Selenium (Se) is an essential trace element for humans and animals, and it plays an important role in immune regulation and disease prevention. Tea is one of the top three beverages in the world, and it contains active ingredients such as polyphenols, theanine, flavonoids, and volatile substances, which have important health benefits. The tea tree has suitable Se aggregation ability, which can absorb inorganic Se and transform it into safe and effective organic Se through absorption by the human body, thereby improving human immunity and preventing the occurrence of many diseases. Recent studies have proven that 50~100.0 mg/L exogenous Se can promote photosynthesis and absorption of mineral elements in tea trees and increase their biomass. The content of total Se and organic selenides in tea leaves significantly increases and promotes the accumulation of polyphenols, theanine, flavonoids, and volatile secondary metabolites, thereby improving the nutritional quality of tea leaves. This paper summarizes previous research on the effects of exogenous Se treatment on the growth and quality of tea trees to provide a theoretical basis and technical support for the germplasm selection and exploitation of Se-rich tea.
- selenium
- Camellia sinensis (L.) O. Kuntze
- growth
- quality
1. Introduction
2. Selenium Uptake and Metabolism in Tea Plant
Inorganic Se uptake by plants primarily consists of selenate and selenite, with selenate primarily existing in alkaline-oxidizing environments and selenite primarily existing in acidic and neutral environments. Tea plants can grow and develop in weakly acidic soils, thereby indicating their stronger uptake of selenite [7]. The organic matter in the soil can fix Se, and when the pH of the soil changes from acidic to alkalescence, the elemental Se and selenides in the soil will slowly oxidize to selenate and selenite. Moreover, the divalent Se produced by the decomposition of organically bound Se will also oxidize to selenate and selenite, thereby increasing the effective Se content in the soil [8]. The roots of tea plants are more efficient at selenite uptake than selenate [9]. When selenite is absorbed by the roots of tea plants, it can be accumulated in the roots through phosphate transport proteins and rapidly converted into organoselenium compounds such as selenocysteine (SeCys), selenocystine (SeCys2), selenomethionine (SeMet), and methylselenocysteine (MeSeCys) before being transported aboveground (Figure 1). The roots of tea trees take up selenate via sulfate transport proteins and transport it rapidly from the xylem to the aboveground. However, selenate is toxic to plants because it is incompletely transformed in the roots [10]. Previous studies have hypothesized that higher selenate concentrations in plant cells can reduce plant water potential, resulting in the rapid translocation of selenate to the aboveground. Selenate is converted into organoselenides, such as SeCys, SeMet, and MeSeCys, in the leaves through the sulfate metabolism pathway. These organoselenides have oxidative, anti-inflammatory, and other biological activities, and they are important components of the nutritional quality of tea leaves [11].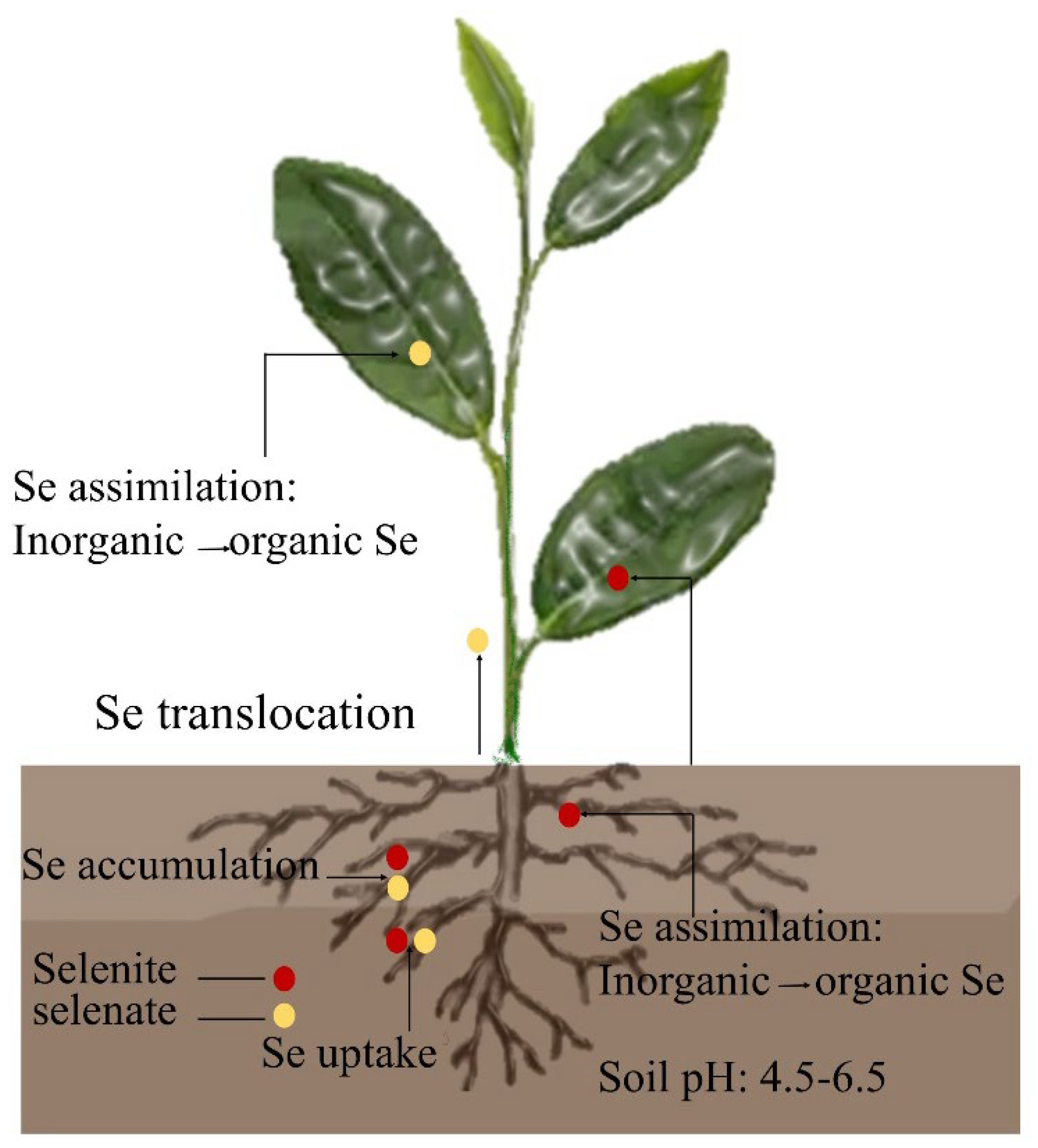
3. Effect of Selenium on Tea Plant Growth
3.1. Effect of Selenium on Tea Plant Biomass
The health functions and pharmacological mechanisms of tea are constantly being explored and utilized by modern science. Tea has become the world’s largest cultivated and most widely consumed beverage crop to improve tea production and promote the early sprouting and expansion of tea buds; thus, the spring tea early market can effectively improve the economic benefits of the market. Investigation shows that exogenous application of Se can significantly increase the yield of tea leaves and promote early germination of tea plants [18]. Huang et al. showed that foliar application of Se could effectively increase the 100-bud weight (weight of one 100 growing buds) of tea trees, and the 100-bud weight gradually increased with the spraying dosage when the concentration of exogenous Se was controlled at 30 and 50 mg/kg, and gradually decreased with the spraying dosage at 100 mg/kg. This result indicates that the tea yield can be significantly increased under the appropriate concentration of Se treatment, but the concentration of Se is excessively high to reduce the 100-bud weight of tea plants [19]. Wu et al. used the tea variety “Baiye No. 1” as the test material for foliar spraying of nano-Se and found that the yield of fresh tea leaves was significantly improved (Table 1). In particular, when the foliar spraying of nano-Se was applied at a concentration of 13.5 g/hm2, the photosynthetic performance and yield of tea trees could be significantly improved. This result could be attributed to the fact that the net photosynthetic rate, transpiration rate, and stomatal conductance of tea trees were enhanced, and the intercellular CO2 con-centration was reduced at this Se concentration, resulting in the greatest increase in photosynthetic performance [20]. Xu et al. also found that Se-containing biologics at a concentration of 100 mg/L and at a rate of 50 g/ha significantly promoted earlier germination of tea plants in early spring and increased the yield of high-grade (Table 1), high-quality tea leaves by a factor of two in early spring [21]. Se treatment not only increases the net photosynthetic rate of tea leaves under low-temperature stress to stabilize plant photosynthesis and membrane systems but also improves the cold tolerance of tea plants. Compared to the CK treatment, the Fv/Fm value of tea leaves increased by 10.63%, and the photochemical quenching value increased by 39.45% under 2 mg/mL exogenous selenium treatment [22]. Therefore, the appropriate Se concentration not only enables tea trees to germinate earlier but also significantly increases the biomass of tea trees and improves tea production.| Tea Species | Se Source | Dose | Type of Treatment | Se Content (DW) | Increased Nutrient Content | References |
|---|---|---|---|---|---|---|
| Early Spring Green Tea | selenite and selenate fertilizer | 60 mg /L | Field foliar spraying | 7.5–10.6 mg/kg | Amino acid; vitamin C FW | [19] |
| Wu Niuzao | organic Se | 100 mg/kg | Field foliar spraying | 4.72 mg/kg | Organic selenium; polyphenol; caffeine DW | [20] |
| Baiye No.1 | Nano-Se | 13.5 g/hm2 | Field foliar spraying | NA | Significant increase in chlorophyll content FW | [21] |
| Early Summer Green Tea | Se-enriched fertilizer | 100 mg/kg | Field foliar spraying | 5.895 mg/kg | Vitamin C FW; tea polyphenol DW | [22] |
| Guilv No.1 | sodium selenite | 100 mg/L | Field foliar spraying | 15.88 mg/kg | Organic selenium; Zn, K, Fe, Ca, and Mg DW | [23] |
| Qiancha 601 | sodium selenate | 0.3 mg/L | hydroponics | ≥0.25 mg/kg | Chlorophyll FW; tea polyphenol DW | [24] |
| Zhongcha 108 | Nano-Se | 10 mg/L | Field foliar spraying | 1–1.5 mg/kg | Tea polyphenol; flavonoids; caffeine DW; amino acid chlorophyll FW | [25] |
| Tea No. 12 | organic Se | 750–2100 g/hm2 | Field foliar spraying | 0.344–1.111 mg/kg | Tea polyphenol; caffeine DW | [26] |
3.2. Effect of Selenium on the Uptake of Mineral Elements by Tea Plant
Tea leaves are rich in a variety of mineral elements, and they have not only an important role in the growth and development of tea trees but are also an important expression of the nutritional value of tea. Based on relevant studies, the exogenous application of Se can have different effects on the uptake of mineral elements in tea plants. Foliar spraying of low concentrations (5.0~50.0 mg/L) of exogenous Se (sodium selenite and sodium selenate) can increase the Zn, K, Ca, and Mg content of tea leaves to some extent, and 50.0 mg/L Se treatment has a significant effect on the Fe content of leaves (Table 1) [27]. In the case of Se deficiency, the fluorine (F) content in tea leaves and roots increased significantly with the increasing exogenous Se concentration. In the case of Se sufficiency, the Fe, Ca, and Al content in tea leaves increased, the Se and Mg content in leaves and roots increased significantly, and the total F, water-soluble F, and malondialdehyde (MDA) content decreased significantly [23][28][29]. There are few reports on the effect of selenium on the absorption of mineral elements in tea plants. The above studies showed that exogenous application of selenium could increase the accumulation of mineral elements such as Zn, Mg, and Fe but inhibit the absorption of F by roots. However, the absorption of other mineral elements has not been reported, so it needs to be verified by subsequent experiments.References
- Razaghi, A.; Poorebrahim, M.; Sarhan, D.; Björnstedt, M. Selenium stimulates the antitumour immunity: Insights to future research. Eur. J. Cancer 2021, 155, 256–267.
- Alcolea, V.; Pérez, S. Selenium as an interesting option for the treatment of Chagas disease: A review. Eur. J. Med. Chem. 2020, 206, 112673.
- Mendil, D.; Demirci, Z.; Uluozlu, O.D.; Tuzen, M.; Soylak, M. A new separation and preconcentration method for selenium in some foods using modified silica gel with 2,6-diamino-4-phenil-1,3,5-triazine. Food Chem. 2017, 221, 1394–1399.
- Ullah, H.; Liu, G.; Yousaf, B.; Ali, M.U.; Abbas, Q.; Munir, M.A.M.; Mian, M.M. Developmental selenium exposure and health risk in daily foodstuffs: A systematic review and meta-analysis. Ecotoxicol. Environ. Saf. 2018, 149, 291–306.
- Zhou, J.; Ho, C.T.; Long, P.; Meng, Q.; Zhang, L.; Wan, X. Preventive efficiency of green tea and its components on nonalcoholic fatty liver disease. J. Agric. Food Chem. 2019, 67, 5306–5317.
- Gao, Y.; Xu, Y.; Ruan, J.; Yin, J. Selenium affects the activity of black tea in preventing metabolic syndrome in high-fat diet-fed Sprague–Dawley rats. J. Sci. Food Agric. 2019, 100, 225–234.
- Gupta, U.C.; Winter, K.A.; McRae, K.B. Selenium enrichment of crops through foliar applications. Can. J. Soil Sci. 1988, 68, 519–526.
- Zhang, L.H.; Song, Y.; Guo, B.; Fan, Y.; Huang, X.; Mao, K.; Liang, Z.; Hu, X.; Sun, Y.; Fang, X.; et al. Benefit–risk assessment of dietary selenium and its associated metals intake in China (2017–2019): Is current selenium-rich agro-food safe enough? J. Hazard. Mater. 2020, 398, 123224.
- Cao, D.; Jin, X.; Gong, Z.; Ma, L.; Zheng, L. Advances in the mechanism of selenium uptake and tolerance in tea plants. Biotechnology. 2017, 7, 445–449.
- White, P.J. Selenium accumulation by plants. Ann. Bot. 2016, 117, 217–235.
- Ren, H.; Li, X.; Guo, L.; Wang, L.; Hao, X.; Zeng, J. Integrative transcriptome and proteome analysis reveals the absorption and metabolism of selenium in tea plants . Front. Plant Sci. 2022, 13, 848349.
- Eichert, T.; Goldbach, H.E. Equivalent pore radii of hydrophilic foliar uptake routes in stomatous and astomatous leaf surfaces--further evidence for a stomatal pathway. Physiol. Plant. 2008, 132, 491–502.
- Kaiser, H. Stomatal uptake of mineral particles from a sprayed suspension containing an organosilicone surfactant. J. Plant Nutr. Soil Sc. 2014, 177, 869–874.
- Guo, X.; Ji, Q.; Rizwan, M.; Li, H.; Li, D.; Chen, G. Effects of biochar and foliar application of selenium on the uptake and subcellular distribution of chromium in Ipomoea aquatica in chromium-polluted soils. Ecotox. Environ. Safe. 2020, 206, 111184.
- Mazej, D.; Falnoga, I.; Veber, M.; Stibilj, V. Determination of selenium species in plant leaves by HPLC–UV–HG-AFS. Talanta 2006, 68, 558–568.
- Trippe, R.C., 3rd; Pilon Smits, E.A.H. Selenium transport and metabolism in plants: Phytoremediation and biofortification implications. J. Hazard Mater. 2021, 404, 124178.
- Zhang, L.; Hu, B.; Li, W.; Che, R.; Deng, K.; Li, H.; Chu, C. OsPT2, a phosphate transporter, is involved in the active uptake of selenite in rice. New Phytol. 2014, 201, 1183–1191.
- Hu, Q.; Xu, J.; Pang, G. Effect of selenium on the yield and quality of green tea leaves harvested in early spring. Agric. Food Chem. 2003, 51, 3379–3381.
- Huang, S.; Kan, Y.; Tang, Y. Effect of bioorganic selenium on yield, quality and selenium content of green tea. J. Tea Commun. 2020, 47, 610–616.
- Wu, H.; Zhang, H.; Ren, Z.H. Effects of Foliar Application of Nano-Selenium on Photosynthetic Characteristics and Yield of Tea. Shandong Agric. Sci. 2021, 53, 64–68.
- Xu, J.; Zhu, F.S.; Yang, L.; Cheng, Y.; Hu, G.; Pan, Q. The influence of selenium on the antioxidant activity of green tea. J. Sci. Food Agric. 2003, 83, 451–455.
- Liu, K.; Li, S.; Han, J.; Zeng, X.; Ling, M.; Mao, J.; Li, Y.; Jiang, J. Effect of selenium on tea (Camellia sinensis) under low temperature: Changes in physiological and biochemical responses and quality. Environ. Exp. Bot. 2021, 188, 104475.
- Ruan, J. The Impact of pH and Calcium on the Uptake of Fluoride by Tea Plants (Camellia sinensis L.). Ann. Bot. 2004, 93, 97–105.
- Hawrylak-Nowak, B. Comparative effects of selenite and selenate on growth and selenium accumulation in lettuce plants under hydroponic conditions. Plant Growth Regul. 2013, 70, 149–157.
- Huang, Y.; Xu, J.; Hu, Q. Effect of Selenium on Preservation Quality of Green Tea during Autumn Tea-Processing Season. Food Chem. 2005, 53, 7444–7447.
- Yang, T.; Li, H.; Hu, X.; Li, J.; Hu, J.; Liu, R.; Deng, Z. Effects of fertilizing with n, p, Se, and Zn on regulating the element and functional component contents and antioxidant activity of tea leaves planted in red soil. Food Chem. 2014, 62, 3823–3830.
- Qin, Y.Y.; Wang, Y.R.; Shi, P.T. Effect of foliar selenium spray on the selenium and mineral element content of tea tree leaves. J. South. Agric. 2019, 50, 622–627.
- Cai, H.; Dong, Y.; Li, Y.; Li, D.; Peng, C.; Zhang, X.Z. Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol. Plant. 2016, 38, 371–374.
- Niu, H.; Zhan, W.K.; Xu, C.; Peng, C.; Hou, Y.; Li, R.; Hou, X.; Wan, H. Selenium treatment modulates fluoride distribution and mitigates fluoride stress in tea plant (Camellia sinensis (L.) O. Kuntze). Environ. Pollut. 2020, 267, 115603.
