microorganisms could increase the risk of sexually transmitted infections. Emerging therapies involve mucosal, intranasal vaccines, which trigger systemic and mucosal immune responses, thus protecting against HPV-induced tumours.
- cervical cancer
- endometrial cancer
- dysbiosis
- Lactobacillus
1. The Microbial Environment of the Vagina and Upper Reproductive Tract
The female reproductive tract is inhabited by various coexisting microorganisms, which influence health or disease states [30][1]. The vaginal microbiota in healthy women of reproductive age is not diverse and usually comprises one or few Lactobacillus spp. [26,31][2][3]. During eubiosis, the vaginal microbiota of reproductive-aged women is primarily composed of various Lactobacillus spp., including Lactobacillus gasseri, Lactobacillus crispatus, Lactobacillus jensenii, and Lactobacillus iners [31,32,33][3][4][5]. Studies have shown that the depletion of beneficial microorganisms could be associated with a higher risk of sexually transmitted infections, pelvic inflammatory disease, preterm births, and spontaneous miscarriages [34][6]. According to studies, the profile of each female vaginal microbiome can be classified into six community state types (CSTs) [3,35][7][8]. Lactobacillus, especially L. crispatus, L. gasseri, L iners, and L. jensenii, is predominant in CST-I, II, and III, Streptococcus and Prevotella dominate in CST IV-A, and Atopobium is highly prevalent in CST IV-B. The presence of bacteria belonging to CST-IV is frequently associated with bacterial vaginosis. The aforementioned Lactobacillus species appear to be adapted for dominance in the vaginal niche since other types of Lactobacillus are not observed there [36,37][9][10]. The explanation of this phenomenon is unknown; however, it could be related to evolutionary issues [38][11]. The predominance of vaginal Lactobacillus spp. protects this microenvironment against the invasion of pathogens. It has been observed that Gardnerella vaginalis can also be dominant in the vaginal microbiome. The vaginal microbiome that is non-Lactobacillus-dominant appears to be more frequent in Hispanic and Black women (30–40%) compared to White and Asian women (10–20%) [39,40,41][12][13][14]. Ethnic and racial disparities can stem from different environmental and socioeconomic factors as well as diverse behaviour, e.g., sexual and hygiene-related [42][15]. However, some reports have indicated that at least one Lactobacillus can be related to disease states. For example, L. iners was identified in females with disorders of the vaginal environment [43,44,45][16][17][18]. The presence of L. iners-dominant vaginal microbiome is frequently observed during the transition to the non-Lactobacillus-dominant communities [46][19]. The vaginal microbiome can be affected by numerous factors, including infections with HPV and other STIs, sexual activity, lubricant use, the number of sexual partners, contraception use, hygiene practices, access to health care, diet and nutrition (fat-rich diet and high glycaemic load), smoking, physical activity, obesity, and alcohol consumption. Age; genetic and epigenetic factors; hormone levels; pregnancy; immune system impairment; stress; and exposure to xenobiotics, carcinogens, toxins, and antibiotics also influence its composition [47,48,49][20][21][22]. The vaginal microbiota profile depends on ethnicity; the Lactobacillus species are more prevalent in Caucasian and Asian women compared to Hispanic and Black women [3][7]. The ethnic differences in microbiota can be associated with either genetic factors affecting mucosal immunity and metabolic pathways or hygiene practices [3][7]. The gut microbiome has been demonstrated to indirectly influence the abundance of Lactobacillus in the vaginal microenvironment via the modulation of oestrogen release, which may imply the existence of a gut–vaginal axis [50,51,52][23][24][25]. β-glucuronidase and β-glucosidase secreted by microorganisms attach to oestrogen, thus leading to its enhanced reabsorption into the circulation [53,54][26][27]. In turn, unbound oestrogen reaches the female reproductive tract where it activates intracellular signalling associated with increased glycogen syntheses, thickening of the genital epithelium, and the production of mucus. Thus, females’ hormones, including oestrogen and progesterone, modulate vaginal colonisation with Lactobacillus spp. Higher levels of these hormones are associated with lower vaginal microbiota diversity and the dominance of Lactobacillus [55,56][28][29]. The relationship between oestrogen levels and the amount of vaginal Lactobacillus is mirrored by the finding of reduced Lactobacillus abundance in females before menstruation, i.e., when oestrogen levels are significantly reduced [57,58][30][31]. In this period of decreased oestrogen levels, some species become enriched, while others are depleted in the vaginal environment [35][8]. Temporal oestrogen deficiency may cause vaginal atrophy, which is partly responsible for higher bacterial diversity [35][8]. The decrease in the lactic acid bacteria pool is associated with the predominance of anaerobic bacteria and the subsequent risk of cervical cancer development. Though the mechanisms underlying hormone-related microbial composition of the vagina are not fully understood, it has been suggested that the dominance of Lactobacillus spp. may be associated with the oestrogen-driven maturation of vaginal epithelium, the production of α-amylase, and the accumulation of glycogen [59][32]. The degradation of glycogen by α-amylase to simple products such as maltose, maltotriose, maltotetraose, and α-dextrins promotes Lactobacillus growth and colony formation [60][33]. The use of synthetic hormones, e.g., contraceptives, has also been reported to decrease the incidence or recurrence of bacterial vaginosis [61][34]. In turn, smoking, sexual intercourse, and vaginal douching appear to diminish the abundance of L. crispatus, increase species diversity, and enhance the risk of bacterial vaginosis [62,63,64][35][36][37]. Data concerning common microbiota inhabiting the uterus, fallopian tubes, or ovaries are limited due to problems with its assessment [65][38]. The microbiota of the female upper reproductive tract was found to be very different from that of the vagina in composition and quantity [42][15]. Chen et al. [66][39] suggested that the number of bacteria in the uterus could be ~10,000-fold lower compared to the number of bacteria in the vagina. However, this estimation could be inexact due to the high risk of cross-contamination with bacteria from the lower part of the tract during transcervical collection. Moreover, it has been suggested that upper reproductive tract microbiota are more diverse compared to that of the lower tract; however, genuine members have not been identified since various studies indicated different microbiota compositions [66][39]. Lactobacillus species were also found in the upper tract, but their abundance gradually reduced with its withdrawal from the vagina and cervix. Numerous studies revealed that various body sites can serve as possible reservoirs of genital microorganisms. For example, common vaginal bacteria, including Lactobacillus, Gardnerella, Sneathia, Prevotella, Atopobium, Gemella, Peptoniphilus, and Finegoldia, are normally found in the urinary tract in both women and men [67,68,69][40][41][42]. Thomas-White et al. [69][42] observed that vaginal and bladder microbiota displayed comparable functional capacities, which differed from gut microbiota. The presence of Lactobacillus spp. in the bladder and the vagina could exert a protective effect against invading uropathogens. Moreover, the co-colonisation of both the vagina and rectum with vaginal Lactobacillus species, including L. crispatus, L. jensenii, L. iners, and L. gasseri, was associated with the lowest prevalence of bacterial vaginosis [70,71][43][44]. Therefore, it was suggested that the rectum might be a vital reservoir for vaginal lactobacilli. The presence of the vaginal microbiome’s members on male penile skin, in semen, and in urine specimens may imply that sexual partners can exchange microbiota residing in their urogenital tracts [72][45]. According to some studies, the composition of endometrial microbiota may affect implantation, pregnancy, and live birth rates [73][46]. Lactobacillus-dominated endometrial fluid and vaginal aspirate correlate with better outcomes. Uterine microbiota was suggested to exert an impact on the immune environment during conception [74][47]. Modifications of microbial composition in the endometrial fluid can elicit an inflammatory response within the endometrium, thus lowering the probability of embryo implantation success [75][48].2. The Role of Lactobacillus in the Female Reproductive Tract
In contrast to many parts of the body in which great microbial diversity appears to be beneficial, in the vagina, a higher diversity of microbiota frequently results in dysbiosis and the development of disease states. Many studies have demonstrated that vaginal microbiota, including Lactobacillus, is involved in the protection of the reproductive tract and gastrointestinal tract against opportunistic infections [1,7][49][50]. The ability of Lactobacillus to produce lactic acid via the fermentation of glucose (glycolysis) supports vaginal eubiosis, as this organic acid helps preserve the vaginal acidic environment [76][51]. The acidic environment constrains the growth of some potentially pathogenic species, including C. trachomatis, G. vaginalis, and Neisseria gonorrhoeae [32,77,78,79][4][52][53][54]. Vaginal pH exceeding 5.0 was found to increase the risk of HPV in premenopausal women by 10–20% [80][55]. This finding could be partly explained by the fact that the HPV protein crucial for viral transformation, E5, is vulnerable to low pH [81][56]. Moreover, it offers optimal conditions for the metabolic functioning of cervical and vaginal cells [82][57]. Apart from affecting the pH of the environment, the chemical structure of lactic acid itself may modulate the HPV infection and the development of squamous intraepithelial lesions [3][7]. As a chiral molecule, lactic acid can be produced in the form of D- and L-isomers. Studies demonstrated that high levels of D-lactic acid could protect against Chlamydia infection and upper reproductive tract infections via the modulation of extracellular matrix metalloproteinase inducer (EMMPRIN) production in vaginal epithelial cells [83,84][58][59]. A higher L-lactate-to-D-lactate ratio is associated with the enhanced expression of EMMPRIN as well as the activation of matrix metalloproteinase 8 (MMP-8), eventually resulting in impaired cervical integrity and the easier entry of HPV into basal keratinocytes [83][58]. Nunn et al. [85][60] revealed that the predominance of L. crispatus and relatively high levels of D-lactic acid could increase the viscosity of cervicovaginal mucus, resulting in viral particle trapping. Lactic acid also limits the cytotoxicity of natural killer (NK) cells, diminishes the synthesis of pro-inflammatory cytokine IL-12, and promotes the release of anti-inflammatory interleukin-10 (IL-10) [86,87][61][62]. Apart from lactic acid, beneficial microbiota can also release other antimicrobial peptides, including bacteriocins and hydrogen peroxide (H2O2) [88,89][63][64]. Bacteriocins exert direct bactericidal effects, but they can also modulate the inflammatory immune response and mediate acquired immune response [1][49]. They possess anti-tumour properties resulting from cytotoxicity and the stimulation of cell lysis. Gassericin (bacteriocin), produced by L. gasseri as well as other strains of L. crispatus and Lactobacillus reuteri, acts on Gram-negative and Gram-positive bacteria [90,91][65][66]. Apart from bacteriocins, some bacteria (e.g., Lactobacillus) can also release biosurfactants, which modify surface tension, therefore hampering bacterial adhesion, biofilm formation, and the excessive growth of pathogenic anaerobes [92][67]. Lactobacillus epithelium adhesin (LEA), produced by L. crispatus, prevents the pilus-mediated adhesion of G. vaginalis [93][68]. The aforementioned bacteriocins and biosurfactants have also been demonstrated to disturb viral infiltration [94][69]. Moreover, both bacteriocin and surface-active components can constrain the synthesis of tumourigenic substances [95][70]. A higher rate of bacterial vaginosis was reported in females with decreased vaginal levels of bacteria capable of producing H2O2 [96][71]. The release of a variety of antimicrobial peptides (AMPs) into the uterine cavity poses a vital defence mechanism, protecting epithelial tissues against proteolytic enzymes secreted by pathogens [97,98][72][73]. Some studies have suggested that hypoxia could also promote the development of bacterial vaginosis since, in such conditions, bacteria are not able to produce H2O2 in a sufficient amount to inhibit pathogenic bacteria growth [99,100][74][75]. The interaction of commensal bacteria with endometrial epithelial cells was found to form an antimicrobial barrier against pathogens [101][76]. The presence of Lactobacillus in the vagina is associated with protection against the adherence of pathogenic bacteria to the epithelial tissue. These bacteria compete against pathogenic microorganisms for territories and nutrients [102][77]. Lactobacillus that occupies the vaginal epithelial cells (VECs) has been found to prevent the conglutination of invasive pathogenic bacteria, thus hampering the initiation of malignant tumours [103,104][78][79]. Lactobacillus was demonstrated to hinder the proliferation of malignant tumours via the secretion of phosphorylated polysaccharides, exopolysaccharides, and peptidoglycans [87,105][62][80]. Moreover, these bacteria can stimulate nitric oxide (NO) production by macrophages and impair energy metabolism in cancer cells [106][81]. Commensal bacteria stimulate the production of neutral, stable mucous by endometrial cells as well as preserve tight junctions [65,107][38][82]. An intact epithelial barrier is crucial for protection against the penetration and colonisation of opportunistic microorganisms. Furthermore, commensal bacteria can modify immune responses at the cellular level [101][76]. Studies have demonstrated that Lactobacillus enhances the proliferation and differentiation of thymus-derived cells (T cells) and ameliorates the immunological recognition and proliferation of B cells [108,109][83][84]. The adhesion of Lactobacillus and the absorption of nutrients have been demonstrated to trigger the complement system, which subsequently regulates microbial growth [110][85].
Motevaseli et al. [111][86] demonstrated that vaginal lactobacilli (L. gasseri and L. crispatus) could exert cytotoxic impact on cervical tumour cells, however, normal cells remained unaffected. Moreover, they observed that this effect was independent of lactic acid and pH. Studies have demonstrated the antimetastatic and antiproliferative properties of Lactobacillus, its subgenera, and its supernatants [87][62]. Via the modulation of HPV oncogenes, Lactobacillus was shown to limit cervical cancer cell viability. Another study has implied that L. crispatus is highly resistant to the co-colonisation of other bacteria and the transition into CST IV [46][19]. These bacteria are rarely found to coexist with other species. Furthermore, females with these bacteria have the lowest vaginal pH and are not susceptible to infections with bacterial STIs, HPV, herpes simplex virus-2 (HSV-2), or HIV [31,112][3][87]. Since bacterial vaginosis promotes the shedding of HIV and HSV-2, it has been suggested that dysbiosis and the reduced abundance of Lactobacillus may support the formation of an environment that induces the persistence of infections and leads to the development of squamous intraepithelial lesions [113][88]. The basic beneficial effects of Lactobacillus in the lower female genital tract are presented in Figure 1.
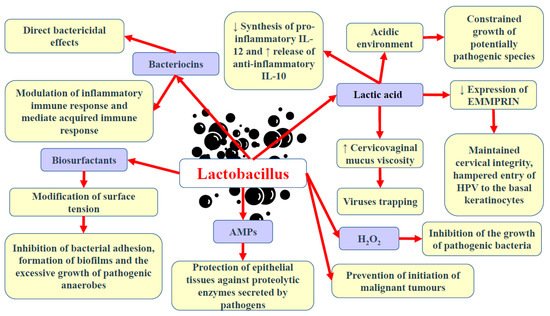
Figure 1. Basic beneficial mechanisms of Lactobacillus in the female genital tract. Abbreviations: ↓—decrease; ↑—increase; AMPs—antimicrobial peptides; H2O2—hydrogen peroxide; EMMPRIN—extracellular matrix metalloproteinase inducer; IL-10 and -12—interleukin-10 and -12.
References
- Foster, K.R.; Schluter, J.; Coyte, K.Z.; Rakoff-Nahoum, S. The evolution of the host microbiome as an ecosystem on a leash. Nature 2017, 548, 43–51.
- Huh, S.Y.; Rifas-Shiman, S.L.; A Zera, C.; Edwards, J.W.R.; Oken, E.; Weiss, S.T.; Gillman, M.W. Delivery by caesarean section and risk of obesity in preschool age children: A prospective cohort study. Arch. Dis. Child. 2012, 97, 610–616.
- Ravel, J.; Gajer, P.; Abdo, Z.; Schneider, G.M.; Koenig, S.S.; McCulle, S.L.; Karlebach, S.; Gorle, R.; Russell, J.; Tacket, C.O. Vaginal microbiome of reproductive-age women. Proc. Natl. Acad. Sci. USA 2011, 108, 4680–4687.
- Tachedjian, G.; Aldunate, M.; Bradshaw, C.S.; Cone, R.A. The role of lactic acid production by probiotic Lactobacillus species in vaginal health. Res. Microbiol. 2017, 168, 782–792.
- Fredricks, D.N.; Fiedler, T.L.; Marrazzo, J.M. Molecular Identification of Bacteria Associated with Bacterial Vaginosis. N. Engl. J. Med. 2005, 353, 1899–1911.
- Martin, D.H.; Marrazzo, J.M. The Vaginal Microbiome: Current Understanding and Future Directions. J. Infect. Dis. 2016, 214, S36–S41.
- Mitra, A.; MacIntyre, D.A.; Marchesi, J.R.; Lee, Y.S.; Bennett, P.R.; Kyrgiou, M. The vaginal microbiota, human papillomavirus infection and cervical intraepithelial neoplasia: What do we know and where are we going next? Microbiome 2016, 4, 58.
- Brotman, R.M.; Shardell, M.D.; Gajer, P.; Fadrosh, D.; Chang, K.; Silver, M.; Viscidi, R.P.; Burke, A.E.; Ravel, J.; Gravitt, P.E. Association between the vaginal microbiota, menopause status, and signs of vulvovaginal atrophy. Menopause 2014, 21, 450–458.
- Antonio, M.A.D.; Hawes, S.E.; Hillier, S.L. The Identification of Vaginal Lactobacillus Species and the Demographic and Microbiologic Characteristics of Women Colonized by These Species. J. Infect. Dis. 1999, 180, 1950–1956.
- Younes, J.A.; Lievens, E.; Hummelen, R.; van der Westen, R.; Reid, G.; Petrova, M.I. Women and Their Microbes: The Unexpected Friendship. Trends Microbiol. 2018, 26, 16–32.
- Miller, E.A.; Beasley, D.E.; Dunn, R.R.; Archie, E.A. Lactobacilli dominance and vaginal pH: Why is the human vaginal microbiome unique? Front. Microbiol. 2016, 7, 1936.
- Fettweis, J.M.; Brooks, J.P.; Serrano, M.G.; Sheth, N.U.; Girerd, P.H.; Edwards, D.J.; Strauss, J.F., III; Jefferson, K.K.; Buck, G.A.; Consortium, V.M. Differences in vaginal microbiome in African American women versus women of European ancestry. Microbiology 2014, 160, 2272.
- Borgdorff, H.; van der Veer, C.; van Houdt, R.; Alberts, C.J.; de Vries, H.J.; Bruisten, S.M.; Snijder, M.B.; Prins, M.; Geerlings, S.E.; van der Loeff, M.F.S.; et al. The association between ethnicity and vaginal microbiota composition in Amsterdam, the Netherlands. PLoS ONE 2017, 12, e0181135.
- Laniewski, P.; Barnes, D.; Goulder, A.; Cui, H.; Roe, D.J.; Chase, D.M.; Herbst-Kralovetz, M.M. Linking cervicovaginal immune signatures, HPV and microbiota composition in cervical carcinogenesis in non-Hispanic and Hispanic women. Sci. Rep. 2018, 8, 7593.
- Łaniewski, P.; Ilhan, Z.E.; Herbst-Kralovetz, M.M. The microbiome and gynaecological cancer development, prevention and therapy. Nat. Rev. Urol. 2020, 17, 232–250.
- Oh, H.; Kim, B.-S.; Seo, S.-S.; Kong, J.-S.; Lee, J.-K.; Park, S.-Y.; Hong, K.-M.; Kim, H.-K.; Kim, M. The association of uterine cervical microbiota with an increased risk for cervical intraepithelial neoplasia in Korea. Clin. Microbiol. Infect. 2015, 21, 674.e1–674.e9.
- Van De Wijgert, J.H.H.M.; Borgdorff, H.; Verhelst, R.; Crucitti, T.; Francis, S.C.; Verstraelen, H.; Jespers, V. The Vaginal Microbiota: What Have We Learned after a Decade of Molecular Characterization? PLoS ONE 2014, 9, e105998.
- Kyrgiou, M.; Mitra, A.; Moscicki, A.-B. Does the vaginal microbiota play a role in the development of cervical cancer? Transl. Res. 2017, 179, 168–182.
- Gajer, P.; Brotman, R.M.; Bai, G.; Sakamoto, J.; Schütte, U.M.E.; Zhong, X.; Koenig, S.S.K.; Fu, L.; Ma, Z.; Zhou, X.; et al. Temporal Dynamics of the Human Vaginal Microbiota. Sci. Transl. Med. 2012, 4, 132ra52.
- Hickey, R.J.; Zhou, X.; Pierson, J.D.; Ravel, J.; Forney, L.J. Understanding vaginal microbiome complexity from an ecological perspective. Transl. Res. 2012, 160, 267–282.
- Lewis, F.M.T.; Bernstein, K.T.; Aral, S.O. Vaginal Microbiome and Its Relationship to Behavior, Sexual Health, and Sexually Transmitted Diseases. Obstet. Gynecol. 2017, 129, 643–654.
- Serrano, M.G.; Parikh, H.I.; Brooks, J.P.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; et al. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011.
- Jespers, V.; Kyongo, J.; Joseph, S.; Hardy, L.; Cools, P.; Crucitti, T.; Mwaura, M.; Ndayisaba, G.; Delany-Moretlwe, S.; Buyze, J.; et al. A longitudinal analysis of the vaginal microbiota and vaginal immune mediators in women from sub-Saharan Africa. Sci. Rep. 2017, 7, 11974.
- Romero, R.; Hassan, S.S.; Gajer, P.; Tarca, A.L.; Fadrosh, D.W.; Nikita, L.; Galuppi, M.; Lamont, R.F.; Chaemsaithong, P.; Miranda, J.; et al. The composition and stability of the vaginal microbiota of normal pregnant women is different from that of non-pregnant women. Microbiome 2014, 2, 4.
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen–gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53.
- Plottel, C.S.; Blaser, M.J. Microbiome and Malignancy. Cell Host Microbe 2011, 10, 324–335.
- Flores, R.; Shi, J.; Fuhrman, B.; Xu, X.; Veenstra, T.D.; Gail, M.H.; Gajer, P.; Ravel, J.; Goedert, J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012, 10, 253.
- Cruickshank, R.; Sharman, A. The biology of the vagina in the human subject. BJOG Int. J. Obstet. Gynaecol. 1934, 41, 208–226.
- Hickey, R.J.; Zhou, X.; Settles, M.L.; Erb, J.; Malone, K.; Hansmann, M.A.; Shew, M.L.; Van Der Pol, B.; Fortenberry, J.D.; Forney, L.J. Vaginal Microbiota of Adolescent Girls Prior to the Onset of Menarche Resemble Those of Reproductive-Age Women. mBio 2015, 6, e00097-15.
- Mirmonsef, P.; Hotton, A.L.; Gilbert, D.; Gioia, C.J.; Maric, D.; Hope, T.J.; Landay, A.L.; Spear, G.T. Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal pH, Estrogen, and Progesterone. PLoS ONE 2016, 11, e0153553.
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50.
- Boskey, E.; Cone, R.; Whaley, K.; Moench, T. Origins of vaginal acidity: High d/l lactate ratio is consistent with bacteria being the primary source. Hum. Reprod. 2001, 16, 1809–1813.
- Spear, G.T.; French, A.L.; Gilbert, D.; Zariffard, M.R.; Mirmonsef, P.; Sullivan, T.H.; Spear, W.W.; Landay, A.; Micci, S.; Lee, B.-H.; et al. Human α-amylase Present in Lower-Genital-Tract Mucosal Fluid Processes Glycogen to Support Vaginal Colonization by Lactobacillus. J. Infect. Dis. 2014, 210, 1019–1028.
- Vodstrcil, L.A.; Hocking, J.S.; Law, M.; Walker, S.; Tabrizi, S.N.; Fairley, C.K.; Bradshaw, C.S. Hormonal Contraception Is Associated with a Reduced Risk of Bacterial Vaginosis: A Systematic Review and Meta-Analysis. PLoS ONE 2013, 8, e73055.
- Brotman, R.M.; He, X.; Gajer, P.; Fadrosh, D.; Sharma, E.; Mongodin, E.F.; Ravel, J.; Glover, E.D.; Rath, J.M. Association between cigarette smoking and the vaginal microbiota: A pilot study. BMC Infect. Dis. 2014, 14, 471.
- Mändar, R.; Punab, M.; Borovkova, N.; Lapp, E.; Kiiker, R.; Korrovits, P.; Metspalu, A.; Krjutškov, K.; Nõlvak, H.; Preem, J.-K.; et al. Complementary seminovaginal microbiome in couples. Res. Microbiol. 2015, 166, 440–447.
- Schwebke, J.R.; Desmond, R.A.; Oh, M.K. Predictors of Bacterial Vaginosis in Adolescent Women Who Douche. Sex Transm Dis 2004, 31, 433–436.
- Baker, J.M.; Chase, D.M.; Herbst-Kralovetz, M.M. Uterine microbiota: Residents, tourists, or invaders? Front. Immunol. 2018, 9, 208.
- Chen, C.; Song, X.; Chunwei, Z.; Zhong, H.; Dai, J.; Lan, Z.; Li, F.; Yu, X.; Feng, Q.; Wang, Z.; et al. The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nat. Commun. 2017, 8, 875.
- E Fouts, D.; Pieper, R.; Szpakowski, S.; Pohl, H.; Knoblach, S.; Suh, M.-J.; Huang, S.-T.; Ljungberg, I.; Sprague, B.M.; Lucas, S.K.; et al. Integrated next-generation sequencing of 16S rDNA and metaproteomics differentiate the healthy urine microbiome from asymptomatic bacteriuria in neuropathic bladder associated with spinal cord injury. J. Transl. Med. 2012, 10, 174.
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine Is Not Sterile: Use of Enhanced Urine Culture Techniques to Detect Resident Bacterial Flora in the Adult Female Bladder. J. Clin. Microbiol. 2014, 52, 871–876.
- Thomas-White, K.; Forster, S.C.; Kumar, N.; Van Kuiken, M.; Putonti, C.; Stares, M.D.; Hilt, E.E.; Price, T.K.; Wolfe, A.J.; Lawley, T.D. Culturing of female bladder bacteria reveals an interconnected urogenital microbiota. Nat. Commun. 2018, 9, 1557.
- Antonio, M.A.D.; Rabe, L.K.; Hillier, S.L. Colonization of the Rectum by Lactobacillus Species and Decreased Risk of Bacterial Vaginosis. J. Infect. Dis. 2005, 192, 394–398.
- El Aila, N.A.; Tency, I.; Claeys, G.; Verstraelen, H.; Saerens, B.; Santiago, G.L.D.S.; De Backer, E.; Cools, P.; Temmerman, M.; Verhelst, R.; et al. Identification and genotyping of bacteria from paired vaginal and rectal samples from pregnant women indicates similarity between vaginal and rectal microflora. BMC Infect. Dis. 2009, 9, 167.
- Dong, Q.; Nelson, D.E.; Toh, E.; Diao, L.; Gao, X.; Fortenberry, J.D.; Van Der Pol, B. The Microbial Communities in Male First Catch Urine Are Highly Similar to Those in Paired Urethral Swab Specimens. PLoS ONE 2011, 6, e19709.
- Moreno, I.; Codoñer, F.M.; Vilella, F.; Valbuena, D.; Martinez-Blanch, J.F.; Jimenez-Almazán, J.; Alonso, R.; Alamá, P.; Remohí, J.; Pellicer, A.; et al. Evidence that the endometrial microbiota has an effect on implantation success or failure. Am. J. Obstet. Gynecol. 2016, 215, 684–703.
- Robertson, S.A.; Chin, P.Y.; Glynn, D.J.; Thompson, J.G. Peri-conceptual cytokines–setting the trajectory for embryo implantation, pregnancy and beyond. Am. J. Reprod. Immunol. 2011, 66, 2–10.
- Dominguez, F.; Gadea, B.; Mercader, A.; Esteban, F.J.; Pellicer, A.; Simón, C. Embryologic outcome and secretome profile of implanted blastocysts obtained after coculture in human endometrial epithelial cells versus the sequential system. Fertil. Steril. 2010, 93, 774–782.e1.
- Zhou, Z.-W.; Long, H.-Z.; Cheng, Y.; Luo, H.-Y.; Wen, D.-D.; Gao, L.-C. From Microbiome to Inflammation: The Key Drivers of Cervical Cancer. Front. Microbiol. 2021, 12, 767931.
- Audirac-Chalifour, A.; Torres-Poveda, K.; Bahena-Román, M.; Téllez-Sosa, J.; Martinez-Barnetche, J.; Cortina-Ceballos, B.; López-Estrada, G.; Delgado-Romero, K.; Burguete-García, A.I.; Cantú, D.; et al. Cervical Microbiome and Cytokine Profile at Various Stages of Cervical Cancer: A Pilot Study. PLoS ONE 2016, 11, e0153274.
- Medina-Colorado, A.A.; Vincent, K.L.; Miller, A.L.; Maxwell, C.A.; Dawson, L.N.; Olive, T.; Kozlova, E.V.; Baum, M.M.; Pyles, R.B. Vaginal ecosystem modeling of growth patterns of anaerobic bacteria in microaerophilic conditions. Anaerobe 2017, 45, 10–18.
- Graver, M.A.; Wade, J.J. The role of acidification in the inhibition of Neisseria gonorrhoeae by vaginal lactobacilli during anaerobic growth. Ann. Clin. Microbiol. Antimicrob. 2011, 10, 8.
- Gong, Z.; Luna, Y.; Yu, P.; Fan, H. Lactobacilli Inactivate Chlamydia trachomatis through Lactic Acid but Not H2O2. PLoS ONE 2014, 9, e107758.
- Breshears, L.M.; Edwards, V.L.; Ravel, J.; Peterson, M.L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 2015, 15, 276.
- A Clarke, M.; Rodriguez, A.C.; Gage, J.C.; Herrero, R.; Hildesheim, A.; Wacholder, S.; Burk, R.; Schiffman, M. A large, population-based study of age-related associations between vaginal pH and human papillomavirus infection. BMC Infect. Dis. 2012, 12, 33.
- Straight, S.W.; Herman, B.; McCance, D.J. The E5 oncoprotein of human papillomavirus type 16 inhibits the acidification of endosomes in human keratinocytes. J. Virol. 1995, 69, 3185–3192.
- Linhares, I.M.; Summers, P.R.; Larsen, B.; Giraldo, P.C.; Witkin, S.S. Contemporary perspectives on vaginal pH and lactobacilli. Am. J. Obstet. Gynecol. 2011, 204, 120.e1–120.e5.
- Witkin, S.S.; Mendes-Soares, H.; Linhares, I.; Jayaram, A.; Ledger, W.J.; Forney, L.J. Influence of Vaginal Bacteria and d- and l-Lactic Acid Isomers on Vaginal Extracellular Matrix Metalloproteinase Inducer: Implications for Protection against Upper Genital Tract Infections. mBio 2013, 4, e00460-13.
- Alvarez-Olmos, M.I.; Barousse, M.M.; Rajan, L.; Van Der Pol, B.J.; Fortenberry, D.; Orr, D.; Fidel Jr, P.L. Vaginal lactobacilli in adolescents: Presence and relationship to local and systemic immunity, and to bacterial vaginosis. Sex. Transm. Dis. 2004, 31, 393–400.
- Nunn, K.L.; Wang, Y.-Y.; Harit, D.; Humphrys, M.S.; Ma, B.; Cone, R.; Ravel, J.; Lai, S.K. Enhanced Trapping of HIV-1 by Human Cervicovaginal Mucus Is Associated with Lactobacillus crispatus-Dominant Microbiota. mBio 2015, 6, e01084-15.
- Sun, S.; Li, H.; Chen, J.; Qian, Q. Lactic Acid: No Longer an Inert and End-Product of Glycolysis. Physiology 2017, 32, 453–463.
- Yang, X.; Da, M.; Zhang, W.; Qi, Q.; Zhang, C.; Han, S. Role of Lactobacillus in cervical cancer. Cancer Manag. Res. 2018, 10, 1219–1229.
- Selle, K.; Klaenhammer, T.R. Genomic and phenotypic evidence for probiotic influences of Lactobacillus gasseri on human health. FEMS Microbiol. Rev. 2013, 37, 915–935.
- Aroutcheva, A.; Gariti, D.; Simon, M.; Shott, S.; Faro, J.; Simoes, J.A.; Gurguis, A.; Faro, S. Defense factors of vaginal lactobacilli. Am. J. Obstet. Gynecol. 2001, 185, 375–379.
- Pandey, N.; Malik, R.K.; Kaushik, J.K.; Singroha, G. Gassericin A: A circular bacteriocin produced by Lactic acid bacteria Lactobacillus gasseri. World J. Microbiol. Biotechnol. 2013, 29, 1977–1987.
- Stoyancheva, G.; Marzotto, M.; Dellaglio, F.; Torriani, S. Bacteriocin production and gene sequencing analysis from vaginal Lactobacillus strains. Arch. Microbiol. 2014, 196, 645–653.
- Reid, G.; Heinemann, C.; Velraeds, M.; van der Mei, H.C.; Busscher, H.J. Biosurfactants produced by Lactobacillus. In Methods in enzymology; Elsevier: Amsterdam, The Netherlands, 1999; Volume 310, pp. 426–433.
- Ojala, T.; Kankainen, M.; Castro, J.; Cerca, N.; Edelman, S.; Westerlund-Wikström, B.; Paulin, L.; Holm, L.; Auvinen, P. Comparative genomics of Lactobacillus crispatus suggests novel mechanisms for the competitive exclusion of Gardnerella vaginalis. BMC Genom. 2014, 15, 1070.
- AL Kassaa, I.; Hober, D.; Hamze, M.; Chihib, N.E.; Drider, D. Antiviral Potential of Lactic Acid Bacteria and Their Bacteriocins. Probiotics Antimicrob. Proteins 2014, 6, 177–185.
- Wang, S.; Wang, Q.; Yang, E.; Yan, L.; Li, T.; Zhuang, H. Antimicrobial Compounds Produced by Vaginal Lactobacillus crispatus Are Able to Strongly Inhibit Candida albicans Growth, Hyphal Formation and Regulate Virulence-related Gene Expressions. Front. Microbiol. 2017, 8, 564.
- Sgibnev, A.V.; Kremleva, E.A. Vaginal Protection by H2O2-Producing Lactobacilli. Jundishapur J. Microbiol. 2015, 8, e22913.
- Hancock, R.E.; Rozek, A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 2002, 206, 143–149.
- Frew, L.; Stock, S.J. Antimicrobial peptides and pregnancy. Reproduction 2011, 141, 725–735.
- O’Hanlon, D.E.; Lanier, B.R.; Moench, T.R.; Cone, R.A. Cervicovaginal fluid and semen block the microbicidal activity of hydrogen peroxide produced by vaginal lactobacilli. BMC Infect. Dis. 2010, 10, 120.
- O’Hanlon, D.E.; Moench, T.R.; Cone, R.A. In vaginal fluid, bacteria associated with bacterial vaginosis can be suppressed with lactic acid but not hydrogen peroxide. BMC Infect. Dis. 2011, 11, 200.
- Al-Nasiry, S.; Ambrosino, E.; Schlaepfer, M.; Morré, S.A.; Wieten, L.; Voncken, J.W.; Spinelli, M.; Mueller, M.; Kramer, B.W. The Interplay Between Reproductive Tract Microbiota and Immunological System in Human Reproduction. Front. Immunol. 2020, 11, 378.
- Maldonado-Barragán, A.; Caballero-Guerrero, B.; Martín, V.; Ruiz-Barba, J.L.; Rodríguez, J.M. Purification and genetic characterization of gassericin E, a novel co-culture inducible bacteriocin from Lactobacillus gasseri EV1461 isolated from the vagina of a healthy woman. BMC Microbiol. 2016, 16, 37.
- Nasioudis, D.; Forney, L.J.; Schneider, G.M.; Gliniewicz, K.; France, M.T.; Boester, A.; Sawai, M.; Scholl, J.; Witkin, S.S. The composition of the vaginal microbiome in first trimester pregnant women influences the level of autophagy and stress in vaginal epithelial cells. J. Reprod. Immunol. 2017, 123, 35–39.
- Niu, X.-X.; Li, T.; Zhang, X.; Wang, S.-X.; Liu, Z.-H. Lactobacillus crispatus Modulates Vaginal Epithelial Cell Innate Response to Candida albicans. Chin. Med. J. 2017, 130, 273–279.
- Zadravec, P.; Štrukelj, B.; Berlec, A. Improvement of LysM-Mediated Surface Display of Designed Ankyrin Repeat Proteins (DARPins) in Recombinant and Nonrecombinant Strains of Lactococcus lactis and Lactobacillus Species. Appl. Environ. Microbiol. 2015, 81, 2098–2106.
- Fichera, G.A.; Fichera, M.; Milone, G. Antitumoural activity of a cytotoxic peptide of Lactobacillus casei peptidoglycan and its interaction with mitochondrial-bound hexokinase. Anti-Cancer Drugs 2016, 27, 609–619.
- Radtke, A.L.; Quayle, A.J.; Herbst-Kralovetz, M.M. Microbial Products Alter the Expression of Membrane-Associated Mucin and Antimicrobial Peptides in a Three-Dimensional Human Endocervical Epithelial Cell Model1. Biol. Reprod. 2012, 87, 132.
- Yao, X.-Y.; Yuan, M.-M.; Li, D.-J. Molecular adjuvant C3d3 improved the anti-hCGβ humoral immune response in vaginal inoculation with live recombinant Lactobacillus expressing hCGβ-C3d3 fusion protein. Vaccine 2007, 25, 6129–6139.
- Lee, T.-Y.; Kim, Y.-H.; Lee, K.-S.; Kim, J.-K.; Lee, I.-H.; Yang, J.-M.; Sung, M.-H.; Park, J.-S.; Poo, H. Human papillomavirus type 16 E6-specific antitumor immunity is induced by oral administration of HPV16 E6-expressing Lactobacillus casei in C57BL/6 mice. Cancer Immunol. Immunother. 2010, 59, 1727–1737.
- Chase, D.; Goulder, A.; Zenhausern, F.; Monk, B.; Herbst-Kralovetz, M. The vaginal and gastrointestinal microbiomes in gynecologic cancers: A review of applications in etiology, symptoms and treatment. Gynecol. Oncol. 2015, 138, 190–200.
- Motevaseli, E.; Shirzad, M.; Akrami, S.M.; Mousavi, A.-S.; Mirsalehian, A.; Modarressi, M.H. Normal and tumour cervical cells respond differently to vaginal lactobacilli, independent of pH and lactate. J. Med. Microbiol. 2013, 62, 1065–1072.
- Borgdorff, H.; Tsivtsivadze, E.; Verhelst, R.; Marzorati, M.; Jurriaans, S.; Ndayisaba, G.F.; Schuren, F.H.; Van De Wijgert, J.H.H.M. Lactobacillus-dominated cervicovaginal microbiota associated with reduced HIV/STI prevalence and genital HIV viral load in African women. ISME J. 2014, 8, 1781–1793.
- Mitchell, C.; Balkus, J.E.; Fredricks, D.; Liu, C.; McKernan-Mullin, J.; Frenkel, L.M.; Mwachari, C.; Luque, A.; Cohn, S.E.; Cohen, C.R. Interaction between lactobacilli, bacterial vaginosis-associated bacteria, and HIV Type 1 RNA and DNA Genital shedding in US and Kenyan women. AIDS Res. Hum. Retrovir. 2013, 29, 13–19.
More
