Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 4 by LinKun Fan and Version 3 by LinKun Fan.
Metal–organic frameworks (MOFs) are coordination polymers with high porosity that are constructed from molecular engineering. Constructing MOFs as photocatalysts for the reduction of nitrogen to ammonia is a newly emerging but fast-growing field, owing to MOFs’ large pore volumes, adjustable pore sizes, controllable structures, wide light harvesting ranges, and high densities of exposed catalytic sites. They are also growing in popularity because of the pristine MOFs that can easily be transformed into advanced composites and derivatives, with enhanced catalytic performance.
- Haber–Bosch process
- solar energy
- photocatalytic nitrogen fixation
1. Introduction
Nitrogen is a necessary element to sustain life. It is abundant in the Earth’s atmosphere in the form of virtually inert dinitrogen (N2) gas that most organisms cannot metabolize [1]; instead, “fixed” forms such as ammonia (NH3) can be metabolized. Before the widespread use of the Haber–Bosch process in the 1950s, fixed nitrogen was mainly derived from natural nitrogen fixation that involved geochemical processes such as lightning, and biological processes that used nitrogenase [2]. The Haber–Bosch process is a traditional and primary means for artificial nitrogen fixation, which converts N2 and H2 to NH3 in the presence of a catalyst (Equation (1)). Now, nitrogen fixation via the Haber–Bosch process has exceeded natural nitrogen fixation. Nearly half of the existing human population will not exist without nitrogen fertilizers from the Haber–Bosch process. However, the Haber–Bosch process requires the expensive raw material hydrogen, intensive energy, and harsh conditions, including high temperatures (400–500 °C) and pressures (20–50 MPa). Therefore, finding a mild and green artificial nitrogen fixation method to replace or replenish the Haber–Bosch process is urgent.
N2(g) + 3H2(g) → 2NH3 (g)
In recent years, photocatalysis technologies have been widely used in various fields [3][4][5][6][7][8][9][10]. With the development of photocatalysts, photocatalysis technology also makes the production of ammonia in mild conditions possible [11]. Currently, several important homogeneous and heterogeneous photocatalysts for nitrogen fixation are available, such as Mo complex [12][13], Fe complex [14], TiO2 [15][16][17][18][19], ZnO [20], La2TiO5 [21], KNbO3 [22], Ti-zeolite [23], BiOBr [24][25], Fe2O3 [26], and g-C3N4 [27]. However, classical organometallic complexes (homogeneous catalysts) have not found practical application due to their low yields (around 10 equivalents of ammonia per equivalent of the catalyst), rapid catalyst deactivation, and the high costs of those catalysts. Thus, homogeneous catalysts for ammonia synthesis are currently the most useful for understanding nitrogenase. Although the stability has dramatically increased for such heterogeneous catalysts, their catalytic performance is still unsatisfactory, especially in the visible light region; most of those photocatalysts suffer from a narrow light absorption range, rapid photogenerated electron–hole combination, and a lack of rich catalytic sites.
Metal–organic frameworks (MOFs) are constructed by inorganic metal ions/metal clusters and multidentate organic ligands [28]. Since MOF-5, a highly porous crystalline material constituted by terephthalic acid (BDC) ligands and Zn4O clusters, was reported in Nature by Yaghi in 1999, MOFs began to develop rapidly [29]. The next two decades witnessed intensive efforts by numerous researchers to reveal new structures and applications of MOFs [30][31][32][33][34][35][36][37][38][39]. The naming of MOFs also has certain rules, mainly in the following four aspects: material composition (i.e., metal–organic framework, abbreviated as MOF) [29], structure (i.e., zeolitic imidazolate framework, abbreviated as ZIF) [40], function (i.e., multivariate metal–organic framework, abbreviated as MTV-MOF) [41], and the name of the laboratory or university (i.e., Materials of Institute Lavoisier, abbreviated as MIL) [33]. This naming method was subsequently adopted by most researchers [42][43][44][45][46]. Owing to their specific structural features such as large specific surface areas, high porosity, well-defined crystallinity, and increased numbers of active sites, MOFs have been widely used in gas adsorption and separation [47][48], fluorescence [49], sensing [44][45][50][51][52], ion conductivity [53], optoelectronics [54], thermal catalysis [55][56][57][58][59], electrocatalysis [60][61], photocatalysis [41][62][63][64], and so on. As photocatalysts, MOFs have many advantages: (i) the large specific surface area and highly ordered pore structure contribute to the mass transfer of reactants; (ii) the adjustable ligands make MOFs possess the ability to harvest light in a wide range; (iii) introducing defects into MOFs can expose more active sites, enhancing their nitrogen fixation activity [65][66][67][68]. Thus far, compared with photocatalytic hydrogen production and photocatalytic carbon dioxide (CO2) reduction, the study of photocatalytic nitrogen fixation by MOFs is still in its initial stages of development.
The recent achievements in state-of-the-art MOF-based materials for photocatalytic nitrogen fixation have been further summarized. Finally, the summary and perspectives of MOF-based materials for photocatalytic nitrogen fixation were presented (Figure 1).
Figure 1. MOF-based materials for photocatalytic nitrogen fixation.
2. MOF-Based Materials for Photocatalytic Nitrogen Fixation
Due to their large pore volumes, adjustable pore sizes, wide light harvesting range, and high densities of exposed catalytic sites, MOFs have been explored for photocatalytic nitrogen fixation. However, it is still in its preliminary stages of development. In addition to pure MOFs, advanced MOF composites and MOF derivatives have also been studied for photocatalytic nitrogen fixation (Table 1).
Table 1. Photocatalytic nitrogen fixation by MOF-based materials.
| Photocatalyst | Light Source | Sacrificial Agents | NH3 Yield | AQY/% | Ref. |
|---|---|---|---|---|---|
| Pure MOFs | |||||
| NH2-MIL-125 (Ti) | Xe Lamp (300 W, L40) | None | 12.25 μmolg−1 h−1 | 0.26 (400 nm) | [69] |
| OH-MIL-125 (Ti) | Xe Lamp (300 W, L40) | None | 5.04 μmolg−1 h−1 | / | [69] |
| CH3-MIL-125 (Ti) | Xe Lamp (300 W, L40) | None | 1.39 μmolg−1 h−1 | / | [69] |
| UiO-66-UV-Vis | Xe Lamp (300 W, UV-vis) | None | 256.60 μmolg−1 h−1 | / | [70] |
| UiO-66(SH)2-200 | Xe Lamp (300 W, L40) | None | 32.40 μmolg−1 h−1 | 0.45 (420 nm) | [71] |
| MIL-101(Fe) | Xe Lamp (300 W, full-spectrum) | None | 50.36 μmolg−1 h−1 | / | [72] |
| MIL-100(Fe) | Xe Lamp (300 W, full-spectrum) | None | 46.53 μmolg−1 h−1 | / | [72] |
| MIL-88(Fe) | Xe Lamp (300 W, full-spectrum) | None | 40.04 μmolg−1 h−1 | / | [72] |
| MIL-53(FeII/FeIII)-0.1 | Xe Lamp (300 W, L42) | K2SO3 | 306.00 μmolg−1 h−1 | 0.12 (420 nm) | [73] |
| Al–PMOF(Fe) | Xe Lamp (100 mWcm−2, L42) | CH3OH | 7.06 μmolg−1 h−1 | / | [74] |
| MOF-76(Ce) | Xe Lamp (300 W, full-spectrum) | None | 34.20 μmolg−1 h−1 | / | [75] |
| Gd-IHEP-8 | Xe Lamp (300 W, AM 1.5 G filter) | None | 220.00 μmolg−1 h−1 | 2.25 (365 nm) | [76] |
| Gd-IHEP-7 | Xe Lamp (300 W, AM 1.5 G filter) | None | 128.00 μmolg−1 h−1 | 1.72 (365 nm) | [76] |
| U(0.5Hf) | Xe Lamp (300 W, full-spectrum) | K2SO3 | 351.80 μmolg−1 h−1 | 0.1 (420 nm) | [77] |
| U(0.5Hf)-2SH | Xe Lamp (300 W, L42) | K2SO3 | 116.10 μmolg−1 h−1 | 0.55 (420 nm) | [77] |
| NU6(Ce–Hf) | Xe Lamp (300 W, full-spectrum) | K2SO3 | 158.4 μmolg−1 h−1 | 0.65 (380 nm) | [78] |
| MOF composites | |||||
| ZIF-67@PMO12 | Xe Lamp (300 W, full-spectrum) | C2H5OH | 39.40 μmolg−1 h−1 | / | [79] |
| ZIF-67@PMO11V | Xe Lamp (300 W, full-spectrum) | C2H5OH | 70.00 μmolg−1 h−1 | / | [79] |
| ZIF-67@PMO10V2 | Xe Lamp (300 W, full-spectrum) | C2H5OH | 74.80 μmolg−1 h−1 | / | [79] |
| ZIF-67@PMO9V3 | Xe Lamp (300 W, full-spectrum) | C2H5OH | 134.60 μmolg−1 h−1 | / | [79] |
| ZIF-67@PMO4V8 | Xe Lamp (300 W, full-spectrum) | C2H5OH | 149.00 μmolg−1 h−1 | / | [79] |
| Au@UiO-66 | Xe Lamp (300 W, L42) | None | 18.90 μmolg−1 h−1 | 1.54 (520 nm) | [80] |
| MOF-74@C3N4 | Xe Lamp (300 W, L40) | CH3OH | 330.00 μmolg−1 h−1 | / | [81] |
| MIL-125@TiO2 | Xe Lamp (300 W, 200 mWcm−2) | None | 102.70 μmolg−1 h−1 | / | [82] |
| GSCe (Graphene@Ce-UiO-66) | LED (6 W, 365 nm) | None | 110.24 μmolg−1 h−1 | 9.25 (365 nm) | [83] |
| 9MX-MOF | Xe Lamp (300 W, full-spectrum) | Na2SO3 | 88.79 μmolg−1 h−1 | / | [84] |
| MOF derivatives | |||||
| Ru–In2O3 HPNs | Xe Lamp (300 W, AM 1.5 G filter) | CH3OH | 44.50 μmolg−1 h−1 | / | [85] |
2.1. Pristine MOFs
2.1.1. Transition Metal-Based MOFs
The unoccupied and occupied d-orbitals in some transition metals-based MOFs have appropriate energy and symmetry, which makes these MOFs effectively adsorb and activate N2. The empty d orbital of open metal sites in MOFs can accept the electrons from the occupied σ orbital of N2. At the same time, the occupied d orbitals of open metal sites donate electrons to the empty π* orbital of N2. The back donated bonds not only weaken N≡N, but also strengthen the metal-nitrogen bond [86], making transition metals-based MOFs for photocatalytic nitrogen fixation possible.
Cerium-Based MOFs
Cerium, with its electron configuration of [Xe]4f26s2, exhibits flexible valence transformation behavior between Ce3+ and Ce4+, with occupied 4f1 and unoccupied 4f0 orbitals, respectively. On this basis, the cerium with empty orbits and filled orbitals can mimic π back donation for further catalysis [87].
Recently, Zhang et al. applied MOF-76(Ce) to effectively transform N2 into NH3, mimicking π back donation [75]. MOF-76(Ce) nanorods with Ce coordinate unsaturated sites (Ce-CUS) were prepared using the solvothermal method (Figure 2a). Under full-spectrum light source irradiation, the photogenerated electrons were first transferred to Ce-CUS sites, then to the π antibonding orbital of adsorbed N2 molecules on it. The electrons entering the π antibonding orbital would weaken the N≡N bond, significantly improving photocatalytic nitrogen fixation efficiency. The photocatalytic nitrogen fixation stability of MOF-76(Ce) is comparable to CeO2 (Figure 2b). Moreover, due to the synergy between Ce-CUS and N2, the photocatalytic nitrogen fixation activity of MOF-76(Ce) is higher than CeO2, with an activity of 34.2 μmolg−1 h−1. It should be noted that this is the first kind of study that involved using MOFs for nitrogen fixation.
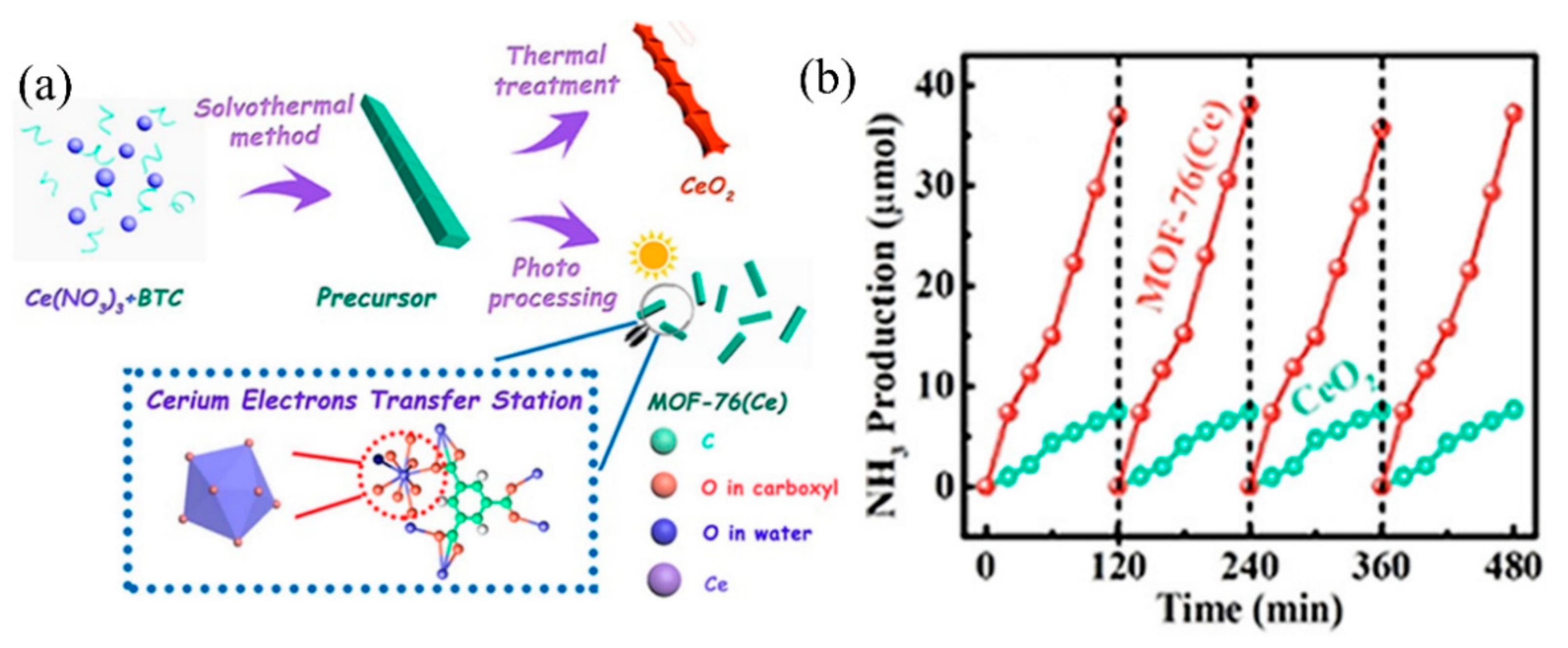
Figure 2. (a) Synthesis diagram of MOFs-76(Ce). (b) MOFs-76(Ce) stability test of photocatalytic nitrogen fixation. Reproduced with permission [75]. Copyright 2019, American Chemical Society.
Titanium Metal-Based MOFs
Ti-based MOFs have been widely explored in photocatalytic hydrogen production [88][89][90], photocatalytic CO2 reduction [91][92], and environmental protection; however, photocatalytic nitrogen fixation is still in its preliminary stages of development.
Recently, the group initially synthesized three functional group-decorated isostructural MOFs (NH2-MIL-125 (Ti), OH-MIL-125 (Ti), and CH3-MIL-125 (Ti)) for visible-light-driven photocatalytic nitrogen fixation [69]. The introduced functional groups effectively enlarged the light-harvesting range of MIL-125 (Ti) from the ultraviolet (UV) region to the visible light region. After being exposed to visible light (400–800 nm) for about 15 h, the produced ammonia of NH2-MIL-125 (Ti), CH3-MIL-125 (Ti), and OH-MIL-125 (Ti) reached 183.76 μmolg−1, 20.88 μmolg−1, and 75.48 μmolg−1, respectively. It should be noted that no sacrificial agent participated in the photocatalytic nitrogen fixation process for those MOFs. The reason for the highest nitrogen fixation rate of NH2-MIL-125 (Ti) can be explained by the introduction of NH2, which significantly increased light absorption to 550 nm, and by the exposed Ti coordinational unsaturated sites induced by a linker defect. The photocatalysis mechanism can be illustrated as follows: under visible light irradiation, photogenerated electrons in organic ligands are transferred to exposed Ti coordinational unsaturated sites and reduce Ti4+ to Ti3+; then, the electron in Ti3+ is further transferred to the π anti-bond of N2 to weaken the strong N≡N bond, eventually reducing the N2 to NH3.
Zirconium-Based MOFs
Defective UiO-66(Zr) series MOFs reflect excellent application prospects in photocatalytic hydrogen production, pollutant degradation, and absorption, which result from their large surface areas and suitable pore structures [93][94][95].
Recently, UiO-66-UV-vis with linker defects induced by light was published by Gao et al. [70] to improve photocatalytic nitrogen fixation activity. In order to improve the increase in photocatalytic nitrogen fixation activity that was attributed to linker defect but not cluster defect, three different types of UiO-66, UiO-66-fresh, UiO-66-UV-vis, and UiO-66-PSE, were studied for photocatalytic nitrogen fixation. UiO-66-fresh, UiO-66-UV-vis, and UiO-66-PSE stand for fresh prepared UiO-66, UV-vis light treated UiO-66, and defect repaired UiO-66 through a post-synthetic ligand exchange process (PSE), respectively. Compared with UiO-66-fresh, the coordinated formic acid and acetic acid were removed in UiO-66-UV-vis after exposure to ultraviolet light. The exposed coordination of unsaturated metal sites greatly enhanced the activity of UiO-66-UV-vis up to 256.6 μmolg−1 h−1. The coordination unsaturated Zr node on UiO-66 can inject photogenerated electrons into the antibonding π-orbitals of N2 to promote the activation and dissociation of N2. In contrast, the exposed coordination unsaturated metal sites were recovered by the terephthalic acid linker in UiO-66-PSE, which resulted in lower photocatalytic activity, even lower than that of UiO-66-fresh.
Guo et al. also introduced defect Zr-based MOFs created by thermal treatment, using UiO-66(SH)2-200 as the photocatalyst for the reduction of N2 [71]. The Zr clusters were dehydrated by thermal treatment, thus providing accessible [Zr6O6] sites for N2 adsorption and activation. The optical temperature was 200 °C. SH groups were also introduced into UiO-66 to improve the absorption edge of the light to visible light. UiO-66(SH)2-200 shows a photocatalytic nitrogen fixation activity up to 32.4 μmolg−1 h−1 under visible light. In-situ DRIFTS (diffuse reflectance infrared Fourier transform spectroscopy) revealed that the N2 molecule was gradually reduced to an NxHy intermediate and to NH3, finally [96]. DFT (density functional theory) calculations further revealed that the photoelectron initiates the reduction of the N2, immediately followed by the protonation of the activated N2.
Iron-Based MOFs
Fe is an essential transition metal in nitrogenase, which plays a vital role in photocatalytic nitrogen fixation. There have been many types of research on reducing N2 by utilizing the Fe active site [97][98][99][100]. When the Fe metal site is exposed for adsorbing N2, the unpaired electrons of the d orbital in Fe will transfer to the π antibonding orbital of the N2 molecule to form a strong Fe–N bond that weakens the N≡N bond.
Recently, in order to prove the importance of the transition metal Fe for photocatalytic nitrogen fixation [72], Li et al. studied the nitrogen fixation performance of MIL-101(Fe), MIL-100(Fe), MIL-88(Fe), and MIL-101(Cr). Notably, MIL-101(Fe) exhibited the highest photocatalytic nitrogen activity (50.36 μmolg−1 h−1), whereas isostructural MIL-101(Cr) had almost no activity. DFT calculations revealed that MIL-101(Fe) showed more electronic supply capacity, higher adsorption energy of N2, and a lower reaction barrier than MIL-101(Cr), confirming the important role Fe has during photocatalytic nitrogen fixation .
Owing to its unique multi-iron metallocluster (Fe2+3Fe3+4M3+, M = Mo, V, Fe), nitrogenase exhibits excellent nitrogen fixation activity. Recently, Zhao et al. synthesized a MIL-53(Fe2+/Fe3+) containing both Fe2+ and Fe3+ for photocatalytic nitrogen fixation [73]. In this MOF, Fe2+ and Fe3+ simulated Fe2+ activity sites and high valence metal ions (M = Mo, V, Fe) in nitrogenase. The Fe3+ in MIL-53(Fe2+/Fe3+) can be partly reduced into Fe2+ by ethylene glycol (EG), and the Fe2+/Fe3+ ratio can be regulated from 0.18:1 to 1.21:1 by changing the EG content. Notably, when the ratio of Fe2+/Fe3+ was 1.06:1, photocatalytic nitrogen fixation activity reached its highest value of 306 μmolh−1 g−1.
The proposed mechanism for photocatalytic nitrogen fixation by MIL-53(Fe2+/Fe3+) can be divided into six steps. (i) N2 adsorbs onto Fe2+ active sites of MOF, in which the electrons transfer to N2 to form the Fe3+-azo intermediate. (ii) Then, the hydrogen transfer quickly occurs in the Fe3+-azo intermediate. (iii) In the photo-excitation stage, holes transfer to the Fe–O− radical, and Fe3+ reduces to Fe2+ by electrons. (iv) The sacrificial agent K2SO3 eliminates the holes, and Fe2+-hydrazo transforms into Fe3+-hydrazo, with H2N–NH2 species appearing. (v) Fe3+-hydrazo transfers to Fe3+-amine with further hydrogenation. (vi) Finally, the NH3 releases gradually, benefitting from continuous hydrogenation.
Transitional Bimetallic MOFs
Similarly to MIL-53(Fe2+/Fe3+), An et al. developed bimetallic MOFs containing Zr and Hf for photocatalytic nitrogen fixation. Zr simulates the Fe2+ active site in nitrogenase, acting as the active site through the π antibonding mechanism. Hf imitates the high valence metal in nitrogenase to promote electron transfer and utilization. An SH group was also introduced to extend the absorption edge to the visible light region [77]. As the SH group expands the absorption spectrum to 502 nm and the synergistic effect of Zr and Hf, U(0.5Hf)-2SH with 50% Hf exhibits extremely superior photocatalytic nitrogen fixation activity (116.1 μmolh−1 g−1) under visible light. The constructed ligand-to-metal-to-metal electron transfer (LMMET) pathway in MOFs promotes the transferring and utilization of photogenerated electrons. Under visible light irradiation, the electrons move from the highest occupied molecular orbit (HOMO) to the lowest occupied molecular orbit (LOMO). At the same time, the holes are consumed by the sacrificial agent K2SO3, the electrons are transferred to the multi-metal clusters, and finally the N2 is reduced to NH3. Except for zirconium- and hafnium-based MOFs, An et al. further synthesized a stable amino-functionalized UiO-66 with bimetallic Ce-Hf nodes for photocatalytic nitrogen fixation [78]. In this MOF, the introduced NH2 group expands the absorption edge, the Ce species acts as an electron buffer tank to enhance electron transfer, and the Hf species plays the part of active catalytic sites to improve the selectivity of the nitrogen fixation reaction. When the molar ratio of Ce-Hf is 1:1, the nitrogen fixation activity was the highest (158.4 μmolh−1 g−1) under visible light, with K2SO3 as the sacrificial agent.
2.1.2. Post-Transition Metal-Based MOFs
Compared with transition metal-based MOFs, the post-transition metal-based variations are rarely reported to reduce nitrogen to ammonia as photocatalysts.
Gadolinium-Based MOFs
Hu et al. developed two viologen-based radical-containing metal–organic frameworks, Gd-IHEP-7 and Gd-IHEP-8 [76]. A single-crystal-to-single-crystal (SCSC) transformation occurred from two-dimensional (2D) Gd-IHEP-7 to three-dimensional (3D) Gd-IHEP-8 when heating the Gd-IHEP-7 in the air at 120 °C. With a rearrangement of the Gd3+ coordination environment, enhanced photocatalytic nitrogen fixation activity emerged with the SCSC transformation. Chemisorption of N2 onto the catalytic sites is a pre-condition for photocatalytic nitrogen fixation. Both the adsorbed N2 on active Gd metal sites for Gd-IHEP-7 and Gd-IHEP-8 were activated, evidenced by the elongated N–N bond length and the shortened Ga–N bond length. Compared with the distal (D) route, the alternative (A) route is more favorable for both Gd-IHEP-7 and Gd-IHEP-8. While even theoretical calculations indicate that similar photocatalytic nitrogen fixation pathways exist for both RMOF (Gd-IHEP-7, Gd-IHEP-8), the intermediates for Gd-IHEP-8 showed better stability, resulting in a better nitrogen fixation activity of 220 µmolh−1 g−1.
Aluminium-Based MOFs
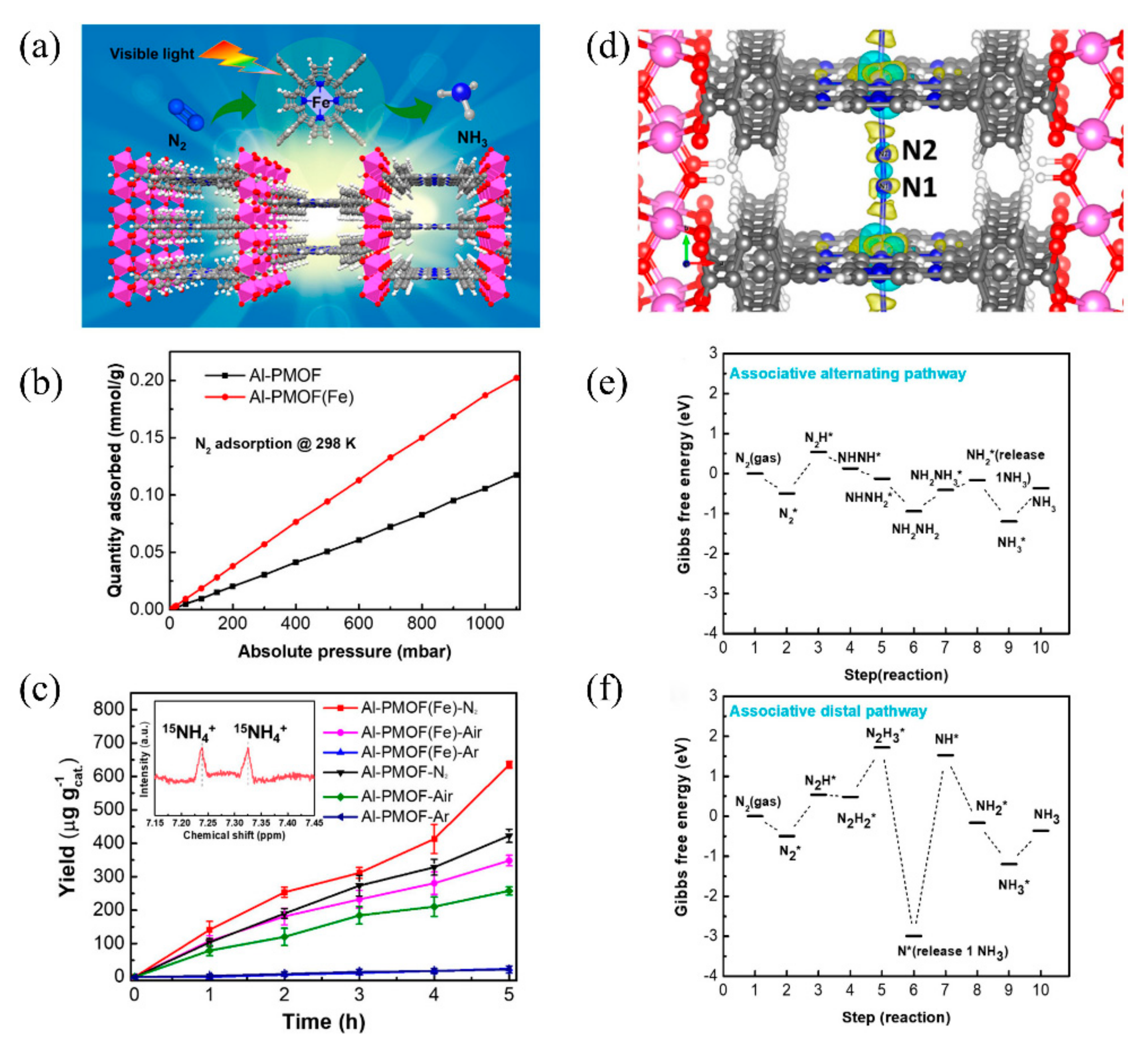
The post-transition metals can not only function as active sites as in Gd-IHEP-8, but they also act as metal nodes that impart high framework stability to MOFs. Recently, Shang et al. developed two porphyrin-based metal–organic frameworks for photocatalytic nitrogen fixation, named Al-PMOF and Al-PMOF(Fe). Compared with Al-PMOF, Al-PMOF(Fe) not only has Al as the metal node to stabilize the framework of the MOF, but also has Fe as the active center to adsorb and reduce the N2 to NH3 [74]. The structure of Al-PMOF(Fe) is shown in Figure 3a. The atomically isolated Fe in Al-PMOF(Fe) was proven through X-ray absorption spectroscopy (XAS) and X-ray photoelectron spectroscopy (XPS). Photocatalytic nitrogen fixation experiments showed that Al-PMOF(Fe) had better activity than Al-PMOF under visible light, and that the produced NH3 originated from N2. It was confirmed that the addition of Fe as the active center effectively increased the adsorption of N2 and further enhanced the photocatalytic performance (Figure 3b,c). DFT calculations were further applied to establish the photocatalytic nitrogen fixation reaction pathway (Figure 3d–f). The first hydrogenation from N2* to N2H* showed no obvious difference between alternating and distal pathways, but further hydrogenation showed that the alternating pathway seemed more likely to occur. Notably, the release of 1NH3 in the distal process requires significant energy, making the reaction difficult.

Figure 3. (a) The structure of Al-PMOF(Fe). (b) N2 adsorption isotherms. (c) Photocatalytic nitrogen fixation activity of Al-PMOF(Fe) and Al-PMOF. (d) Charge different maps for Al-PMOF(Fe) adsorbing N2. (e) Alternating and (f) distal pathways in Al-PMOF(Fe). Reproduced with permission [74]. Copyright 2021, American Chemical Society.
MOFs can also function as photosensitizers and transfer the photogenerated electrons to active sites in other functional materials that are in contact with them. For example, Ding et al. combined N-defect thin film g-C3N4 (DF-C3N4) with nano MOF to form Nano MOF-74@DF-C3N4 composite for enhanced photocatalytic nitrogen reduction. A Z-type heterojunction was formed between Nano-MOF-74 and DF-C3N4, which clearly improved the separation efficiency of photogenerated electrons and holes. The Z-type heterojunction photocatalyst structure first proposed by Bard et al. in 1979, can provide the entire system with a stronger redox ability to promote photocatalytic activity. It should be noted that another carrier transfer process of type II is competitive with the Z-type process, and the fluorescence lifetime results indicated that the Z-type is the main process. In this Z-type carrier transfer process, the photogenerated electrons in the conduction band of MOFs are firstly transferred to the valence band of DF-C3N4, then become re-excited to the conduction band of DF-C3N4, and finally anticipate the reduction of N2 adsorbed on N defect sites of DF-C3N4.
Another similar example is the MOFs composite MIL-125@TiO2 with core-shell structure synthesized by Wang et al., using the post thermal solvent method (Figure 10a) [95]. The thickness of TiO2 nanosheets can be controlled by the reaction time with thioacetamide. Under the synergistic effect of MIL-125 and TiO2, MIL-125@TiO2-2 h exhibited an activity up to 89.5 µmolg−1 h−1 under visible light, which was much higher than the pristine MIL-125 (Figure 10b). In MIL-125@TiO2, photogenerated electrons can be transferred to both the coordination unsaturated Ti sites of defect MIL-125 and TiO2, and further reduce the adsorbed N2 to NH3.
MXene is a new type of two-dimensional material with a graphene-like structure. It has the advantages of a large specific surface area, excellent conductivity, and abundant surface groups. Qin et al. synthesized MXene/MOF composites (Ti3C2-QD/Ni-MOF, formed as type II heterojunction; QD stands for quantum dot) by combining MXene QDs and Ni-MOF through self-assembly, which improved the photocatalytic nitrogen fixation efficiency to 88.79 μmol g−1 h−1 [97]. Under light irrigation, the electrons and holes in Ti3C2-QD were separated. Then, the photo-excited electrons were transferred to the conduction band (CB) of Ni-MOF to participate in the photocatalytic nitrogen fixation reaction. In this system, the heterojunction formed by Ti3C2-QD and Ni-MOF effectively promotes the separation of electrons and holes. Ni effectively adsorbed N2 as an active site, and promoted the nitrogen fixation process.
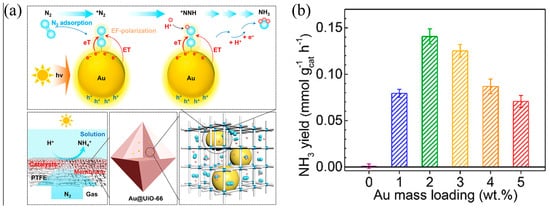
MOFs can also function as porous scaffolds to support functional units, and enhance the mass transfer of N2 to produce NH3. For example, Chen et al. used the UiO-66 membrane as a nanoreactor to support gold nanoparticles (AuNPs) [93] and realized a direct plasma photocatalytic nitrogen reduction reaction at room temperature and ordinary pressure. Notably, in the Au@UiO-66, UiO-66 not only effectively restricts the highly dispersed AuNPs but also ensures effective contact between AuNPs and N2 molecules in the aqueous solvent. In the gas film solution reaction interface, the N2 molecule can proceed straight into the Au@UiO-66 membrane. Each AuNP in UiO-66 could not only generate electrons through photo-excitation, but also promote the reduction of N2 as a co-catalyst. (Figure 4a). The mass loading of gold was the critical factor that affected photocatalytic nitrogen fixation. When the loading amount of Au is 1.9 wt %, the conversion of nitrogen to ammonia reaches its highest level of 0.14 mmol g−1 h−1 (Figure 4b).

Figure 4. (a) Schematic diagram of direct plasma photocatalytic nitrogen reduction on AuNP encapsulated by UiO-66 matrix. (b) Direct plasma photocatalytic nitrogen reduction performances in the PiS system. Reproduced with permission [93]. Copyright 2021, American Chemical Society.
The introduced functional materials into MOFs can increase the photocatalytic nitrogen fixation activity and enhance the stability of composite materials. For example. Pan et al. reported on a graphene-embedded Ce-based UiO-66 photocatalyst (GSCe) [96]. With ultraviolet light irradiation, a breakage of benzene-C bonds inside Ce-UiO-66 formed active sites, which was evidenced by C K-edge X-ray absorption near its edge structure (reduced of C 1s → σ* at 292.0 eV). Even if the introduced graphene did not have any active sites, it could help control the activation process and hence stabilize the entire photocatalyst structure. With a graphene ratio of 0.35, GSCe reached its best performance, with a rate and apparent quantum efficiency (AQE) of 110.24 μmolL−1 h−1 and 9.25%, respectively. The stability of GSCe even reached 7 × 24 h.
4.3. MOF Derivatives
MOFs have been proposed as a potential precursor for synthesizing nanostructured materials with porosity [73,114]. After pyrolysis in different atmospheres, MOFs can be transformed into derivatives, including metal oxides, metal carbides, metal nitrides, carbon nanostructures, etc. Those MOF derivatives can be used for electrocatalysis [115], sensing [116,117], electrode materials [118], photocatalysis [119], etc. As an example of photocatalytic nitrogen fixation, Vu et al. synthesized an In-based hollow peanut-like photocatalyst (Ru-In2O3 HPNs) through pyrolysis of MIL-68-In(Ru) precursors in the air [98]. Under solar simulator (AM 1.5 G filter) irradiation, the nitrogen fixation activity of Ru-In2O3 HPNs reached 44.5 μmolg−1 h−1. This was the result of the synergistic effects of hollow structure and Ru. Under the light, electrons and holes were separated. The electrons in the conduction band were immediately captured by Ru and oxygen vacancies after migrating to In2O3, which activated and weakened N≡N, and finally reduced N2 to NH3.5. Summary and Future Perspective
The photocatalytic fixation of nitrogen to ammonia is a significant challenge, but it is a potential technology for green ammonia production. MOF-based materials, including pristine MOFs, MOF composites, and MOF derivatives, are excellent candidates for photocatalytic nitrogen fixation. This comprehensive review highlights recent advances in applying MOF-based materials toward photocatalytic nitrogen fixation. First, this review introduced the determination method of produced ammonia. Then, the synthetic methods of MOF-based materials were summarized. Finally, the recent achievements in MOF-based materials for photocatalytic nitrogen fixation were systematically introduced. Despite considerable progress in MOF-based materials toward photocatalysis with nitrogen fixation being achieved, many challenges remain. Improving Reliability of NH3 Production. Before or during photocatalytic nitrogen fixation, the equipment, nitrogen sources, catalysts, solvents, etc., may be polluted. Therefore, in order to avoid unreliable conclusions, the following control experiments should be conducted: using inert Ar as a gas source, conducting the experiments under dark conditions, replacing protonic solvents (such as water) with aprotic solvents (such as DMF, DMSO), and conducting 15N2 isotope labeling experiments. Furthermore, different ammonia detection methods have their advantages and limitations; therefore, suitable detection methods should be applied in different reaction systems, and several methods should be used to detect ammonia accurately. Reporting data in photocatalytic systems accurately. Typically, catalytic rates are expressed in terms of the amount of product divided by the amount of catalyst per unit of time. Nevertheless, this parameter can be easily manipulated to the authors’ convenience, especially in multicomponent systems where only a fraction of the catalyst represents the genuine active site. For this reason, other figures of merit such as apparent quantum yield (AQY) are also useful when comparing activities between different photocatalysts. The combination of rates plus AQY seems to be a more robust way to report catalytic performances and establish fair comparisons between different materials [120]. Developing efficient photocatalysts. The coordination of unsaturated sites in MOFs can effectively improve N2 adsorption/activation, which can be created with the defects generated. Therefore, creating abundant defects in MOFs while maintaining their highly porous structure are required for highly efficient photocatalytic nitrogen fixation. Moreover, broadening the light-harvesting range of MOFs can also increase the photocatalytic nitrogen fixation efficiency. Introducing light-absorbing functional groups into linkers such as amino groups, sulfhydryl groups, etc., or selecting strong photoactive ligands as linkers, such as porphyrin derivatives, phthalocyanine derivatives, naphthalene derivatives, etc., can greatly broaden the spectral responses of MOFs, thereby enhancing their light absorption efficiency. Constructing reasonable structures of MOFs to enhance the photogenerated electron and hole separation and avoid recombination can also dramatically enhance their photocatalytic nitrogen fixation efficiency. Finally, a rational combination with other functional materials (graphene, g-C3N4, MXene, etc.) to form advanced MOF composites, or as precursors to prepare MOF-derived materials ranging from carbon-based materials to metal-based materials, is another option to enhance their photocatalytic nitrogen fixation efficiency. Discovering photocatalytic mechanisms using in-situ characterization techniques. Traditional characterization techniques can only reflect the state of the catalyst before and after the reaction. However, the catalytic reaction requires both a steady state and a transient state. Therefore, in-situ characterization techniques have become indispensable in catalytic reactions. Compared with traditional characterization techniques, in-situ techniques are much easier to use in capturing the complex intermediates with short lifetimes to reveal the real active sites. In-situ characterization techniques, such as in-situ extended X-ray absorption fine structure (EXAFS), infrared absorption spectra, diffuse reflectance infrared Fourier transform spectroscopy (DRIFTS), thermal gravimetric analysis (TGA), XPS, Raman spectroscopy, etc., can greatly deepen our understanding of the reaction mechanisms, and further guide us to design and synthesize efficient photocatalysts. Although part of those in-situ techniques had been applied to study the photocatalytic nitrogen fixation by MOF-based materials, the research is still in its early stages. Delving into the photocatalytic mechanism by theoretical calculation. Theoretical calculations and experiments always coexist and promote each other. MOFs with predicated structures are ideal platforms for constructing models used for theoretical calculations. With the help of theoretical calculations, important information including the electronic structures of materials, densities of states, adsorption energies of N2, reaction pathways of N2 and H2O, changes in bond lengths and angles, etc. can be obtained to enhance our understanding of the specific relationships between the structures of photocatalysts and their corresponding performances, guiding highly efficient photocatalyst design and promoting development of the photocatalytic nitrogen fixation field. Although MOF-based materials for photocatalytic nitrogen fixation are still in their initial stages of development, it is a rapidly growing field owing to their remarkable advantages. We hope this comprehensive review will contribute to further developments in MOF-based materials for nitrogen fixation that benefit researchers who are interested or involved in this field.References
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem. Rev 2014, 114, 4041–4062. [Google Scholar] [PubMed]
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196. [Google Scholar] [PubMed]
- Xiong, Q.; Chen, Y.; Xu, T.; Zhu, Z.; Chen, W.; Lu, W. Highly efficient purification of emerging pollutants and bacteria in natural water by g-C3N4-sheltered fibers containing TiO2. Appl. Surf. Sci. 2021, 559, 149839. [Google Scholar]
- Wang, T.; Dai, Z.; Kang, J.; Fu, F.; Zhang, T.; Wang, S. A TiO2 nanocomposite hydrogel for Hydroponic plants in efficient water improvement. Mater. Chem. Phys. 2018, 215, 242–250. [Google Scholar] [CrossRef]
- Sun, Y.; Luo, Y.; Zhu, Y.; Fu, Y. Improved visible-light photocatalytic activity of sodium tantalum oxide via biomass-derived silk fibroin doping. Text. Res. J. 2018, 89, 1332–1339. [Google Scholar]
- Jiang, T.; Jiang, G.; Li, L.; Chen, H.; Zhou, H.; Yao, J.; Kong, X.; Chen, W. N-Doped carbon hybrid conjugates as vectors for photocatalytic CS2 production. Mater. Res. Express 2015, 2, 045603. [Google Scholar]
- Liu, B.; Xu, Y.; Cui, J.; Wang, S.; Wang, T. Carbon nanotubes-dispersed TiO2 nanoparticles with their enhanced photocatalytic activity. Mater. Res. Bull. 2014, 59, 278–282. [Google Scholar]
- Wang, S.; Wang, T.; Ding, Y.; Su, Q.; Xu, Y.; Xu, Z.; Jiang, G.; Chen, W. Air-Water Interface Photocatalysis: A Realizable Approach for Decomposition of Aqueous Organic Pollutants. Sci. Adv. Mater. 2013, 5, 1006–1012. [Google Scholar]
- Jiang, G.; Wang, R.; Wang, X.; Xi, X.; Hu, R.; Zhou, Y.; Wang, S.; Wang, T.; Chen, W. Novel highly active visible-light-induced photocatalysts based on BiOBr with Ti doping and Ag decorating. ACS Appl. Mater. Inter. 2012, 4, 4440–4444. [Google Scholar]
- Fang, Y.; Wang, R.; Jiang, G.; Jin, H.E.; Wang, Y.I.N.; Sun, X.; Wang, S.; Wang, T.A.O. CuO/TiO2 nanocrystals grown on graphene as visible-light responsive photocatalytic hybrid materials. Bull. Mater. Sci. 2012, 35, 495–499. [Google Scholar]
- Novas, N.; Garcia, R.M.; Camacho, J.M.; Alcayde, A. Advances in Solar Energy towards Efficient and Sustainable Energy. Sustainability 2021, 13, 6295. [Google Scholar]
- Yandulov, D.V.; Schrock, R.R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 2003, 301, 76–78. [Google Scholar] [CrossRef]
- Arashiba, K.; Miyake, Y.; Nishibayashi, Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem. 2011, 3, 120–125. [Google Scholar] [PubMed]
- Rodriguez, M.M.; Bill, E.; Brennessel, W.W.; Holland, P.L. N(2)reduction and hydrogenation to ammonia by a molecular iron-potassium complex. Science 2011, 334, 780–783. [Google Scholar]
- Zhang, G.; Yang, X.; He, C.; Zhang, P.; Mi, H. Constructing a tunable defect structure in TiO2 for photocatalytic nitrogen fixation. J. Mater. Chem. A 2020, 8, 334–341. [Google Scholar] [CrossRef]
- Yang, J.; Guo, Y.; Jiang, R.; Qin, F.; Zhang, H.; Lu, W.; Wang, J.; Yu, J.C. High-Efficiency "Working-in-Tandem" Nitrogen Photofixation Achieved by Assembling Plasmonic Gold Nanocrystals on Ultrathin Titania Nanosheets. J. Am. Chem. Soc. 2018, 140, 8497–8508. [Google Scholar] [PubMed]
- Li, C.; Wang, T.; Zhao, Z.-J.; Yang, W.; Li, J.-F.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted Fixation of Molecular Nitrogen with Surface Oxygen Vacancies on Plasmon-Enhanced TiO2 Photoelectrodes. Angew. Chem. Int. Ed. 2018, 130, 5376–5380. [Google Scholar] [CrossRef]
- Comer, B.M.; Liu, Y.H.; Dixit, M.B.; Hatzell, K.B.; Ye, Y.; Crumlin, E.J.; Hatzell, M.C.; Medford, A.J. The Role of Adventitious Carbon in Photo-catalytic Nitrogen Fixation by Titania. J. Am. Chem. Soc. 2018, 140, 15157–15160. [Google Scholar] [PubMed]
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Oxygen Vacancies of Titanium Dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936. [Google Scholar] [CrossRef]
- Janet, C.M.; Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. Heterogeneous Wet Chemical Synthesis of Superlattice-Type Hierarchical ZnO Architectures for Concurrent H2 Production and N2 Reduction. J. Phys. Chem. C 2010, 114, 2622–2632. [Google Scholar]
- Song, M.; Wang, L.; Li, J.; Sun, D.; Guan, R.; Zhai, H.; Gao, X.; Li, X.; Zhao, Z.; Sun, Z. Defect density modulation of La2TiO5: An effective method to suppress electron-hole recombination and improve photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2021, 602, 748–755. [Google Scholar] [PubMed]
- Zhang, W.; Xing, P.; Zhang, J.; Chen, L.; Yang, J.; Hu, X.; Zhao, L.; Wu, Y.; He, Y. Facile preparation of novel nickel sulfide modified KNbO3 heterojunction composite and its enhanced performance in photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2021, 590, 548–560. [Google Scholar] [PubMed]
- Khan, F.; Yue, P.; Rizzuti, L.; Augugliaro, V.; Brucato, A. Photoassisted water cleavage and nitrogen fixation over titanium-exchanged zeolites. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 238–241. [Google Scholar]
- Wang, S.; Hai, X.; Ding, X.; Chang, K.; Xiang, Y.; Meng, X.; Yang, Z.; Chen, H.; Ye, J. Light-Switchable Oxygen Vacancies in Ultrafine Bi5O7Br Nanotubes for Boosting Solar-Driven Nitrogen Fixation in Pure Water. Adv. Mater. 2017, 29, 1701774. [Google Scholar]
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed {001} Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399. [Google Scholar]
- Khader, M.M.; Lichtin, N.N.; Vurens, G.H.; Salmeron, M.; Somorjai, G.A. Photoassisted catalytic dissociation of water and reduction of nitrogen to ammonia on partially reduced ferric oxide. Langmuir 1987, 3, 303–304. [Google Scholar]
- Dong, G.; Ho, W.; Wang, C. Selective photocatalytic N2 fixation dependent on g-C3N4 induced by nitrogen vacancies. J. Mater. Chem. A 2015, 3, 23435–23441. [Google Scholar]
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157. [Google Scholar]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y.; et al. Ordered macro-microporous metal-organic framework single crystals. Science 2018, 359, 206–210. [Google Scholar]
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332. [Google Scholar] [PubMed]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69. [Google Scholar] [PubMed]
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040. [Google Scholar] [PubMed]
- Zhang, J.; Huang, J.; Wang, L.; Sun, P.; Wang, P.; Yao, Z.; Yang, Y. Coupling Bimetallic NiMn-MOF Nanosheets on NiCo2O4 Nanowire Arrays with Boosted Electrochemical Performance for Hybrid Supercapacitor. Mater. Res. Bull. 2022, 149, 111707. [Google Scholar]
- Yu, Z.; Kang, S.; Wang, J.; Tai, M.; Wang, X.; Ding, Y.; Jin, D.; Wang, L. Highly improved photoluminescence properties of novel ternary Eu(cpioa)phen metal–organic frameworks. Funct. Mater. Lett. 2022, 15, 2251026. [Google Scholar]
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196. [Google Scholar]
- Chen, W.; Wei, W.; Wang, K.; Zhang, N.; Chen, G.; Hu, Y.; Ostrikov, K.K. Plasma-engineered bifunctional cobalt-metal organic framework derivatives for high-performance complete water electrolysis. Nanoscale 2021, 13, 6201–6211. [Google Scholar]
- Lei, Z.; Tang, Q.; Ju, Y.; Lin, Y.; Bai, X.; Luo, H.; Tong, Z. Block copolymer@ZIF-8 nanocomposites as a pH-responsive multi-steps release system for controlled drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 695–711. [Google Scholar]
- Li, G.; Cai, H.; Li, X.; Zhang, J.; Zhang, D.; Yang, Y.; Xiong, J. Construction of Hierarchical NiCo2O4@Ni-MOF Hybrid Arrays on Carbon Cloth as Superior Battery-Type Electrodes for Flexible Solid-State Hybrid Supercapacitors. ACS Appl. Mater. Inter. 2019, 11, 37675–37684. [Google Scholar]
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67. [Google Scholar]
- Wang, X.-S.; Chen, C.-H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of adsorption and photosensitivity capabilities into a cationic multivariate metal-organic framework for enhanced visible-light photoreduction reaction. Appl. Catal. B Environ. 2019, 253, 323–330. [Google Scholar] [CrossRef]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Xu, H.; Gao, J.; Du, W.; Shangguan, L.; Zhang, X.; Lin, R.-B.; Wu, H.; Zhou, W.; Liu, X.; et al. Tunable titanium metal–organic frameworks with infinite 1D Ti–O rods for efficient visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A 2019, 7, 11928–11933. [Google Scholar]
- Chen, C.-H.; Wang, X.-S.; Li, L.; Huang, Y.-B.; Cao, R. Highly selective sensing of Fe(3+) by an anionic metal-organic framework containing uncoordinated nitrogen and carboxylate oxygen sites. Dalton Trans. 2018, 47, 3452–3458. [Google Scholar] [PubMed]
- Wang, X.-S.; Li, L.; Yuan, D.-Q.; Huang, Y.-B.; Cao, R. Fast, highly selective and sensitive anionic metal-organic framework with nitrogen-rich sites fluorescent chemosensor for nitro explosives detection. J. Hazard. Mater. 2017, 344, 283–290. [Google Scholar]
- Wang, X.-S.; Liang, J.; Li, L.; Lin, Z.-J.; Bag, P.P.; Gao, S.-Y.; Huang, Y.-B.; Cao, R. An Anion Metal-Organic Framework with Lewis Basic Sites-Rich toward Charge-Exclusive Cationic Dyes Separation and Size-Selective Catalytic Reaction. Inorg. Chem. 2016, 55, 2641–2649. [Google Scholar]
- Gao, J.; Cai, Y.; Qian, X.; Liu, P.; Wu, H.; Zhou, W.; Liu, D.X.; Li, L.; Lin, R.B.; Chen, B. A Microporous Hydrogen-Bonded Organic Framework for the Efficient Capture and Purification of Propylene. Angew. Chem. Int. Ed. 2021, 60, 20400–20406. [Google Scholar] [CrossRef]
- Gao, J.; Qian, X.; Lin, R.B.; Krishna, R.; Wu, H.; Zhou, W.; Chen, B. Mixed Metal-Organic Framework with Multiple Binding Sites for Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2020, 59, 4396–4400. [Google Scholar]
- Mohanty, A.; Singh, U.P.; Butcher, R.J.; Das, N.; Roy, P. Synthesis of fluorescent MOFs: Live-Cell imaging and sensing of a herbicide. CrystEngComm 2020, 22, 4468–4477. [Google Scholar]
- Xu, K.; Zhan, C.; Zhao, W.; Yu, X.; Zhu, Q.; Yang, L. Tunable resistance of MOFs films via an anion exchange strategy for advanced gas sensing. J. Hazard. Mater. 2021, 416, 125906. [Google Scholar]
- Xu, H.; Gao, J.; Qian, X.; Wang, J.; He, H.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. Metal–organic framework nanosheets for fast-response and highly sensitive luminescent sensing of Fe3+. J. Mater. Chem. A 2016, 4, 10900–10905. [Google Scholar] [CrossRef]
- Deng, Z.; Zhang, H.; Yuan, P.; Su, Z.; Bai, Y.; Yin, Z.; He, J. Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate. Catalysts 2022, 12, 679. [Google Scholar] [CrossRef]
- Montoro, C.; Ocon, P.; Zamora, F.; Navarro, J.A. Metal-Organic Frameworks Containing Missing-Linker Defects Leading to High Hydroxide-Ion Conductivity. Chem. Eur. J. 2016, 22, 1646–1651. [Google Scholar] [CrossRef]
- Ren, X.; Liao, G.; Li, Z.; Qiao, H.; Zhang, Y.; Yu, X.; Wang, B.; Tan, H.; Shi, L.; Qi, X.; et al. Two-dimensional MOF and COF nanosheets for next-generation optoelectronic applications. Coord. Chem. Rev. 2021, 435, 213781. [Google Scholar] [CrossRef]
- Jiang, X.L.; Jiao, Y.E.; Hou, S.L.; Geng, L.C.; Wang, H.Z.; Zhao, B. Green Conversion of CO2 and Propargylamines Triggered by Triply Synergistic Catalytic Effects in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 20417–20423. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Li, L.; Liang, J.; Huang, Y.-B.; Cao, R. Boosting Oxidative Desulfurization of Model and Real Gasoline over Phosphotungstic Acid Encapsulated in Metal-Organic Frameworks: The Window Size Matters. ChemCatChem 2017, 9, 971–979. [Google Scholar] [CrossRef]
- Wang, X.-S.; Huang, Y.-B.; Lin, Z.-J.; Cao, R. Phosphotungstic acid encapsulated in the mesocages of amine-functionalized metal-organic frameworks for catalytic oxidative desulfurization. Dalton Trans. 2014, 43, 11950–11958. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, G.; Chen, J.; Niu, H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts 2022, 12, 775. [Google Scholar] [CrossRef]
- Panda, J.; Biswal, S.P.; Jena, H.S.; Mitra, A.; Samantray, R.; Sahu, R. Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol. Catalysts 2022, 12, 494. [Google Scholar] [CrossRef]
- Wang, B.; Wang, X.; Yong, J.Y.; Song, Z.R.; Chen, J.Z.; Wang, X.S.; Gao, J.K. Hofmann-type Metal-Organic Framework Based Bimetal/Carbon Nanosheets for Efficient Electrocatalytic Oxygen Evolution. Z. Anorg. Allg. Chem. 2022, 648, 12–17. [Google Scholar] [CrossRef]
- Khan, U.; Nairan, A.; Gao, J.; Zhang, Q. Current Progress in Two-Dimensional Metal-Organic Frameworks for Electrocatalysis. Small Struct. 2022. [Google Scholar] [CrossRef]
- Bag, P.P.; Wang, X.-S.; Sahoo, P.; Xiong, J.; Cao, R. Efficient photocatalytic hydrogen evolution under visible light by ternary composite CdS@NU-1000/RGO. Catal. Sci. Technol. 2017, 7, 5113–5119. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Chen, C.; Li, H.; Huang, Y.; Cao, R. Graphene Quantum Dots Supported on Fe-based Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Acta Chim. Sinica 2022, 80, 22–28. [Google Scholar] [CrossRef]
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-Functionalized Titanium Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Molecules 2022, 27, 4241. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Huang, Q.; Wu, Y.; Lan, Y.-Q.; Chen, B. Metal–Organic Frameworks for Photo/Electrocatalysis. Adv. Energy Sustain. Res. 2021, 2, 2100033. [Google Scholar] [CrossRef]
- Yan, Y.; Abazari, R.; Yao, J.; Gao, J. Recent strategies to improve the photoactivity of metal-organic frameworks. Dalton Trans. 2021, 50, 2342–2349. [Google Scholar] [CrossRef]
- Li, L.; Wang, X.S.; Liu, T.F.; Ye, J. Titanium-Based MOF Materials: From Crystal Engineering to Photocatalysis. Small Methods 2020, 4, 2000486. [Google Scholar] [CrossRef]
- Wang, X.S.; Li, L.; Li, D.; Ye, J. Recent Progress on Exploring Stable Metal–Organic Frameworks for Photocatalytic Solar Fuel Production. Sol. RRL 2020, 4, 1900547. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, R.; Bian, X.; Zhou, C.; Zhao, Y.; Zhang, S.; Wu, F.; Waterhouse, G.I.N.; Wu, L.Z.; Tung, C.H.; et al. Ammonia Detection Methods in Photocatalytic and Electrocatalytic Experiments: How to Improve the Reliability of NH3 Production Rates? Adv. Sci. 2019, 6, 1802109. [Google Scholar] [CrossRef]
- Ivancic, I. An optimal manual procedure for ammonia analysis in natural waters by the indophenol blue method. Water Res. 1984, 18, 1143–1147. [Google Scholar] [CrossRef]
- Verdouw, H.; Van Echteld, C.J.A.; Dekkers, E.M.J. Ammonia determination based on indophenol formation with sodium salicylate. Water Res. 1978, 12, 399–402. [Google Scholar] [CrossRef]
- Fritz, J.S. Ion chromatography. Anal. Chem. 1987, 59, 335A–344A. [Google Scholar] [CrossRef]
- Zhang, H.; Nai, J.; Yu, L.; Lou, X.W. Metal-Organic-Framework-Based Materials as Platforms for Renewable Energy and Environmental Applications. Joule 2017, 1, 77–107. [Google Scholar] [CrossRef]
- Ni, Z.; Masel, R.I. Rapid production of metal-organic frameworks via microwave-assisted solvothermal synthesis. J. Am. Chem. Soc. 2006, 128, 12394–12395. [Google Scholar] [CrossRef]
- Lin, J.B.; Lin, R.B.; Cheng, X.N.; Zhang, J.P.; Chen, X.M. Solvent/additive-free synthesis of porous/zeolitic metal azolate frameworks from metal oxide/hydroxide. Chem. Commun. 2011, 47, 9185–9187. [Google Scholar] [CrossRef]
- Stassen, I.; Styles, M.; Grenci, G.; Gorp, H.V.; Vanderlinden, W.; Feyter, S.D.; Falcaro, P.; Vos, D.D.; Vereecken, P.; Ameloot, R. Chemical vapour deposition of zeolitic imidazolate framework thin films. Nat. Mater. 2016, 15, 304–310. [Google Scholar] [CrossRef]
- Bermudez-Garcia, J.M.; Vicent-Luna, J.M.; Yanez-Vilar, S.; Hamad, S.; Sanchez-Andujar, M.; Castro-Garcia, S.; Calero, S.; Senaris-Rodriguez, M.A. Liquid self-diffusion of H2O and DMF molecules in Co-MOF-74: Molecular dynamics simulations and dielectric spectroscopy studies. Phys. Chem. Chem. Phys. 2016, 18, 19605–19612. [Google Scholar] [CrossRef]
- Xiao, J.-D.; Qiu, L.-G.; Ke, F.; Yuan, Y.-P.; Xu, G.-S.; Wang, Y.-M.; Jiang, X. Rapid synthesis of nanoscale terbium-based metal–organic frameworks by a combined ultrasound-vapour phase diffusion method for highly selective sensing of picric acid. J. Mater. Chem. A 2013, 1, 8745. [Google Scholar] [CrossRef]
- Ameloot, R.; Stappers, L.; Fransaer, J.; Alaerts, L.; Sels, B.F.; De Vos, D.E. Patterned Growth of Metal-Organic Framework Coatings by Electrochemical Synthesis. Chem. Mater. 2009, 21, 2580–2582. [Google Scholar] [CrossRef]
- Friscic, T.; Reid, D.G.; Halasz, I.; Stein, R.S.; Dinnebier, R.E.; Duer, M.J. Ion- and liquid-assisted grinding: Improved mechanochemical synthesis of metal-organic frameworks reveals salt inclusion and anion templating. Angew. Chem. Int. Ed. 2010, 49, 712–715. [Google Scholar] [CrossRef]
- Samal, M.; Panda, J.; Biswal, B.P.; Sahu, R. Kitchen grinder: A tool for the synthesis of metal–organic frameworks towards size selective dye adsorption. CrystEngComm 2018, 20, 2486–2490. [Google Scholar] [CrossRef]
- Huang, H.; Wang, X.-S.; Philo, D.; Ichihara, F.; Song, H.; Li, Y.; Li, D.; Qiu, T.; Wang, S.; Ye, J. Toward visible-light-assisted photocatalytic nitrogen fixation: A titanium metal organic framework with functionalized ligands. Appl. Catal. B Environ. 2020, 267, 118686. [Google Scholar] [CrossRef]
- Gao, W.; Li, X.; Zhang, X.; Su, S.; Luo, S.; Huang, R.; Jing, Y.; Luo, M. Photocatalytic nitrogen fixation of metal-organic frameworks (MOFs) excited by ultraviolet light: Insights into the nitrogen fixation mechanism of missing metal cluster or linker defects. Nanoscale 2021, 13, 7801–7809. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Cheng, X.; Tang, Y.; Guo, W.; Deng, S.; Wu, L.; Fu, X. Dehydrated UiO-66(SH)2: The Zr-O Cluster and Its Photocatalytic Role Mimicking the Biological Nitrogen Fixation. Angew. Chem. Int. Ed. 2022, 61, e202117244. [Google Scholar]
- Li, G.; Li, F.; Liu, J.; Fan, C. Fe-based MOFs for photocatalytic N2 reduction: Key role of transition metal iron in nitrogen activation. J. Solid State Chem. 2020, 285, 121245. [Google Scholar] [CrossRef]
- Zhao, Z.; Yang, D.; Ren, H.; An, K.; Chen, Y.; Zhou, Z.; Wang, W.; Jiang, Z. Nitrogenase-inspired mixed-valence MIL-53(FeII/FeIII) for photocatalytic nitrogen fixation. Chem. Eng. J. 2020, 400, 125929. [Google Scholar] [CrossRef]
- Shang, S.; Xiong, W.; Yang, C.; Johannessen, B.; Liu, R.; Hsu, H.Y.; Gu, Q.; Leung, M.K.H.; Shang, J. Atomically Dispersed Iron Metal Site in a Porphyrin-Based Metal-Organic Framework for Photocatalytic Nitrogen Fixation. ACS nano 2021, 15, 9670–9678. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, Y.; Lv, C.; Zhou, X.; Wang, Y.; Xing, W.; Meng, Q.; Kong, Y.; Chen, G. Mimicking pi Backdonation in Ce-MOFs for Solar-Driven Ammonia Synthesis. ACS Appl. Mater. Inter. 2019, 11, 29917–29923. [Google Scholar] [CrossRef]
- Hu, K.Q.; Qiu, P.X.; Zeng, L.W.; Hu, S.X.; Mei, L.; An, S.W.; Huang, Z.W.; Kong, X.H.; Lan, J.H.; Yu, J.P.; et al. Solar-Driven Nitrogen Fixation Catalyzed by Stable Radical-Containing MOFs: Improved Efficiency Induced by a Structural Transformation. Angew. Chem. Int. Ed. 2020, 59, 20666–20671. [Google Scholar] [CrossRef]
- An, K.; Ren, H.; Yang, D.; Zhao, Z.; Gao, Y.; Chen, Y.; Tan, J.; Wang, W.; Jiang, Z. Nitrogenase-inspired bimetallic metal organic frameworks for visible-light-driven nitrogen fixation. Appl. Catal. B: Environ. 2021, 292, 120167. [Google Scholar] [CrossRef]
- An, K.; Tan, J.; Yang, D.; Ren, H.; Zhao, Z.; Chen, Y.; Wang, W.; Xin, X.; Shi, Y.; Jiang, Z. Modular assembly of electron transfer pathways in bimetallic MOFs for photocatalytic ammonia synthesis. Catal. Sci. Technol. 2022, 12, 2015–2022. [Google Scholar] [CrossRef]
- Li, X.H.; He, P.; Wang, T.; Zhang, X.W.; Chen, W.L.; Li, Y.G. Keggin-Type Polyoxometalate-Based ZIF-67 for Enhanced Photocatalytic Nitrogen Fixation. ChemSusChem 2020, 13, 2769–2778. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.W.; Hao, Y.C.; Guo, Y.; Zhang, Q.; Li, J.; Gao, W.Y.; Ren, L.; Su, X.; Hu, L.; Zhang, N.; et al. Metal-Organic Framework Membranes Encapsulating Gold Nanoparticles for Direct Plasmonic Photocatalytic Nitrogen Fixation. J. Am. Chem. Soc. 2021, 143, 5727–5736. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Wang, S.; Chang, X.; Wang, D.H.; Zhang, T. Nano-MOF@defected film C3N4 Z-scheme composite for visible-light photocatalytic nitrogen fixation. RSC Adv. 2020, 10, 26246–26255. [Google Scholar] [CrossRef]
- Wang, L.; Wang, S.; Li, M.; Yang, X.; Li, F.; Xu, L.; Zou, Y. Constructing oxygen vacancies and linker defects in MIL-125@TiO2 for efficient photocatalytic nitrogen fixation. J. Alloys Compd. 2022, 909, 164751. [Google Scholar] [CrossRef]
- Liu, S.; Teng, Z.; Liu, H.; Wang, T.; Wang, G.; Xu, Q.; Zhang, X.; Jiang, M.; Wang, C.; Huang, W.; et al. A Ce-UiO-66 Metal-Organic Framework-Based Graphene-Embedded Photocatalyst with Controllable Activation for Solar Ammonia Fertilizer Production. Angew. Chem. Int. Ed. 2022, 134, e202207026. [Google Scholar] [CrossRef]
- Qin, J.; Liu, B.; Lam, K.-H.; Song, S.; Li, X.; Hu, X. 0D/2D MXene Quantum Dot/Ni-MOF Ultrathin Nanosheets for Enhanced N2 Photoreduction. ACS Sustain. Chem. Eng. 2020, 8, 17791–17799. [Google Scholar] [CrossRef]
- Vu, M.-H.; Quach, T.-A.; Do, T.-O. The construction of Ru-doped In2O3 hollow peanut-like structure for an enhanced photocatalytic nitrogen reduction under solar light irradiation. Sustain. Energ. Fuels 2021, 5, 2528–2536. [Google Scholar] [CrossRef]
- Legare, M.A.; Belanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900. [Google Scholar] [CrossRef]
- Wang, S.; Ichihara, F.; Pang, H.; Chen, H.; Ye, J. Nitrogen Fixation Reaction Derived from Nanostructured Catalytic Materials. Adv. Funct. Mater. 2018, 28, 1803309. [Google Scholar] [CrossRef]
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-Light-Promoted Photocatalytic Hydrogen Production by Using an Amino-Functionalized Ti(IV) Metal–Organic Framework. J. Phys. Chem. C 2012, 116, 20848–20853. [Google Scholar] [CrossRef]
- Nasalevich, M.A.; Becker, R.; Ramos-Fernandez, E.V.; Castellanos, S.; Veber, S.L.; Fedin, M.V.; Kapteijn, F.; Reek, J.N.H.; van der Vlugt, J.I.; Gascon, J. Co@NH2-MIL-125(Ti): Cobaloxime-Derived metal–organic framework-based composite for light-driven H2 production. Energy Environ. Sci. 2015, 8, 364–375. [Google Scholar] [CrossRef]
- Xiao, J.D.; Han, L.; Luo, J.; Yu, S.H.; Jiang, H.L. Integration of Plasmonic Effects and Schottky Junctions into Metal-Organic Framework Composites: Steering Charge Flow for Enhanced Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1103–1107. [Google Scholar] [CrossRef]
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367. [Google Scholar] [CrossRef]
- Logan, M.W.; Ayad, S.; Adamson, J.D.; Dilbeck, T.; Hanson, K.; Uribe-Romo, F.J. Systematic variation of the optical bandgap in titanium based isoreticular metal–organic frameworks for photocatalytic reduction of CO2 under blue light. J. Mater. Chem. A 2017, 5, 11854–11863. [Google Scholar] [CrossRef]
- Abou-Elyazed, A.S.; Ye, G.; Sun, Y.; El-Nahas, A.M. A Series of UiO-66(Zr)-Structured Materials with Defects as Heterogeneous Catalysts for Biodiesel Production. Ind. Eng. Chem. Res. 2019, 58, 21961–21971. [Google Scholar] [CrossRef]
- Cho, K.Y.; Seo, J.Y.; Kim, H.-J.; Pai, S.J.; Do, X.H.; Yoon, H.G.; Hwang, S.S.; Han, S.S.; Baek, K.-Y. Facile control of defect site density and particle size of UiO-66 for enhanced hydrolysis rates: Insights into feasibility of Zr(IV)-based metal-organic framework (MOF) catalysts. Appl. Catal. B: Environ. 2019, 245, 635–647. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, Q.; Jiang, M.; Yao, J. Tailoring the Properties of UiO-66 through Defect Engineering: A Review. Ind. Eng. Chem. Res. 2019, 58, 17646–17659. [Google Scholar] [CrossRef]
- Burgess, B.K.; Lowe, D.J. Mechanism of Molybdenum Nitrogenase. Chem Rev 1996, 96, 2983–3012. [Google Scholar] [CrossRef]
- Smith, J.M.; Lachicotte, R.J.; Pittard, K.A.; Cundari, T.R.; Lukat-Rodgers, G.; Rodgers, K.R.; Holland, P.L. Stepwise reduction of dinitrogen bond order by a low-coordinate iron complex. J. Am. Chem. Soc. 2001, 123, 9222–9223. [Google Scholar] [CrossRef]
- Smith, J.M.; Sadique, A.R.; Cundari, T.R.; Rodgers, K.R.; Lukat-Rodgers, G.; Lachicotte, R.J.; Flaschenriem, C.J.; Vela, J.; Holland, P.L. Studies of low-coordinate iron dinitrogen complexes. J. Am. Chem. Soc. 2006, 128, 756–769. [Google Scholar] [CrossRef] [PubMed]
- Stoian, S.A.; Vela, J.; Smith, J.M.; Sadique, A.R.; Holland, P.L.; Munck, E.; Bominaar, E.L. Mossbauer and computational study of an N2-bridged diiron diketiminate complex: Parallel alignment of the iron spins by direct antiferromagnetic exchange with activated dinitrogen. J. Am. Chem. Soc. 2006, 128, 10181–10192. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.S.; Cutsail, G.E., 3rd; Rittle, J.; Connor, B.A.; Gunderson, W.A.; Zhang, L.; Hoffman, B.M.; Peters, J.C. Characterization of an Fe identical withN-NH2 Intermediate Relevant to Catalytic N2 Reduction to NH3. J. Am. Chem. Soc. 2015, 137, 7803–7809. [Google Scholar] [CrossRef]
- Ma, X.; Chen, F.; Zhang, X.; Wang, T.; Yuan, S.; Wang, X.; Li, T.; Gao, J. Hierarchical Co@C-N synthesized by the confined pyrolysis of ionic liquid@metal–organic frameworks for the aerobic oxidation of alcohols. New J. Chem. 2022, 46, 7528–7536. [Google Scholar] [CrossRef]
- Cheng, N.; Wang, N.; Ren, L.; Casillas-Garcia, G.; Liu, N.; Liu, Y.; Xu, X.; Hao, W.; Dou, S.X.; Du, Y. In-situ grafting of N-doped carbon nanotubes with Ni encapsulation onto MOF-derived hierarchical hybrids for efficient electrocatalytic hydrogen evolution. Carbon 2020, 163, 178–185. [Google Scholar] [CrossRef]
- Xu, K.; Zhao, W.; Yu, X.; Duan, S.; Zeng, W. MOF-derived Co3O4/Fe2O3 p-n hollow cubes for improved acetone sensing characteristics. Phys. E: Low-Dimens. Syst. Nanostructures 2020, 118, 113869. [Google Scholar] [CrossRef]
- Zhai, C.; Zhang, H.; Du, L.; Wang, D.; Xing, D.; Zhang, M. Nickel/iron-based bimetallic MOF-derived nickel ferrite materials for triethylamine sensing. CrystEngComm 2020, 22, 1286–1293. [Google Scholar] [CrossRef]
- Wang, C.; Mutahir, S.; Wang, L.; Lei, W.; Xia, X.; Jiao, X.; Hao, Q. Hierarchical MOF-derived layered Fe3O4 QDs@C imbedded on graphene sheets as a high-performance anode for Lithium-ion storage. Appl. Surf. Sci. 2020, 509, 144882. [Google Scholar] [CrossRef]
- Ren, X.; Wei, S.; Wang, Q.; Shi, L.; Wang, X.-S.; Wei, Y.; Yang, G.; Philo, D.; Ichihara, F.; Ye, J. Rational construction of dual cobalt active species encapsulated by ultrathin carbon matrix from MOF for boosting photocatalytic H2 generation. Appl. Catal. B: Environ. 2021, 286, 119924. [Google Scholar] [CrossRef]
- Melchionna, M.; Fornasiero, P. Updates on the Roadmap for Photocatalysis. ACS Catal. 2020, 10, 5493–5501. [Google Scholar] [CrossRef]
References
- Hoffman, B.M.; Lukoyanov, D.; Yang, Z.Y.; Dean, D.R.; Seefeldt, L.C. Mechanism of nitrogen fixation by nitrogenase: The next stage. Chem. Rev 2014, 114, 4041–4062.
- Canfield, D.E.; Glazer, A.N.; Falkowski, P.G. The evolution and future of Earth’s nitrogen cycle. Science 2010, 330, 192–196.
- Xiong, Q.; Chen, Y.; Xu, T.; Zhu, Z.; Chen, W.; Lu, W. Highly efficient purification of emerging pollutants and bacteria in natural water by g-C3N4-sheltered fibers containing TiO2. Appl. Surf. Sci. 2021, 559, 149839.
- Wang, T.; Dai, Z.; Kang, J.; Fu, F.; Zhang, T.; Wang, S. A TiO2 nanocomposite hydrogel for Hydroponic plants in efficient water improvement. Mater. Chem. Phys. 2018, 215, 242–250.
- Sun, Y.; Luo, Y.; Zhu, Y.; Fu, Y. Improved visible-light photocatalytic activity of sodium tantalum oxide via biomass-derived silk fibroin doping. Text. Res. J. 2018, 89, 1332–1339.
- Jiang, T.; Jiang, G.; Li, L.; Chen, H.; Zhou, H.; Yao, J.; Kong, X.; Chen, W. N-Doped carbon hybrid conjugates as vectors for photocatalytic CS2 production. Mater. Res. Express 2015, 2, 045603.
- Liu, B.; Xu, Y.; Cui, J.; Wang, S.; Wang, T. Carbon nanotubes-dispersed TiO2 nanoparticles with their enhanced photocatalytic activity. Mater. Res. Bull. 2014, 59, 278–282.
- Wang, S.; Wang, T.; Ding, Y.; Su, Q.; Xu, Y.; Xu, Z.; Jiang, G.; Chen, W. Air-Water Interface Photocatalysis: A Realizable Approach for Decomposition of Aqueous Organic Pollutants. Sci. Adv. Mater. 2013, 5, 1006–1012.
- Jiang, G.; Wang, R.; Wang, X.; Xi, X.; Hu, R.; Zhou, Y.; Wang, S.; Wang, T.; Chen, W. Novel highly active visible-light-induced photocatalysts based on BiOBr with Ti doping and Ag decorating. ACS Appl. Mater. Inter. 2012, 4, 4440–4444.
- Fang, Y.; Wang, R.; Jiang, G.; Jin, H.E.; Wang, Y.I.N.; Sun, X.; Wang, S.; Wang, T.A.O. CuO/TiO2 nanocrystals grown on graphene as visible-light responsive photocatalytic hybrid materials. Bull. Mater. Sci. 2012, 35, 495–499.
- Novas, N.; Garcia, R.M.; Camacho, J.M.; Alcayde, A. Advances in Solar Energy towards Efficient and Sustainable Energy. Sustainability 2021, 13, 6295.
- Yandulov, D.V.; Schrock, R.R. Catalytic reduction of dinitrogen to ammonia at a single molybdenum center. Science 2003, 301, 76–78.
- Arashiba, K.; Miyake, Y.; Nishibayashi, Y. A molybdenum complex bearing PNP-type pincer ligands leads to the catalytic reduction of dinitrogen into ammonia. Nat. Chem. 2011, 3, 120–125.
- Rodriguez, M.M.; Bill, E.; Brennessel, W.W.; Holland, P.L. N(2)reduction and hydrogenation to ammonia by a molecular iron-potassium complex. Science 2011, 334, 780–783.
- Zhang, G.; Yang, X.; He, C.; Zhang, P.; Mi, H. Constructing a tunable defect structure in TiO2 for photocatalytic nitrogen fixation. J. Mater. Chem. A 2020, 8, 334–341.
- Yang, J.; Guo, Y.; Jiang, R.; Qin, F.; Zhang, H.; Lu, W.; Wang, J.; Yu, J.C. High-Efficiency "Working-in-Tandem" Nitrogen Photofixation Achieved by Assembling Plasmonic Gold Nanocrystals on Ultrathin Titania Nanosheets. J. Am. Chem. Soc. 2018, 140, 8497–8508.
- Li, C.; Wang, T.; Zhao, Z.-J.; Yang, W.; Li, J.-F.; Li, A.; Yang, Z.; Ozin, G.A.; Gong, J. Promoted Fixation of Molecular Nitrogen with Surface Oxygen Vacancies on Plasmon-Enhanced TiO2 Photoelectrodes. Angew. Chem. Int. Ed. 2018, 130, 5376–5380.
- Comer, B.M.; Liu, Y.H.; Dixit, M.B.; Hatzell, K.B.; Ye, Y.; Crumlin, E.J.; Hatzell, M.C.; Medford, A.J. The Role of Adventitious Carbon in Photo-catalytic Nitrogen Fixation by Titania. J. Am. Chem. Soc. 2018, 140, 15157–15160.
- Hirakawa, H.; Hashimoto, M.; Shiraishi, Y.; Hirai, T. Photocatalytic Conversion of Nitrogen to Ammonia with Water on Surface Oxygen Vacancies of Titanium Dioxide. J. Am. Chem. Soc. 2017, 139, 10929–10936.
- Janet, C.M.; Navaladian, S.; Viswanathan, B.; Varadarajan, T.K.; Viswanath, R.P. Heterogeneous Wet Chemical Synthesis of Superlattice-Type Hierarchical ZnO Architectures for Concurrent H2 Production and N2 Reduction. J. Phys. Chem. C 2010, 114, 2622–2632.
- Song, M.; Wang, L.; Li, J.; Sun, D.; Guan, R.; Zhai, H.; Gao, X.; Li, X.; Zhao, Z.; Sun, Z. Defect density modulation of La2TiO5: An effective method to suppress electron-hole recombination and improve photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2021, 602, 748–755.
- Zhang, W.; Xing, P.; Zhang, J.; Chen, L.; Yang, J.; Hu, X.; Zhao, L.; Wu, Y.; He, Y. Facile preparation of novel nickel sulfide modified KNbO3 heterojunction composite and its enhanced performance in photocatalytic nitrogen fixation. J. Colloid Interface Sci. 2021, 590, 548–560.
- Khan, F.; Yue, P.; Rizzuti, L.; Augugliaro, V.; Brucato, A. Photoassisted water cleavage and nitrogen fixation over titanium-exchanged zeolites. Ind. Eng. Chem. Prod. Res. Dev. 1983, 22, 238–241.
- Wang, S.; Hai, X.; Ding, X.; Chang, K.; Xiang, Y.; Meng, X.; Yang, Z.; Chen, H.; Ye, J. Light-Switchable Oxygen Vacancies in Ultrafine Bi5O7Br Nanotubes for Boosting Solar-Driven Nitrogen Fixation in Pure Water. Adv. Mater. 2017, 29, 1701774.
- Li, H.; Shang, J.; Ai, Z.; Zhang, L. Efficient Visible Light Nitrogen Fixation with BiOBr Nanosheets of Oxygen Vacancies on the Exposed Facets. J. Am. Chem. Soc. 2015, 137, 6393–6399.
- Khader, M.M.; Lichtin, N.N.; Vurens, G.H.; Salmeron, M.; Somorjai, G.A. Photoassisted catalytic dissociation of water and reduction of nitrogen to ammonia on partially reduced ferric oxide. Langmuir 1987, 3, 303–304.
- Dong, G.; Ho, W.; Wang, C. Selective photocatalytic N2 fixation dependent on g-C3N4 induced by nitrogen vacancies. J. Mater. Chem. A 2015, 3, 23435–23441.
- Huang, Y.B.; Liang, J.; Wang, X.S.; Cao, R. Multifunctional metal-organic framework catalysts: Synergistic catalysis and tandem reactions. Chem. Soc. Rev. 2017, 46, 126–157.
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279.
- Shen, K.; Zhang, L.; Chen, X.; Liu, L.; Zhang, D.; Han, Y.; Chen, J.; Long, J.; Luque, R.; Li, Y.; et al. Ordered macro-microporous metal-organic framework single crystals. Science 2018, 359, 206–210.
- Jagadeesh, R.V.; Murugesan, K.; Alshammari, A.S.; Neumann, H.; Pohl, M.M.; Radnik, J.; Beller, M. MOF-derived cobalt nanoparticles catalyze a general synthesis of amines. Science 2017, 358, 326–332.
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable electrical conductivity in metal-organic framework thin-film devices. Science 2014, 343, 66–69.
- Ferey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040.
- Zhang, J.; Huang, J.; Wang, L.; Sun, P.; Wang, P.; Yao, Z.; Yang, Y. Coupling Bimetallic NiMn-MOF Nanosheets on NiCo2O4 Nanowire Arrays with Boosted Electrochemical Performance for Hybrid Supercapacitor. Mater. Res. Bull. 2022, 149, 111707.
- Yu, Z.; Kang, S.; Wang, J.; Tai, M.; Wang, X.; Ding, Y.; Jin, D.; Wang, L. Highly improved photoluminescence properties of novel ternary Eu(cpioa)phen metal–organic frameworks. Funct. Mater. Lett. 2022, 15, 2251026.
- Sun, P.; Zhang, J.; Huang, J.; Wang, L.; Wang, P.; Cai, C.; Lu, M.; Yao, Z.; Yang, Y. Bimetallic MOF-derived (CuCo)Se nanoparticles embedded in nitrogen-doped carbon framework with boosted electrochemical performance for hybrid supercapacitor. Mater. Res. Bull. 2021, 137, 111196.
- Chen, W.; Wei, W.; Wang, K.; Zhang, N.; Chen, G.; Hu, Y.; Ostrikov, K.K. Plasma-engineered bifunctional cobalt-metal organic framework derivatives for high-performance complete water electrolysis. Nanoscale 2021, 13, 6201–6211.
- Lei, Z.; Tang, Q.; Ju, Y.; Lin, Y.; Bai, X.; Luo, H.; Tong, Z. Block nanocomposites as a pH-responsive multi-steps release system for controlled drug delivery. J. Biomater. Sci. Polym. Ed. 2020, 31, 695–711.
- Li, G.; Cai, H.; Li, X.; Zhang, J.; Zhang, D.; Yang, Y.; Xiong, J. Construction of Hierarchical NiCo2O4@Ni-MOF Hybrid Arrays on Carbon Cloth as Superior Battery-Type Electrodes for Flexible Solid-State Hybrid Supercapacitors. ACS Appl. Mater. Inter. 2019, 11, 37675–37684.
- Phan, A.; Doonan, C.J.; Uribe-Romo, F.J.; Knobler, C.B.; O’Keeffe, M.; Yaghi, O.M. Synthesis, structure, and carbon dioxide capture properties of zeolitic imidazolate frameworks. Acc. Chem. Res. 2010, 43, 58–67.
- Wang, X.-S.; Chen, C.-H.; Ichihara, F.; Oshikiri, M.; Liang, J.; Li, L.; Li, Y.; Song, H.; Wang, S.; Zhang, T.; et al. Integration of adsorption and photosensitivity capabilities into a cationic multivariate metal-organic framework for enhanced visible-light photoreduction reaction. Appl. Catal. B Environ. 2019, 253, 323–330.
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851.
- Li, C.; Xu, H.; Gao, J.; Du, W.; Shangguan, L.; Zhang, X.; Lin, R.-B.; Wu, H.; Zhou, W.; Liu, X.; et al. Tunable titanium metal–organic frameworks with infinite 1D Ti–O rods for efficient visible-light-driven photocatalytic H2 evolution. J. Mater. Chem. A 2019, 7, 11928–11933.
- Chen, C.-H.; Wang, X.-S.; Li, L.; Huang, Y.-B.; Cao, R. Highly selective sensing of Fe(3+) by an anionic metal-organic framework containing uncoordinated nitrogen and carboxylate oxygen sites. Dalton Trans. 2018, 47, 3452–3458.
- Wang, X.-S.; Li, L.; Yuan, D.-Q.; Huang, Y.-B.; Cao, R. Fast, highly selective and sensitive anionic metal-organic framework with nitrogen-rich sites fluorescent chemosensor for nitro explosives detection. J. Hazard. Mater. 2017, 344, 283–290.
- Wang, X.-S.; Liang, J.; Li, L.; Lin, Z.-J.; Bag, P.P.; Gao, S.-Y.; Huang, Y.-B.; Cao, R. An Anion Metal-Organic Framework with Lewis Basic Sites-Rich toward Charge-Exclusive Cationic Dyes Separation and Size-Selective Catalytic Reaction. Inorg. Chem. 2016, 55, 2641–2649.
- Gao, J.; Cai, Y.; Qian, X.; Liu, P.; Wu, H.; Zhou, W.; Liu, D.X.; Li, L.; Lin, R.B.; Chen, B. A Microporous Hydrogen-Bonded Organic Framework for the Efficient Capture and Purification of Propylene. Angew. Chem. Int. Ed. 2021, 60, 20400–20406.
- Gao, J.; Qian, X.; Lin, R.B.; Krishna, R.; Wu, H.; Zhou, W.; Chen, B. Mixed Metal-Organic Framework with Multiple Binding Sites for Efficient C2H2/CO2 Separation. Angew. Chem. Int. Ed. 2020, 59, 4396–4400.
- Mohanty, A.; Singh, U.P.; Butcher, R.J.; Das, N.; Roy, P. Synthesis of fluorescent MOFs: Live-Cell imaging and sensing of a herbicide. CrystEngComm 2020, 22, 4468–4477.
- Xu, K.; Zhan, C.; Zhao, W.; Yu, X.; Zhu, Q.; Yang, L. Tunable resistance of MOFs films via an anion exchange strategy for advanced gas sensing. J. Hazard. Mater. 2021, 416, 125906.
- Xu, H.; Gao, J.; Qian, X.; Wang, J.; He, H.; Cui, Y.; Yang, Y.; Wang, Z.; Qian, G. Metal–organic framework nanosheets for fast-response and highly sensitive luminescent sensing of Fe3+. J. Mater. Chem. A 2016, 4, 10900–10905.
- Deng, Z.; Zhang, H.; Yuan, P.; Su, Z.; Bai, Y.; Yin, Z.; He, J. Cobalt-Based Metal-Organic Framework Nanoparticles with Peroxidase-like Catalytic Activity for Sensitive Colorimetric Detection of Phosphate. Catalysts 2022, 12, 679.
- Montoro, C.; Ocon, P.; Zamora, F.; Navarro, J.A. Metal-Organic Frameworks Containing Missing-Linker Defects Leading to High Hydroxide-Ion Conductivity. Chem. Eur. J. 2016, 22, 1646–1651.
- Ren, X.; Liao, G.; Li, Z.; Qiao, H.; Zhang, Y.; Yu, X.; Wang, B.; Tan, H.; Shi, L.; Qi, X.; et al. Two-dimensional MOF and COF nanosheets for next-generation optoelectronic applications. Coord. Chem. Rev. 2021, 435, 213781.
- Jiang, X.L.; Jiao, Y.E.; Hou, S.L.; Geng, L.C.; Wang, H.Z.; Zhao, B. Green Conversion of CO2 and Propargylamines Triggered by Triply Synergistic Catalytic Effects in Metal-Organic Frameworks. Angew. Chem. Int. Ed. 2021, 60, 20417–20423.
- Wang, X.-S.; Li, L.; Liang, J.; Huang, Y.-B.; Cao, R. Boosting Oxidative Desulfurization of Model and Real Gasoline over Phosphotungstic Acid Encapsulated in Metal-Organic Frameworks: The Window Size Matters. ChemCatChem 2017, 9, 971–979.
- Wang, X.-S.; Huang, Y.-B.; Lin, Z.-J.; Cao, R. Phosphotungstic acid encapsulated in the mesocages of amine-functionalized metal-organic frameworks for catalytic oxidative desulfurization. Dalton Trans. 2014, 43, 11950–11958.
- Liu, Y.; Chen, G.; Chen, J.; Niu, H. Excellent Catalytic Performance of Ce–MOF with Abundant Oxygen Vacancies Supported Noble Metal Pt in the Oxidation of Toluene. Catalysts 2022, 12, 775.
- Panda, J.; Biswal, S.P.; Jena, H.S.; Mitra, A.; Samantray, R.; Sahu, R. Role of Lewis Acid Metal Centers in Metal–Organic Frameworks for Ultrafast Reduction of 4-Nitrophenol. Catalysts 2022, 12, 494.
- Wang, B.; Wang, X.; Yong, J.Y.; Song, Z.R.; Chen, J.Z.; Wang, X.S.; Gao, J.K. Hofmann-type Metal-Organic Framework Based Bimetal/Carbon Nanosheets for Efficient Electrocatalytic Oxygen Evolution. Z. Anorg. Allg. Chem. 2022, 648, 12–17.
- Khan, U.; Nairan, A.; Gao, J.; Zhang, Q. Current Progress in Two-Dimensional Metal-Organic Frameworks for Electrocatalysis. Small Struct. 2022.
- Bag, P.P.; Wang, X.-S.; Sahoo, P.; Xiong, J.; Cao, R. Efficient photocatalytic hydrogen evolution under visible light by ternary composite /RGO. Catal. Sci. Technol. 2017, 7, 5113–5119.
- Wang, X.; Yang, X.; Chen, C.; Li, H.; Huang, Y.; Cao, R. Graphene Quantum Dots Supported on Fe-based Metal-Organic Frameworks for Efficient Photocatalytic CO2 Reduction. Acta Chim. Sinica 2022, 80, 22–28.
- Hu, N.; Cai, Y.; Li, L.; Wang, X.; Gao, J. Amino-Functionalized Titanium Based Metal-Organic Framework for Photocatalytic Hydrogen Production. Molecules 2022, 27, 4241.
- Gao, J.; Huang, Q.; Wu, Y.; Lan, Y.-Q.; Chen, B. Metal–Organic Frameworks for Photo/Electrocatalysis. Adv. Energy Sustain. Res. 2021, 2, 2100033.
- Yan, Y.; Abazari, R.; Yao, J.; Gao, J. Recent strategies to improve the photoactivity of metal-organic frameworks. Dalton Trans. 2021, 50, 2342–2349.
- Li, L.; Wang, X.S.; Liu, T.F.; Ye, J. Titanium-Based MOF Materials: From Crystal Engineering to Photocatalysis. Small Methods 2020, 4, 2000486.
- Wang, X.S.; Li, L.; Li, D.; Ye, J. Recent Progress on Exploring Stable Metal–Organic Frameworks for Photocatalytic Solar Fuel Production. Sol. RRL 2020, 4, 1900547.
- Huang, H.; Wang, X.-S.; Philo, D.; Ichihara, F.; Song, H.; Li, Y.; Li, D.; Qiu, T.; Wang, S.; Ye, J. Toward visible-light-assisted photocatalytic nitrogen fixation: A titanium metal organic framework with functionalized ligands. Appl. Catal. B Environ. 2020, 267, 118686.
- Gao, W.; Li, X.; Zhang, X.; Su, S.; Luo, S.; Huang, R.; Jing, Y.; Luo, M. Photocatalytic nitrogen fixation of metal-organic frameworks (MOFs) excited by ultraviolet light: Insights into the nitrogen fixation mechanism of missing metal cluster or linker defects. Nanoscale 2021, 13, 7801–7809.
- Guo, B.; Cheng, X.; Tang, Y.; Guo, W.; Deng, S.; Wu, L.; Fu, X. Dehydrated UiO-66(SH)2: The Zr-O Cluster and Its Photocatalytic Role Mimicking the Biological Nitrogen Fixation. Angew. Chem. Int. Ed. 2022, 61, e202117244.
- Li, G.; Li, F.; Liu, J.; Fan, C. Fe-based MOFs for photocatalytic N2 reduction: Key role of transition metal iron in nitrogen activation. J. Solid State Chem. 2020, 285, 121245.
- Zhao, Z.; Yang, D.; Ren, H.; An, K.; Chen, Y.; Zhou, Z.; Wang, W.; Jiang, Z. Nitrogenase-inspired mixed-valence MIL-53(FeII/FeIII) for photocatalytic nitrogen fixation. Chem. Eng. J. 2020, 400, 125929.
- Shang, S.; Xiong, W.; Yang, C.; Johannessen, B.; Liu, R.; Hsu, H.Y.; Gu, Q.; Leung, M.K.H.; Shang, J. Atomically Dispersed Iron Metal Site in a Porphyrin-Based Metal-Organic Framework for Photocatalytic Nitrogen Fixation. ACS nano 2021, 15, 9670–9678.
- Zhang, C.; Xu, Y.; Lv, C.; Zhou, X.; Wang, Y.; Xing, W.; Meng, Q.; Kong, Y.; Chen, G. Mimicking pi Backdonation in Ce-MOFs for Solar-Driven Ammonia Synthesis. ACS Appl. Mater. Inter. 2019, 11, 29917–29923.
- Hu, K.Q.; Qiu, P.X.; Zeng, L.W.; Hu, S.X.; Mei, L.; An, S.W.; Huang, Z.W.; Kong, X.H.; Lan, J.H.; Yu, J.P.; et al. Solar-Driven Nitrogen Fixation Catalyzed by Stable Radical-Containing MOFs: Improved Efficiency Induced by a Structural Transformation. Angew. Chem. Int. Ed. 2020, 59, 20666–20671.
- An, K.; Ren, H.; Yang, D.; Zhao, Z.; Gao, Y.; Chen, Y.; Tan, J.; Wang, W.; Jiang, Z. Nitrogenase-inspired bimetallic metal organic frameworks for visible-light-driven nitrogen fixation. Appl. Catal. B: Environ. 2021, 292, 120167.
- An, K.; Tan, J.; Yang, D.; Ren, H.; Zhao, Z.; Chen, Y.; Wang, W.; Xin, X.; Shi, Y.; Jiang, Z. Modular assembly of electron transfer pathways in bimetallic MOFs for photocatalytic ammonia synthesis. Catal. Sci. Technol. 2022, 12, 2015–2022.
- Li, X.H.; He, P.; Wang, T.; Zhang, X.W.; Chen, W.L.; Li, Y.G. Keggin-Type Polyoxometalate-Based ZIF-67 for Enhanced Photocatalytic Nitrogen Fixation. ChemSusChem 2020, 13, 2769–2778.
- Chen, L.W.; Hao, Y.C.; Guo, Y.; Zhang, Q.; Li, J.; Gao, W.Y.; Ren, L.; Su, X.; Hu, L.; Zhang, N.; et al. Metal-Organic Framework Membranes Encapsulating Gold Nanoparticles for Direct Plasmonic Photocatalytic Nitrogen Fixation. J. Am. Chem. Soc. 2021, 143, 5727–5736.
- Ding, Z.; Wang, S.; Chang, X.; Wang, D.H.; Zhang, T. film C3N4 Z-scheme composite for visible-light photocatalytic nitrogen fixation. RSC Adv. 2020, 10, 26246–26255.
- Wang, L.; Wang, S.; Li, M.; Yang, X.; Li, F.; Xu, L.; Zou, Y. Constructing oxygen vacancies and linker defects in 2 for efficient photocatalytic nitrogen fixation. J. Alloys Compd. 2022, 909, 164751.
- Liu, S.; Teng, Z.; Liu, H.; Wang, T.; Wang, G.; Xu, Q.; Zhang, X.; Jiang, M.; Wang, C.; Huang, W.; et al. A Ce-UiO-66 Metal-Organic Framework-Based Graphene-Embedded Photocatalyst with Controllable Activation for Solar Ammonia Fertilizer Production. Angew. Chem. Int. Ed. 2022, 134, e202207026.
- Qin, J.; Liu, B.; Lam, K.-H.; Song, S.; Li, X.; Hu, X. 0D/2D MXene Quantum Dot/Ni-MOF Ultrathin Nanosheets for Enhanced N2 Photoreduction. ACS Sustain. Chem. Eng. 2020, 8, 17791–17799.
- Vu, M.-H.; Quach, T.-A.; Do, T.-O. The construction of Ru-doped In2O3 hollow peanut-like structure for an enhanced photocatalytic nitrogen reduction under solar light irradiation. Sustain. Energ. Fuels 2021, 5, 2528–2536.
- Legare, M.A.; Belanger-Chabot, G.; Dewhurst, R.D.; Welz, E.; Krummenacher, I.; Engels, B.; Braunschweig, H. Nitrogen fixation and reduction at boron. Science 2018, 359, 896–900.
- Wang, S.; Ichihara, F.; Pang, H.; Chen, H.; Ye, J. Nitrogen Fixation Reaction Derived from Nanostructured Catalytic Materials. Adv. Funct. Mater. 2018, 28, 1803309.
- Horiuchi, Y.; Toyao, T.; Saito, M.; Mochizuki, K.; Iwata, M.; Higashimura, H.; Anpo, M.; Matsuoka, M. Visible-Light-Promoted Photocatalytic Hydrogen Production by Using an Amino-Functionalized Ti(IV) Metal–Organic Framework. J. Phys. Chem. C 2012, 116, 20848–20853.
- Nasalevich, M.A.; Becker, R.; Ramos-Fernandez, E.V.; Castellanos, S.; Veber, S.L.; Fedin, M.V.; Kapteijn, F.; Reek, J.N.H.; van der Vlugt, J.I.; Gascon, J. 2-MIL-125(Ti): Cobaloxime-Derived metal–organic framework-based composite for light-driven H2 production. Energy Environ. Sci. 2015, 8, 364–375.
- Xiao, J.D.; Han, L.; Luo, J.; Yu, S.H.; Jiang, H.L. Integration of Plasmonic Effects and Schottky Junctions into Metal-Organic Framework Composites: Steering Charge Flow for Enhanced Visible-Light Photocatalysis. Angew. Chem. Int. Ed. 2018, 57, 1103–1107.
- Fu, Y.; Sun, D.; Chen, Y.; Huang, R.; Ding, Z.; Fu, X.; Li, Z. An amine-functionalized titanium metal-organic framework photocatalyst with visible-light-induced activity for CO2 reduction. Angew. Chem. Int. Ed. 2012, 51, 3364–3367.
- Logan, M.W.; Ayad, S.; Adamson, J.D.; Dilbeck, T.; Hanson, K.; Uribe-Romo, F.J. Systematic variation of the optical bandgap in titanium based isoreticular metal–organic frameworks for photocatalytic reduction of CO2 under blue light. J. Mater. Chem. A 2017, 5, 11854–11863.
- Abou-Elyazed, A.S.; Ye, G.; Sun, Y.; El-Nahas, A.M. A Series of UiO-66(Zr)-Structured Materials with Defects as Heterogeneous Catalysts for Biodiesel Production. Ind. Eng. Chem. Res. 2019, 58, 21961–21971.
- Cho, K.Y.; Seo, J.Y.; Kim, H.-J.; Pai, S.J.; Do, X.H.; Yoon, H.G.; Hwang, S.S.; Han, S.S.; Baek, K.-Y. Facile control of defect site density and particle size of UiO-66 for enhanced hydrolysis rates: Insights into feasibility of Zr(IV)-based metal-organic framework (MOF) catalysts. Appl. Catal. B: Environ. 2019, 245, 635–647.
- Feng, Y.; Chen, Q.; Jiang, M.; Yao, J. Tailoring the Properties of UiO-66 through Defect Engineering: A Review. Ind. Eng. Chem. Res. 2019, 58, 17646–17659.
- Burgess, B.K.; Lowe, D.J. Mechanism of Molybdenum Nitrogenase. Chem Rev 1996, 96, 2983–3012.
- Smith, J.M.; Lachicotte, R.J.; Pittard, K.A.; Cundari, T.R.; Lukat-Rodgers, G.; Rodgers, K.R.; Holland, P.L. Stepwise reduction of dinitrogen bond order by a low-coordinate iron complex. J. Am. Chem. Soc. 2001, 123, 9222–9223.
- Smith, J.M.; Sadique, A.R.; Cundari, T.R.; Rodgers, K.R.; Lukat-Rodgers, G.; Lachicotte, R.J.; Flaschenriem, C.J.; Vela, J.; Holland, P.L. Studies of low-coordinate iron dinitrogen complexes. J. Am. Chem. Soc. 2006, 128, 756–769.
- Stoian, S.A.; Vela, J.; Smith, J.M.; Sadique, A.R.; Holland, P.L.; Munck, E.; Bominaar, E.L. Mossbauer and computational study of an N2-bridged diiron diketiminate complex: Parallel alignment of the iron spins by direct antiferromagnetic exchange with activated dinitrogen. J. Am. Chem. Soc. 2006, 128, 10181–10192.
- Anderson, J.S.; Cutsail, G.E., 3rd; Rittle, J.; Connor, B.A.; Gunderson, W.A.; Zhang, L.; Hoffman, B.M.; Peters, J.C. Characterization of an Fe identical withN-NH2 Intermediate Relevant to Catalytic N2 Reduction to NH3. J. Am. Chem. Soc. 2015, 137, 7803–7809.
More
