1. Embryonic Stem Cells
Pluripotent ESCs forming the blastocyst inner cell mass are the predecessors of all types of cells in the body. Not surprisingly, ESCs have long telomeres and exhibit high telomerase activity
[1][2][96,112], making ESC cultures immortal. Moreover, the length of telomeres in animals cloned by SCNT is indistinguishable from the telomere length in normally born animals matched by age
[3][113]. In iPSCs obtained by transfection with genes encoding for the Yamanaka factors (Oct4, Sox2, Klf4, c-Myc), telomeres are longer than in mother somatic cells
[4][5][6][114,115,116]. Taken together, these data suggest the ability of pluripotent cells not only to maintain but also to extend the length of telomeres. Probably, at the blastocyst stage the pluripotent inner cell mass and epiblast cells restore the telomere length and, accordingly, recover their full proliferative potential. At the earlier cleavage stage of ontogenesis, the recombination-based alternative lengthening of telomeres (ALT) mechanism may be at work, as in some telomerase-negative tumors
[7][117]. Later, at the blastocyst stage, telomerase activity is greatly enhanced, and, consequently, ALT elongation is not detected in human ESC cultures
[8][118], though it occurs in mouse ESC lines
[9][119] (
Figure 1).
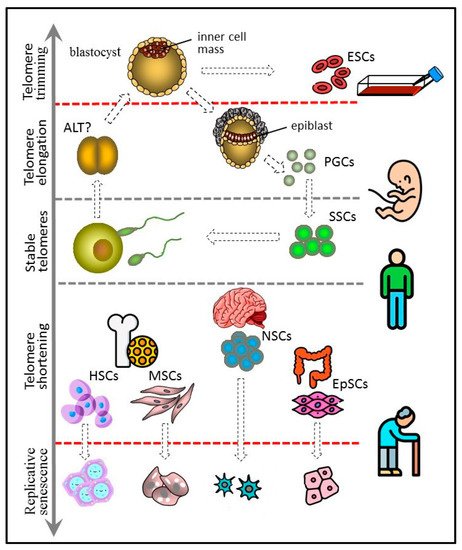
Figure 1. Telomere length dynamics in various types of stem cells. The figure 1 has been designed using icons made by Freepik and Smashicons from
Figure 1. Telomere length dynamics in various types of stem cells.
(The figure 1 has been designed using icons made by Freepik and Smashicons from
www.flaticon.com.
.)
In mouse ESCs, the ALT takes place via telomere sister chromatid exchange-dependent homologous recombination and starts with the expression of the Zscan4 gene, expressed in the two-cell embryo and ESCs
[10][120]. Zscan4 activation is responsible for the prolonged culture of telomerase-deficient late-generation murine ESCs and human ALT U2OS cancer cells with stably short telomeres
[11][121]. Expression of this gene is enhanced upon telomere shortening and is controlled by several molecular actors, including the Rif1 telomere-associated protein, which also controls the level of other two-cell embryonic-specific factors. In mouse ESCs, Rif1 upholds the H3K9me3 histone methylation at the subtelomeric zones, thus inhibiting the expression of Zscan4
[12][122]. The expression of Zscan4 is positively controlled by Dcaf11 (Ddb1- and Cul4-associated factor 11) through the ubiquitination-mediated degradation of Kap1 (KRAB-associated protein 1), resulting in the switching on of the Zscan4 downstream enhancer and the removal of H3K9me3 histone at the subtelomeric regions
[13][123]. Recently, it was confirmed that totipotent two-cell stage mouse blastomeres and murine ESCs maintain a robust transcriptional program, which includes high Zscan4 expression and ALT-like telomere extension
[14][124].
Due to high telomerase activity, ESCs need to control the upper, rather than the lower, limit of telomere length, since exceedingly long telomeres can compromise genome stability. Telomere shortening is achieved by so-called telomere trimming
[15][125]. The amount of available shelterin being constant, its concentration across the hyper-elongated telomere regions is reduced
[16][126], allowing direct binding of the telomeric zinc finger-associated protein (TZAP) to TTAGGG repeats, resulting in the removal of the telomere part corresponding to the t-loop
[17][18][127,128]. Telomere shortening initiates the extrachromosomal DNA t-circle release marking the process. Thus, the upper threshold of the telomere length, which will further determine the proliferative potential and differentiation capacity, is set up in early embryogenesis.
2. Germline Stem Cells
Since the germline cells constitute the material link between parents and progeny, maintenance of their stable telomere length across generations seems to be very important. However, the telomere length can be extended at the blastocyst, and probably also at the cleavage stage. Accordingly, even if telomeres are abnormally short in the zygote, they can regain regular length in the germline cells. In humans, primordial germ cells (PGCs) arise from the epiblast cells and initially are located within the yolk sac dorsal endoderm area
[19][129]. Though PGCs do not produce somatic cell progeny, they express Oct4 and NANOG
[20][130], suggesting that they are potentially pluripotent. Indeed, PGC-derived cell lines under specific culture conditions exhibit ESC-like differentiation properties
[21][131]. In the course of gonadal development, PGCs migrate to the genital ridge to form the indifferent gonad and then undergo male or female sex specification
[22][132].
Cells of the inner cell mass of the blastocyst can increase the length of their telomeres due to high telomerase activity. They probably control the upper limit of telomere length by trimming and can give rise to immortal cultures of embryonic stem cells (ESCs). PGCs arise from the epiblast cells and have properties similar to those of ESCs, including long telomeres and high telomerase activity. PGCs form a germline, which in adult males includes spermatogonial stem cells (SSCs). SSCs express telomerase and are characterized by either stable telomeres or weak telomere shortening that can be compensated later at the offspring blastocyst stage. Other types of adult stem cells, such as hematopoietic (HSC), mesenchymal (MSC), neural (NSC), and epithelial (EpSC), have shorter telomeres and generally low telomerase activity. They undergo replicative senescence, although more slowly than other actively proliferating somatic cells.
Male PGCs are localized in the testes, have long telomeres, high telomerase activity, and display multipotent stem cell differentiation potential
[23][133]. After passing several intermediate differentiation stages, PGCs form a population of SSCs. This cell population exhibits the features of unipotent stem cells, since it is self-sustaining and gives rise to more differentiated spermatocytes and, finally, to spermatozoa. Unlike PGCs, SSCs do not express the pluripotency markers
[20][130]. However, they continue to express telomerase
[24][134]. Mouse SSC-derived cell lines retain the ability of spermatogenesis after transplantation into the testes. High proliferative potency allows them to proliferate for more than 2 years. However, their immortality is questionable since telomere shortening takes place during this period
[25][135]. Telomere shortening is found in patients with oligospermia, a major cause of male infertility
[26][136], while normally the telomere length in spermatozoa is substantially higher than in somatic cells.
Female PGCs located in the ovaries undergo step-by-step differentiation into the oogonia. After meiosis, oogonia become oocytes. Some authors claim the existence of oogonial stem cells in the gonads of adult women
[27][137]. However, there is no hard evidence supporting this assumption
[28][138]. The telomere length of immature oocytes exceeds that of mature oocytes
[29][139]. Furthermore, telomerase activity can be detected during maturation but not in mature oocytes
[30][140].
3. Hematopoietic Stem Cells
Telomerase activity is present in the multipotent HSCs, but its level is insufficient to completely prevent telomere erosion, resulting in the shortening of blood cell telomeres throughout a lifetime
[31][141]. Telomerase activity has been found not only in HSCs but also in some of their more differentiated descendants. The ability of fast propagation of certain cell types in response to blood loss or infection is a characteristic functional feature of the hematopoietic system. To prevent crucial telomere shortening in actively proliferating cells, telomerase activation can occur in response to cytokine or antigen exposure
[32][142]. This is most clearly seen in the example of the clonal expansion of lymphocytes. Upon contact with an antigen, a naïve lymphocyte acquires antigen specificity and further acts like a typical stem cell reproducing itself and forming a clone of more differentiated effector cells in order to sustain sensibility of the immune system to the antigen. Given this, it is no surprise that telomerase is active in both naïve and activated lymphocytes
[33][143]. During aging, lymphocyte production is impaired because of the accumulation of SASP cells in the bone marrow stroma forming the HSC niche
[34][144]. Because of that, aging causes a shift in HSC differentiation towards the myeloid lineage
[35][145]. The impairment of the HSCs’ ability to support the telomere length leads to the development of pathological states, such as aplastic anemia, specific lymphopenias, or even total bone marrow failure
[36][37][146,147].
4. Mesenchymal Stem Cells
Mesenchymal stem cells (MSCs) are multipotent cells with a fibroblast-like morphology that are capable of differentiation in at least three directions: osteogenic, chondrogenic, and adipogenic
[38][148]. Originally, MSCs were isolated from bone marrow but were later found in other organs and tissues, including fat, dental pulp, endometrium, umbilical cord Wharton’s jelly, and others
[39][149]. The efficacy of osteogenic differentiation of MSCs isolated from adults is diminished compared to MSCs isolated from children
[40][150], suggesting that MSCs undergo aging in vivo. The proliferative potential of MSCs in culture is limited and close to the margin determined by Hayflick
[41][151], and telomere shortening is due to cellular divisions rather than other age-related processes
[42][152]. Telomerase activity in MSCs is low or altogether absent
[43][44][45][108,153,154], and its varying level may be due to the heterogeneity of MSC populations
[46][155]. In culture, MSCs gradually lose multipotency and homing ability but retain competence for adipogenic differentiation
[47][156]. TERT overexpression makes MSCs immortal, sustains their differentiation and immunomodulation properties
[48][157], and promotes tolerance to oxidative stress
[49][158]. This provides favorable opportunities for telomerized MSC applications in regenerative medicine.
5. Neural Stem Cells
NSCs are oligopotent cells able to differentiate into neurons and glial cells, astrocytes, and oligodendrocytes. Like MSCs, transplanted NSCs exert therapeutic effects, especially in central nervous system disorders
[50][159]. However, their applications are more restricted, since it is practically impossible to isolate them from adult donors, while the use of fetal NSCs is limited by ethical reasons and fears of teratomas.
Telomere shortening can interfere with the neuronal differentiation of NSCs
[51][160]. TRF2, an essential component of the shelterin complex, slows down NSC differentiation by suppressing the transcriptional regulator REST in the self-renewing NSC subpopulation. The inhibition of REST induces down-regulation of a number of neuron-specific genes. NSC entry into differentiation is induced in the presence of the truncated isoform of TRF2 located in the cytoplasm and lacking the suppressive activity
[52][161]. Remarkably, TRF2 disfunction and related genome instability, including chromosome fusion, has no impact on the functional activity of the affected terminally differentiated neurons in vivo
[53][162]. Embryonic NSCs have high levels of telomerase expression
[54][163]. The enzyme makes an important contribution to neurogenesis by enhancing the efficacy of neural differentiation
[55][164]. The noncanonical telomerase function, unrelated to the support of the telomere length, also has an important impact, for instance, in spatial memory formation
[56][165]. After the differentiation process has been initiated, the telomerase interferes even more with further differentiation at the progenitor cell stage, rather than helps it
[57][166]. Notably, TERT has been detected in the cytoplasm of the mature neurons in the adult human hippocampus
[58][167]. Probably, this kind of ectopic TERT expression protects neurons from apoptosis, as it happens in embryonic neurogenesis
[59][168].
In the adult brain, NSCs are located in the neurogenic niches of the subgranular zone of the hippocampus dentate gyrus and the subventricular zone of the lateral ventricles. These niches exhibit telomerase activity but much less pronounced than in the embryonic NSCs
[60][169]. In the rat brain, new neurons are produced constantly
[61][170], and there is much evidence in favor of ongoing neurogenesis in the adult human brain
[62][63][171,172]. However, recent studies have demonstrated that in the human hippocampus neurogenesis drops sharply over time, and in adulthood the production of new neurons is hard to detect
[64][173]. The rate of the telomere attrition in the brain is slower than in other tissues, probably because of sluggish proliferation
[65][174]. At the same time, many brain disorders, including cognitive disfunction, dementia, schizophrenia, and autism, are associated with critically short telomeres
[66][175].
6. Tissue-Specific Epithelial Stem Cells
Epithelial cells are located at the border of tissues and the environment and are constantly exposed to negative external influences. Therefore, the presence of epithelial stem cells (EpSCs) possessing proliferative potential, ensuring rapid renewal and repair of the epithelium, is a prerequisite of its normal functioning. Usually, EpSCs have longer telomeres compared to other cells of the same tissue, and this can be applied to the search for tissue stem cell niches
[67][176].
Normal aging is associated with skin atrophy and the loss of hair follicles because of epidermal stem cell dysfunction and telomere shortening
[68][177]. Stem cells causing the renewal of skin epidermis persist in the stratum basale during a lifetime. They slowly proliferate and produce more actively proliferating stem cells, which in turn give rise to rapidly dividing progenitor cells, finally differentiating into keratinocytes
[69][178]. EpSCs express the stem cell hallmarks, including genes regulating the telomerase activity
[70][179]. Interestingly, TERT can directly activate quiescent hair follicle stem cells via its noncanonical activity in the absence of TERC
[71][180]. Nevertheless, patients with dyskeratosis congenita, who have shortened telomeres because of a TERC mutation, are subject to skin atrophy and premature hair graying
[72][181]. Their keratinocytes are characterized by a reduced potential for proliferation and colony formation in vitro
[73][182]. In contrast, in patients with Werner’s syndrome (mutation of the WRN helicase gene) displaying analogous symptoms, keratinocytes have normal proliferative potential, while the in vitro proliferation potential of their skin fibroblasts is reduced
[74][183]. The poor functioning of EpSCs in patients with progeroid disorders, unrelated directly to the mutations in genes encoding for the telomerase complex factors, may be associated with the failure of mesenchymal cells to adequately support epidermal cell growth
[75][184]. A closer insight into the molecular mechanisms of the dermal epithelium stem cell suppression caused by the telomere dysfunction revealed the upregulation of the Follistatin protein, a Smad signaling inhibitor which blocks skin stem cells, including hair follicle stem cell differentiation, via the BMP regulatory pathway
[76][185].
Quiescent and actively proliferating stem cells were identified in the intestine
[77][78][186,187]. They are located in the basal part of crypts and, as differentiation progresses, migrate towards the villi, where they give rise to enteroendocrine cells, tuft cells, goblet cells, M cells, and Paneth cells
[79][188]. Thus, unlike skin unipotent EpSCs, intestinal stem cells are oligopotent and can differentiate into different cell types present within the tissue. Intestinal epithelium is the fastest self-renewing tissue with a cell turnover time of about 4–5 days
[80][189]. Not surprisingly, intestinal crypts, which home the stem cell niches, display telomerase activity
[81][190]. It was reported that active telomerase is confined to the slowly proliferating stem cells, which undergo asymmetric divisions reproducing themselves and yielding Lrg-positive stem cells lacking telomerase activity
[77][186]. The Lrg-positive stem cells divide symmetrically and, while moving apically towards the villi, generate a progenitor cell population. These data contest the opinion that more actively proliferating cells, including the Lrg-positive intestinal stem cells, have higher levels of telomerase expression. However, during the mouse lifespan an intestinal stem cell and its progeny must go through 700–1000 divisions, suggesting crucial telomere shortening in the absence of telomerase activity in actively dividing cells
[82][191]. It should be taken into account that in intestinal epithelial cells the telomere attrition is dependent upon intratissue and external factors specific to the intestine. In this organ, stem cells are in complex interactions with the mesenchymal and immune cells controlling local inflammatory reactions
[83][192] as well as with the microbiota acting through ROS production or otherwise
[84][193].
Aging of the regional stem cells and associated telomere shortening are involved in the pathogenesis of a number of lung diseases, including idiopathic pulmonary fibrosis, chronic obstructive pulmonary disease, SARS-CoV-2 infection, and lung cancer
[85][194]. Lung tissue contains several types of poorly characterized EpSCs, located mainly in the distal parts of the bronchi
[86][87][195,196]. Unlike them, type 2 alveolar epithelial cells (AEC2s) reside within the alveolar epithelium and have been studied much better because they are the prime target of the SARS-CoV-2 virus. AEC2s constitute 15% of the alveolar epithelium and are capable of self-renewal. Type 1 alveolar epithelial cells (AEC1s), the cell type predominant in the alveolar epithelium, are the product of proliferation and the consecutive terminal differentiation of AEC2s. It has been suggested that AEC2 aging leads not only to impaired AEC1 replenishment but also to aberrant transdifferentiation and pulmonary fibrosis
[88][89][197,198]. Interestingly, in addition to AEC2 depletion, the SARS-CoV-2 virus can impair alveolar regeneration by the induction of AEC2 proliferative aging
[90][199]. The molecular mechanism underlying AEC2 aging induction involves the TGF-β signaling pathway inducing suppression of the TERT expression, as well as the shelterin TPP1 degradation-mediated DNA damage response. TPP1 degradation can be associated with different environmental stress factors, including smoking, bacterial toxins, and ionizing radiation
[88][197].
The possible role of the regional EpSCs in the initiation of solid tumors is one of the significant problems related to their aging
[91][200]. These long-living cells have the proliferative potential and activity required to accumulate oncogenic mutation in numbers sufficient for malignant transformation. Proliferative senescence and telomere erosion can lead to genomic instability, substantially enhancing mutagenesis. The ensuing cancer stem cells can undergo asymmetrical divisions, supporting a pool of tumor cells highly resistant to therapy and capable of initiating de novo tumor growth.