1. Clerodendrum bungei
In Chinese folk medicine,
C. bungei (eng. name: rose glory bower, Chinese name: Chou mu dan) is a plant raw material whose roots and leaves are used to treat boils, haemorrhoids, hypertension, lung cancer and eczema
[34][1]. This species is known to be rich in diterpenoids, some of which exhibit potential biological activities
[34,35,36][1][2][3].
This plant species was first investigated for diterpenoid content by Fan et al. in 1999
[35][2]. The
resea
uthorchers isolated two new royleanone-type compounds from
C. bungei roots: 9,10-dihydro-3,4,9-trimethyl phenanthro [3,2-b]pyran (7H)-7, 12(8H)-dione (bungone A
(1)) and 9,10-dihydro-8-hydroxymethyl-3,4,9-trimethylphenanthro [3,2-β]pyran(2H)-7,12-dione (bungone B
(2)). While these abietane diterpenoids are structurally similar to the royleanones, with both possessing an 11,14-para benzoquinone group, the C-12 has an oxygen enclosed by an additional aliphatic ring instead of a hydroxyl group, which is typical for roylanones. Due to their cytotoxic activities, these compounds are very interesting for further research; like other diterpenes, including horminone or acetyl-horminone, royleanone is able to damage DNA and inhibit topoisomerase I and II
[31,40,41][4][5][6](
Figure 1).
Figure 1. The chemical structure of the compounds isolated from Clerodendrum genus.
Liu et al. (2008)
[36][3] isolated other abietane-type diterpenoids from the roots of
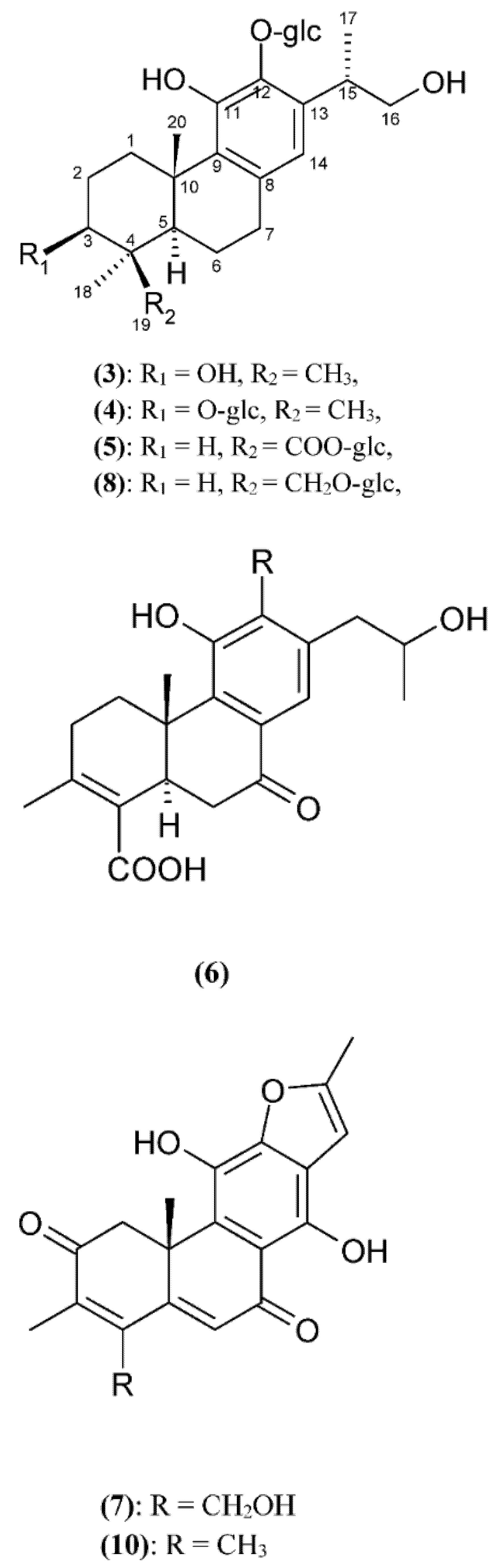
Clerodendrum bungei. Five were new structures: 12-
O-
β-D-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene
(3), 3,12-
O-
β-D-diglucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
(4), 19-
O-
β-D-carboxyglucopyranosyl-12-
O-
β-D-glucopyranosyl-11,16-dihydroxyabieta-8,11,13-triene
(5), 11,16-dihydroxy-12-
O-
β-D-glucopyranosyl-17(15→16),18(4→3)-
abeo-4-carboxy-3,8,11,13-abietatetraen-7-one
(6) and 19-hydroxyteuvincenone F
(7). All are glycosides, apart from compound
(7), and all contain aglycone, either as an abietatriene or abietatetraene.
In addition, the diterpenoids ajugaside A
(8), uncinatone
(9) and teuvincenone F
(10), first isolated from other plant materials, were also isolated, purified and identified from the aqueous acetone crude extract of
C. bungei roots. The identified compounds were tested for their potential cytotoxic activity against three cell lines: B16 murine melanoma, HGC-27 human gastric, and HEK-293 human epithelial kidney. Of the tested compounds, only uncinatone
(9), a rearranged abietane derivative containing a 17(15→16), 18(4→3)-
diabeo-abietane framework, was found to demonstrate moderate cytotoxicity against tested cell lines: the IC
50 value ranged from 1.2 to 6.4 µM depending on the treated cell line, as indicated by MTT, i.e., 3-(4,5-dimethylythiazol-2-yl)-2,5-diphenyl-2
H-tetrazolium bromide. This diterpenoid also inhibited cell proliferation and induced cell-cycle G2/M phase arrest
[36][3].
In addition, 12-
O-
β-D-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene
(3), 3,12-
O-
β-D-diglucopyranosyl-11,16-dihydroxyabieta- 8,11,13-triene
(4), ajugaside A
(8), uncinatone
(9) and 19-hydroxyteuvincenone F
(7) demonstrated significant anti-complement activity on the classical pathway complement system, as expressed by total hemolytic activity
[37][7]. The inhibitory activity of these compounds against the complement system recorded an IC
50 range from 24 µM to 232 µM. The most active compound was found to be 12-
O-
β-D-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene
(3) [37][7]. Kim et al. (2010) postulate that the hydroxyl group in position 3 of this compound may play an important role in its high anti-complement activity. Other diterpenes with glucose, methyl, or hydrogen moieties at position 3 demonstrated significantly lower anti-complement activities
[37][7]. In addition, another two new diterpenoids were isolated from
C. bungei: 3
β-(
β-D-glucopyranosyl)isopimara-7,15-diene-11α,12α-diol
(11) and 16-
O-
β-D- D-glucopyranosyl-3
β-20-epoxy-3-hydroxyabieta-8,11,13-triene
(12) together with other known compounds, such as 12-
O-
β-D-glucopyranosyl-3,11,16-trihydroxyabieta-8,11,13-triene
(3) and 3,12-
O-
β-D-diglucopyranosyl-11,16-dihydroxy-abieta-8,11,13-triene
(4) [34][1]. All isolated, purified and identified secondary metabolites were evaluated for cytotoxicity against the following tumour cell lines: B16 murine melanoma, HGC-27 human gastric and BEL-7402 human hepatocellular carcinoma. Sun et al. (2014) report that only 16-
O-
β-D-glucopyranosyl-3
β-20-epoxy-3-hydroxyabieta-8,11,13-triene
(12) appeared to be active among all tested compounds; it demonstrated moderate cytotoxicity against B16, HGC-27, and BEL-7402 cells, manifested with IC
50 values of 8.8, 9.8, and 7.1 µM, respectively
[34][1]. The
resea
uthorchers emphasise the structural similarities between this diterpenoid and bioactive compounds isolated from the same plant material
[34,35,37][1][2][7]. It is worth adding, that this metabolite has a hydroxyl group at the third carbon, which is believed to be responsible for the biological activities of compounds isolated from
C. bungei roots
[37][7].
Further studies on
C. bundei resulted in the isolation and identification of the following diterpenoids: bungnate A
(13) (12,16-epoxy-6-methoxy-11,14-dihydroxy-17(15→16)-abeo-5,8,11,13,15-abietapentaen-7-one-17-carboxylate), bungnate B
(14) (19-O-β-D-carboxyglucopyranosyl-11,12,16-trihydroxy-abieta-8,11,13-triene-7-one), 15-dehydrocyrtophyllone A
(15) (12,16-epoxy-6-methoxy-11,14-dihydroxy-17(15→16)-abeo-5,8,11,13,15-abietapentaen-7-one) 15-dehydro-17-hydroxycyrtophyllone A
(16) (12,16-epoxy-6-methoxy-11,14,17-trihydroxy-17(15→16)-abeo-5,8,11,13,15-abietapentaen-7-one), and cyrtophyllone A
(17) [38][8]. Of these, 15-dehydrocyrtophyllone A
(15) demonstrated ACE (Angiotensin Converting Enzyme) inhibition activity, with an IC
50 value of 42.7 µM. Among the tested diterpenoids, none inhibited α-glucosidase
[38][8].
2. Clerodendrum cyrtophyllum
This genus, known in Chinese medicine as “Da quing”, is recommended for treating infectious diseases, common cold and malaria
[42][9]. Many relevant compounds have been extracted from the plant, including the diterpenoids teuvincenone F
(10), uncinatone
(9) and sugiol
(18), the triterpenoids friedelin
(19) and clerodolone
(20) and the phytosteroids stigmasta-5,22,25-trien-3
β-ol and clerosterol. In addition, two new abietane derivatives, cyrtophyllone A
(17) (16(
S)-12,16-epoxy-11,13-dihydroxy-6-methoxy-17(15-16)-
abeo-abieta-5,8,11,13-tetraen-7-one) and cyrtophyllone B
(21) ((+)-11,12,16-trihydroxy-abieta-8,11,13-trien-7-one) have been isolated from ethanolic extract of the entire
C. cyrtophyllum plant following cleaning by water and chloroform mix
[42][9]. The former has a 17(15-16)-
abeo-abietane framework.
The diterpenes sugiol
(18), uncinatone
(9) and cyrtophyllone B
(21), also isolated from
C. cyrtophyllum, have also been identified in
Aegiphila lhotzkyan roots. These phytocompounds were tested for antiproliferative activity against leukaemia (CEM and HL-60), breast (MCF-7), colon (HCT-8) and skin (B-16) cancer cell lines in three independent experiments
[43][10]. Of these, only cyrtophyllone B
(21) is able to inhibit the proliferation of all tested tumour cell lines; however, it did not demonstrate strong inhibition (IC
50 values above 1 µg mL
−1)
[43][10]. In addition, diterpenoids isolated from
Caryopteris mongolica roots were found to inhibit acethyl- and butyrylcholineesterase (AChE and BChE)
[44][11].
3. Clerodendrum eriophyllum
This unusual plant was previously used in malaria treatment in Kenya
[45][12]. An alcoholic
C. eriophyllum root bark extract demonstrated significant chemosuppressive properties against
Plasmodium berghei in infected experimental mice
[46][13]. The first phytochemical study of
Clerodendrum eriophyllum was recorded by Machumi et. al. in 2010
[47][14]. The dichloromethane-methanolic root extract was found to contain ten abietane diterpenoids, with one being a new discovery: 12-hydroxy-8,12-abietadiene-3,11,14-trione
(22). The remaining nine diterpenes had previously been isolated from other plant materials: royleanone
(23), taxodione
(24), 6-deoxy-taxodione
(25) (11-hydroxy-7,9(11),13-abietatrien-12-one), sugiol
(18), ferruginol
(26), 6-hydroxysalvinolone
(27), 6,11,12,16-tetrahydroxy-5,8,11,13-abietatetra-en-7-one
(28), uncinatone
(9) and 11-hydroxy-8,11,13-abietatriene-12-O-β-xylopyranoside
(29) [47][14].
One of the abietane diterpenoids, royleanone
(23), was first isolated from
Inula royleana roots
[48][15]. However, its presence has also been confirmed in other plant species, e.g., in transformed
Salvia austriaca roots
[49][16] and non-transformed
Salvia officinalis roots
[32][17]. Royleanone
(23), the diterpenoid characterised by the presence of a
p-quinone grouping in the C ring, is also well known for its various biological activities. It has been found to demonstrate cytotoxicity against the cancer cell lines HeLa and Hep-2, particularly against Hep-2, with an IC
50 value of 34 µg mL
−1 [50][18]. It has also been found to demonstrate some antibacterial activity, but with weaker activity against methycyllin- and vancomycin-resistant
S. aureus strains (MRSA and VRE) compared to other diterpenoids from outside the
Clerodendrum genus (MIC = 32 and above 64 µg mL
−1, respectively)
[51][19].
Taxodione
(24) is a very well-known abietane-type diterpenoid with a metide-quinone moiety, which was first isolated from entire
Taxodium distichum plant
[52][20]. This compound has been found to demonstrate in vivo cytotoxic activity against Walker intramuscular carcinosarcoma 256 in rats and in vitro activity against human nosopharynx carcinoma cells KB
[52][20]. Its high cytotoxicity was confirmed in further studies on Hep-2 and HeLa
[50][18] and A549
[30][21]. This compound also demonstrates weak AChE and BChE inhibition. Computer modelling found the phytocompound to demonstrate low cardio- and genotoxicity and good permeability of the blood–brain barrier
[30][21]. It has also been found to demonstrate strong antibacterial activity, particularly against MRSA and VRE strains (MIC = 4–10 µg mL
−1)
[51][19].
6-deoxy-taxodione
(25), isolated from
C. eriophyllum roots, is also detected in various parts of other plant species, e.g., in winter cones of
Taxodium distichum and fruits of
Cupressus sempervirens [52,53,54][20][22][23]. Like taxodione
(24), both isolated from
Cupressus sempervirens cones, this compound demonstrates potent anti-leishmanial activity, with IC
50 values of 0.077 µg mL
−1 for 6-deoxy-taxodione
(25) and 0.025 µg mL
−1 for taxodione
(24). The two diterpenoids demonstrated much stronger activity against
Leishmania donovani and its promastigotes than the anti-leishmanial drugs used as controls: pentamidine (IC
50 1.62 µg mL
−1) and amphotericin B (IC
50 0.11 µg mL
−1)
[53][22]. In addition, 6-deoxy-taxodione
(25) was found to demonstrate potent antibacterial activities against methicillin-resistant
Staphylococcus aureus (MRSA), with IC
50 values being 0.80 μg mL
−1 for
(25) and 0.85 μg mL
−1 for
(26) [53][22].
Another abietane-type diterpenoid is sugiol
(18), isolated from
Clerodendrum eriophyllum roots. This compound has an oxygen atom connected to the B ring and an aromatic C ring. This unusual aromatic diterpene demonstrates various antioxidant, antibacterial, antiviral, anticancer, anti-tumour and anti-inflammatory activities
[55][24]. Its antioxidant activity is similar to those of α-tocopherol and ascorbic acid based on DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay (84% and 82%, respectively)
[56][25]. Sugiol
(18) also demonstrates a concentration-dependent inhibitory effect (72.4%) against NO (nitric oxide), at a concentration of 100 μg mL
−1; it also demonstrated similar superoxide radical scavenging activity at a concentration of 250 μg mL
−1, to ascorbic acid and α-tocopherol activities (73% for sugiol compared to 73% and 74.5%, respectively)
[56][25]. Sugiol
(18) is also active against various foodborne pathogenic bacteria but neutralises Gram-positive bacteria more effectively than Gram-negative bacteria. When isolated from
Metasequoia glyptostroboides cones, the compound was also found to demonstrate stronger antibacterial action against Gram-positive bacteria than the streptomycin used as a control
[57][26]. Sugiol
(18) has also been found to exhibit antiviral activity against the H1N1 virus in infected Madin-Darby canine kidney (MDCK) cells: no cytopathic changes were observed following 72 h of exposure following treatment with 500 μg mL
−1 sugiol
(18). Hence, sugiol
(18) could be a potential antiviral compound that can prevent H1N1-mediated cytopathy in MDCK cells
[58][27].
The diterpenoid sugiol
(18) also demonstrated cytotoxic activity against tumour cell lines, inhibiting the growth of three prostate tumour cell lines (LNCap, PC3 and DU145) and a non-tumorigenic cell line (MCF10A)
[55][24]. Similarly, sugiol
(18) treatment was found to reduce tumour weight and volume by as much as 75% in mice subcutaneously injected with DU145 cells in comparison with the control group. However, sugiol
(18) did not affect the body weight of the mouse
[55][24].
The abietane diterpenoid ferruginol
(26) was first isolated in 1939 from the
Podocarpus ferruginea tree. Structurally, ferruginol is similar to sugiol
(18), although it lacks an oxygen in the B ring. The biologically active ferruginol has been recorded in many plants including those of the Podocarpaceae, Cupressaceae, Lamiaceae and Verbenaceae
[59][28]. This diterpenoid exhibits antibacterial and antifungal activities
[60][29]. It has been found to inhibit the growth of
Bacillus brevis,
B. subtilis and
Staphylococcus aureus, with inhibition zone diameters of 18, 10 and 9 mm, respectively. Ferruginol
(26) demonstrated fungicidal activity against the pathogenic
Paecilomyces variotii, with an inhibition zone of 10 mm
[60][29], and Ferruginol
(26) isolated from
Chamaecyparis lawsoniana cones also demonstrated antibacterial activity against
S. aureus, with MIC values ranging from 4 to 16 μg mL
−1 depending on the strain
[61][30]. It has demonstrated potent antimalarial activity
[59][28], with EC
50 values against
Plasmodium falciparum ranging from 2.47 to 19.57 μM, depending on the strain
[59][28]. In addition, ferruginol
(26) has displayed moderate cytotoxic activity against NALM-6 human leukaemia lymphoblastic cells (IC
50 27.2 μg mL
−1) and promyelocytic HL-60 cells (IC
50 33.6 μg mL
−1)
[62][31].
The abietane diterpenoid 6-hydroxysalvinolone
(27), containing oxygen and hydroxyl groups in the B ring, demonstrates strong cytotoxicity against carcinoma cell lines. Following isolation from
Salvia chorassanica roots, the compound exhibited strong cytotoxic activity against HL-60 and K562 cell lines with IC
50 values of 36.3 and 33.3 μM, respectively. It appeared to demonstrate a substantially less cytotoxic effect on non-cancerous human cell lines. When administered at concentrations of 2.5 and 5.0 μM for 48 h, it also enhanced the expression of the proapoptotic protein Bax, and cleaved caspase-3 and PARP
[63][32]. It also was found to exhibit moderate cytotoxic activity against monkey kidney fibroblasts (VERO) with an IC
50 level of 4.5 μg mL
−1 [47][14]. Similarly to taxodione
(24), 6-hydroxysalvinolone
(27) also demonstrated antifungal activity, especially against
Candida neoformans with an IC
50 value of 0.96 μg mL
−1. In the same assay, the IC
50 of taxodione
(24) was found to be 0.58 μg mL
−1, which is comparable with that of standard amphotericin B (IC
50 = 0.44 μg mL
−1)
[47][14].
Another abietane-type diterpenoid is 6,11,12,16-tetrahydroxy-5,8,11,13-abietatetra-en-7-one
(28), isolated from
Avicennia marina twigs; it differs from 6-hydroxysalvinolone
(27) by the presence of a hydroxyl group in the isopropyl moiety. It demonstrated moderate antiproliferative properties against L-929 (mouse fibroblasts) and K562 (human chronic myeloid leukaemia), and cytotoxic activities against the HeLa (human cervix carcinoma) cell line
[64][33]. In biological tests, 6,11,12,16-tetrahydroxy-5,8,11,13-abietatetra-en-7-one
(28) demonstrated GI
50 (concentration causing 50% cell growth inhibition) values of 9.6 and 8.9 μg mL
−1, against L-929 (DSM ACC 2, mouse fibroblasts) and K562 cell lines (DSM ACC 10, human chronic myeloid leukaemia), and a CC
50 (concentration that reduced the cell viability by 50%) of 18 μg mL
−1 against the HeLa cell line
[64][33]. The compound also demonstrated antibacterial activity against Gram-positive and Gram-negative bacteria and antifungal potential. A study of its antibacterial activity against
Bacillus subtilis ATTC 6 633 (IMET) NA,
Bacillus subtilis ATTC 6 633 (IMET) AS,
Escherichia coli SG 458,
Pseudomonas aeruginosa K 799/61,
Mycobacterium vaccae IMET 10 670,
Sporobolomyces salmonicolor SBUG 549,
Candida albicans BMSY 212 and
Penicillium notatum JP
[64][33] found zone inhibition to range from 12 mm (for
C. albicans) to 25 mm (for
B. subtilis ATTC 6 633 (IMET) AS)
[64][33].
Uncinatone
(9), a diterpenoid known for its biological activity, also exhibits potent antileishmanial activity. The IC
50 value for
L. donovani is 0.2 μg mL
−1 [47][14].
4. C. formicarum
The
abeo-abietane diterpenoid formidiol
(30) was first obtained by methanolic extraction of
Clerodendrum formicarum leaves and chromatographic separation of its triterpenoid constituents
[65][34]. It was accompanied by the diterpenoid 12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13,15-pentanene-3,7-dione
(31), which had been previously isolated from a hexane extract of
Aegiphila lhotzkiana roots. It was found to demonstrate antiproliferative activity against the leukaemia cell lines HL-60 (IC
50 4.4 μM) and CEM (IC
50 8.4 μM)
[43][10]. Due to its structural similarity to formidiol
(30), compound
(31) should be included in future studies of anti-proliferative activity.
5. Clerodendrum inerme
Studies on the aerial parts of
Clerodendrum inerme resulted in the isolation of cleroinermin
(32) a
neo-clerodane diterpenoid
[66][35] consisting of a bicyclic ring decalin moiety and a six-carbon side chain including a furane skeleton. The compound, first isolated from
Heteroplexis micocephala, showed neuroprotective activity against MPP+ induced PC12-syn cell damage, with a relative cell proliferation rate of 104.32%
[67][36]. Elsewhere, the
neo-clerodane diterpenoids clerodendrin B
(33), 3-epicaryoptin
(34), clerodendrin C
(35), 2-acetoxyclerodendrin B
(36) and 15-hydroxyepicaryoptin
(37) have since been isolated
[68][37].
C. inerme has become an interesting subject of research for diterpenoid isolation. The aerial parts are a source of the neo-clerodane-type diterpenoids: clerodermic acid
(38), inermes A
(39) and B
(40), as well as 14,15-dihydro-15β-methoxy-3-epicaryoptin
(41) [69,71][38][39]. Among these compounds, clerodermic acid
(38) deserves special attention due to its strong biological activity. The compound, isolated from the dichloromethane extract of the aerial part of
Salvia nemorosa, was found to reduce the viability of A549 cells in a concentration-dependent manner, with an IC
50 of 35 µg mL
−1 at 48 h, based on the MTT assay
[72][40]. Furthermore, clerodermic acid treatment resulted in various morphological changes, including diminished cell density, membrane blebbing and an increased number of floating cells, all of them being a manifestation of cell death
(38). DNA ladder, DAPI staining, cell cycle analysis, and annexin V/PI testing indicated that clerodermic acid demonstrates strong geno- and cytotoxicity and is able to induce apoptosis in A549 cells, as evidenced also by DNA fragmentation and chromatin condensation
[72][40].
C. inerme aerial parts have also been found to include a newly rearranged abietane diterpenoid, crolerodendrum B
(42), as well as other known diterpenoids, such as crolerodendrum A
(43), uncinatone
(9) and harwickiic acid
(44) [70][41]. Harwickiic acid
(44) was first isolated from
Sindora sumatrana MIQ fruits
[73][42]. This clerodane-type diterpenoid, obtained from the stem bark of
Croton sylvaticus, was found to demonstrate significant antileishmanial activity against
L. donovani promastigotes with an IC
50 of 31.57 µM, as well as cytotoxic activity against RAW 264.7 (CC
50 = 247.83 μM)
[74][43]. Harwickiic acid
(44), isolated from
C. inerme aerial parts, together with crolerodendrum B
(42) and uncinatone
(9) also demonstrates strong antioxidant activity measured as DPPH radical-scavenging activity; these compounds have been found to have respective ED
50 values of 11.3 µM
(44), 17.6 µM
(42) and 10.1 µM
(9) [70][41].
6. Clerodendrum infortunatum
Crystallization and chromatographic separation of the leaf extract resulted in the isolation and identification of the clerodane diterpenoids clerodin
(45), 15-methoxy-14,15-dihydroclerodin
(46) and 15-hydroxy-14,15-dihyroclerodin
(47) [75][44]. The extraction methods used for the phytochemical analyses of this plant species are shown in
Table 6. The isolated compounds were tested against
Helicoverpa armigera. Studies on the growth inhibition potential of these diterpenoids found topical application of clerodin
(45), 15-methoxy-14,15-dihydroclerodin
(46) and 15-hydroxy-14,15-dihyroclerodin
(47) to yield GI
50 values of 13, 21 and 11 ppm, respectively; in contrast, azadirachtin was found to have a GI
50 value of 15 ppm
[75][44].
The purified diterpenoids, together with their extracts and fractions, also demonstrated insecticidal activity against the highly polyphagous cotton bollworm (
Helicoverpa armigera)
[77][45]. The antifeedant activity of the isolated diterpenoids was tested using choice and no-choice tests with 24- and 48-h observation intervals. In the no-choice test conditions, clerodin
(45) and 15-methoxy-14,15-dihydroclerodin
(46) demonstrated significantly higher antifeedant activity compared to high concentration azadirachtin, the key ingredient in many commercial pesticides
[77][45], with the second diterpenoid demonstrating similar antifeedant activity to that of azadirachtin. In the choice test conditions, all isolated and identified compounds, as well as azadirachtin, demonstrated 100% antifeedant activity at the highest concentration. Furthermore, clerodin
(45) has also been found to demonstrate antifeedant activity against
Earias vitella and
Spodoptera litura [68][37]. The antifeedant index (AI
50) values for clerodin
(45), 15-methoxy-14,15-dihydroclerodin
(46) and 15-hydroxy-14,15-dihyroclerodin
(47) were found to be 6, 6, and 8 ppm in the choice tests, and 8, 9, and 11 ppm in the no-choice tests, respectively.
The antifeedant activity of clerodanes has been attributed to the presence of a perhydrofuranofuran moiety and the degree of its unsaturation; a significant role may also be played by the presence of a trans-decalin ring system bearing an epoxide, together with acetate groups
[78,79][46][47]. These results suggest that the diterpenoids isolated from
Clerodendrum infortunatum leaf extract offer promise as biopesticides and require further studies
[77][45].
7. Clerodendrum kaichianum
Clerodendrum kaichianum P. S. Hsu is known to be the source of two new abietane-type compounds,
viz. 17-hydroxyteuvincenone G
(51) and 17-hydroxyteuvincen-5(6)-enone G
(52), as well as four known diterpenoids: teuvincenone A
(48), 11,14-dihydroxyabieta-8,11,13-trien-7-one
(49), dehydroabietan-7-one
(50) and sugiol
(18) [80][48]. These new secondary metabolites demonstrated relatively strong cytotoxic activities against HL-60 and A-549 cell lines in vitro based on the MTT assay. This action was compared to
cis-platin, which was used as a control compound. In addition, 17-hydroxyteuvincenone G
(51) yielded IC
50 scores of 5.95 and 9.37 µM for HL-60 and A-549 cells, respectively; this activity was slightly higher than that of 17-hydroxyteuvincen-5(6)-enone G
(52) (IC
50 of 15.91 and 10.35 µM against the same cell lines)
[80][48].
Further chromatographic separation from
C. kaichianum stem extract resulted in the isolation of a newly rearranged abietane diterpenoid with five known compounds: villosin A
(53), salvinolone
(54), 14-deoxyloleon U
(55), 5,6-dehydrosugiol
(56), and coleon U
(57). This new diterpenoid was identified as (16R)-12,16-epoxy-11,14,17-trihydroxy-17(15→16)-abeo-8,11,13-abietatrien-7-one
(58) [81][49]. Villosin A
(53), salvinolone
(54) and 5,6-dehydrosugiol
(56) were noted in the
Clerodendrum genus for the first time. All extraction methods used for the phytochemical analyses of this plant species are shown in
Table 7. All isolated constituents were tested for their cytotoxic activities against the viable HL-60 tumour cell line based on the MTT assay. The highest cytotoxic activity was demonstrated by (16R)-12,16-epoxy-11,14,17-trihydroxy-17(15→16)-abeo-8,11,13-abietatrien-7-one
(58) with an IC
50 value of 18.5 µM, with villosin A
(53) and coleon U
(57) demonstrating IC
50 values of 20.1 and 24.1 µM, respectively. Salvinolone
(54), 14-deoxyloleon U
(55) and 5,6-dehydrosugiol
(56) demonstrated more than two-fold weaker cytotoxic activity, with IC
50 values over 40 µM
[81][49].
8. Clerodendrum kiangsiense and C. mandarinorum
A phytochemical study on the aerial parts of
C. kiangsiense resulted in the isolation of eight diterpenoids, one of which was a novel
abeo-abietane diterpenoid. Spectroscopic analyses resulted in its identification as 12-methoxy-6,11,14,16-tetrahydroxy-17(15→16)-abeo-5,8,11,13-abietatetraen-3,7-dione
(59) [82][50]. The remaining secondary metabolites were identified as mandarone A
(60) ((5
R,10
S)-12-hydroxy-8,11,13-abietatriene-37-dione), taxusabietane A
(61), 12-O-demethylcryptojaponol
(62), cryptojaponol
(63), 11,14-dihydroxy-8,11,13-abietatrien-7-one
(64), fortunin E
(65) and fortunin F
(66) [82][50]. Mandarone A
(60) had previously been isolated from
Clerodendrum mandarinorum stem
[83][51] and
Euonymus lutchuensis roots
[84][52].
Various other mandarones have also been isolated from
C. mandarinorum stem, including mandarone B
(67) ((16
S)-12,16-epoxy-11,14-dihydroxy-17(15→16)-abeo-abieta-5,8,11,13-tetraene-7-one), mandarone C
(68) (12,16-epoxy-11,14-dihydroxy-17(15→16)-abeo-abieta-2,5,8,11,13,15-hexaene-7-one)
[84][52], mandarone D
(69) (16
S)-12,16-epoxy-l1-hydroxy-17(15→16),18(4→3)-diabeo-abieta-3,5.8,11,13-pentaene-7-one, mandarone E
(70) (12.l6-epoxy-l1,14-dihydroxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13,15-hexaene-7-one), mandarone F
(71) (12,16-epoxy-6,11,14-trihydroxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13,15-hexaene-7-one), mandarone G
(72) (12,16-epoxp-11,14-dihydroxy-6-methoxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13,15-hexaene-2,7-dione) and mandarone H
(73) (12,16-epoxy-11,14-dihydroxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13,15-hexaene-1,7-dione)
[85][53].
Taxusabietane A
(61), isolated from bark extract of
Taxus wallichiana Zucc. (in addition to taxusabietane C and taxamairin F), was found to demonstrate considerable lipoxygenase (LOX) inhibitory activity at an IC
50 of 57 μM compared to controls (baicalein IC
50 22.1 μM) based on in vitro lipooxygenase inhibition assay and in vivo carrageenan-induced paw oedema model
[86][54]. Cryptojaponol
(63), isolated from extracted
Taxodium distichum bark, demonstrated moderate cytotoxic activity against human pancreatic carcinoma (PANC-1)
[87][55] with an EC
50 of about 38 μM and selective index (SI) of 7.9
[87][55].
In addition, 11,14-dihydroxy-8,11,13-abietatrien-7-one
(64), an abietane diterpenoid found in
Clerodendrum kiangsiense aerial parts, exhibits some interesting biological activities. Costa-Lotufo et al. (2004) found it to demonstrate moderate cytotoxic activity against tumour cell lines, together with as well as carnasol, isolated from
Hyptis martiusii roots
[88][56]. Zadali et al. (2020) also reported it to be present in the aerial parts and roots of
Zhumeria majdae and to show promising antiprotozoal activity; the IC
50 value was found to be 8.65 μM, with a selectivity index (SI) of 4.6
[89][57]. Additionally, it has also been found to demonstrate greater binding affinity at the active site of AChE in comparison to donepezil
[90][58].
9. Clerodendrum splendens
Scientific research on this species allowed to isolate and identify four new clerodane diterpenoids, namely 2α-acetoxy-3β-(2′,3′-diacetoxy-2′-methyl)-butanoyloxy-14-hydro-15-hydroxyclerodin
(74), 3β,15-dihydroxy-14-hydro-clerodin
(75), 2α,15-dihydroxy-3β-(2′-hydroxy-2′-methyl-3′-acetoxy)-butanoyloxy-6α,18-diacetoxy-4α,17-epoxy-clerodan-11,16-lactone
(76) and 3β,14S,15-trihydroxy-6α,18-diacetoxy-4α,17-epoxy-clerodan-11,16-lactone
(77) [91][59]. Faiella et al. (2013) tested these compounds for their potential antiproliferative activity against HeLa cells. Briefly, the HeLa cells were incubated for 24 h with the diterpenoids at a concentration of 50 μM, and the results were compared with 15 μM phenethylisothiocyanate (PEITC) as a control. The results indicate that 2α-acetoxy-3β-(2′,3′-diacetoxy-2′-methyl)-butanoyloxy-14-hydro-15-hydroxyclerodin
(74) and 2α,15-dihydroxy-3β-(2′-hydroxy-2′-methyl-3′-acetoxy)-butanoyloxy-6α,18-diacetoxy-4α,17-epoxy-clerodan-11,16-lactone
(76) exhibit cell growth inhibition activity. In addition, the IC
50 values for the two compounds, viz.,
(76) and
(74), were found to be 101 μM and 98 μM, respectively, after 72 h incubation
[91][59].
10. Clerodendrum trichotomum
Trichotomone
(78) was first isolated from
Clerodendrum trichotomum roots by careful semi-preparative chromatographical analysis. This diterpenoid is a rare phenolic ketal of a regular abietane derivative, cyrtophyllone B
(21), and a rearranged abietane derivative related to uncinatone
(9) [89][57]. Trichotomone
(78) demonstrates moderate cytotoxic activity against some tumour cell lines (A549, Jurkat, BGC-823 and 293T WT) with IC
50 values ranging between 7.51 and 19.38 µM
[92][60].
Wang et al. (2013) report the isolation of various other diterpenoid compounds from the species, including 17(15→16)-
abeo-abietane (6-methoxyvillosin C
(79) (=(10
R,16
R)-12,16-epoxy-11,14,17-trihydroxy-6-methoxy-17(15→16)-abeoabieta-5,8,11,13-tetraene-7-one), 18-hydroxy-6-methoxyvillosin C
(80) (=(10
R,16
R)-12,16-epoxy-6-methoxy-11,14,17,18-tetrahydroxy-17(15→16)-abeo-abieta-5,8,11,13-tetraene-7-one) and (10
R,16
S)-12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13-tetraene-3,7-dione
(81) and 17(15→16),18(4→3)-
diabeo-abietane diterpenoids (trichotomone D
(82) (=10
R,16
S)-12,16-epoxy-11,14-dihydroxy-18-oxo-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13-pentaene-7-one, (10
R,16
R)-12,16-epoxy-11,14,17-trihydroxy-17(15→16),18(4→3)-diabeo-abieta-3,5,8,11,13-pentaene-2,7-dione
(83) and trichotomone F
(84) =(3
S,4
R,10
R,16
S)-3,4:12,16-diepoxy-11,14-dihydroxy-17(15→16),18(4→3)-
diabeo-abieta-5,8,11,13-tetraene-7-one)
[93][61]. In addition, the following known diterpenoids were also isolated: villosin C
(85), 12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13,15-pentanene-3,7-dione
(31), uncinatone
(9), mandarone E
(70), formidiol
(30), teuvincenone E
(86), teuvincenone F
(10) and Trichotomone H
(87) (=12,16-epoxy-17(15→16),18(4→3)-
diabeo-abieta-3,5,8,12,15-pentaene-7,11,14-trione)
[93][61].
Of the 14 isolated compounds, (10
R,16
S)-12,16-epoxy-11,14-dihydroxy-6-methoxy-17(15→16)-abeo-abieta-5,8,11,13-tetraene-3,7-dione
(81) is a newly discovered naturally occurring compound. All the extraction methods used for the phytochemical analyses of this plant species are shown in
Table 11. The cytotoxic activities of these diterpenoids were studied against tumour cell lines BGC-823, Huh-7, KB, KE-97, and Jurkat based on CellTiter Glo™ Luminescent cell viability assay. Of all the tested compounds, trichotomone D
(82), F
(84) and H
(87), teuvincenone E and H
(88), uncinatone
(9) and mandarone E
(70) showed cytotoxic activity. IC
50 values ranged from 0.83 to 50.99 µM. The most active diterpenoid was found to be Teuvincenone E
(86), with IC
50 values of 3.95, 5.37, 1.18, 1.27, and 0.83 µM against the BGC-823, Huh-7, KB, KE-97, and Jurkat lines, respectively. The authors attribute the high cytotoxic activity of this compound to its rearranged A ring and intact 2-methyl-3-dihydro-furan fragment
[93][61].
In further phytochemical studies, air-dried stems of
Clerodendrum trichotomum were extracted and chromatographically separated. Eleven compounds were identified, including seven abietane diterpenes: sugiol
(18), teuvincenone A
(48), teuvincenone B
(89), teuvincenone F
(10), teuvincenone H
(88), uncinatone
(9) and cyrtophyllone B
(21) [94][62]. In further studies on
C. trichotomum stems, the same authors also identified the diterpenoids villosin B
(90) and villosin C
(85); these demonstrate remarkable cytotoxic activities against tumour cell lines A549, HepG-2, MCF-7 and 4T1 with IC
50 values ranging from 14.93 to 29.74 µM
[95][63].
Hu et al. (2018) isolated twelve new abietane diterpenoids from
C. trichotomum roots: 15,16-dehydroteuvincenone G
(91), 3-dihydroteuvincenone G
(92), 17-hydroxymandarone B
(93), trichotomin A
(94), 15,16-dihydroformidiol
(95), 18-hydroxyteuvincenone E
(96), 2α-hydrocaryopincaolide F
(97), 15α-hydroxyuncinatone
(98), 15α-hydroxyteuvincenone E
(99), trichotomin B
(100), trichotomside A
(101) and B
(102) [96][64]. As earlier studies indicate that
C. trichotomum roots possess anti-inflammatory properties
[17][65], all the secondary metabolites isolated by Hu et al. (2018) were tested for their ability to inhibit NO production in LPS-stimulated RAW 264.7 cells, a marker of inflammation
[96][64]. Of the tested substances, 15,16-dehydroteuvincenone G, trichotomin A, 2α-hydrocaryopincaolide F, as well as other isolated compounds, such as villosin C
(85), 15-dehydro-17-hydroxycyrtophyllone A
(16), demethylcryptojaponol, 6β-hydroxydemethylcryptojaponol and trichotomone
(78), exhibited IC
50 values ranging from 6.0 to 16.1 µM, with 15,16-dehydroteuvincenone G being the most active diterpenoid (IC
50 value 6.0 µM). It is worth adding that all these active compounds acted at non-cytotoxic concentrations and demonstrated stronger activity than aminoguanidine hydrochloride (IC
50 26.2 µM)
[96][64].