Time-lapse microscopy imaging is a key approach for an increasing number of biological and biomedical studies to observe the dynamic behavior of cells over time which helps quantify important data, such as the number of cells and their sizes, shapes, and dynamic interactions across time. Label-free imaging is an essential strategy for such studies as it ensures that native cell behavior remains uninfluenced by the recording process. Computer vision and machine/deep learning approaches have made significant progress in this area.
- label-free microscopy
- cell segmentation
- cell classification
- cell event detection
- cell tracking
1. Introduction
2. Cell Segmentation
Image segmentation is the main task for producing numerical data from live-cell imaging experiments, thus providing direct insight into the living system from the quantitative cell information [23,24][19][20]. Cell segmentation is the process of splitting a microscopy image into “segments”, i.e., Regions Of Interest (ROIs), and produces an image where cells are separated from the image background by cell contours or cell labels. Accurate cell segmentations are crucial for many challenges involved in cellular analysis, including but not limited to cell tracking [25][21], cellular features quantification, proliferation, morphology, migration, interactions, and counting [26,27,28,29][22][23][24][25]. Several steps are considered in the literature for achieving cell segmentation. Some authors [8,20,30][8][26][27] consider crucial to initially perform an image reconstruction step, which produces images with higher contrast between foreground and background, increasing the success of the subsequent image processing tasks. The image formation model in phase contrast microscopy can be studied to reduce artefacts and solve the inverse problem through a regularization approach [8]. Some ML-based methods are reviewed by Vicar et al. [20][26] for PhC and DIC images, while De Haan et al. [30][27] present an overview of how DL-based frameworks solve these inverse problems in optical microscopy. Cell detection (or identification) is also frequently adopted previous to segmentation [20[26][28],22], with the aim of locating the cells in the image (e.g., via bounding boxes, as exemplified in Figure 2a).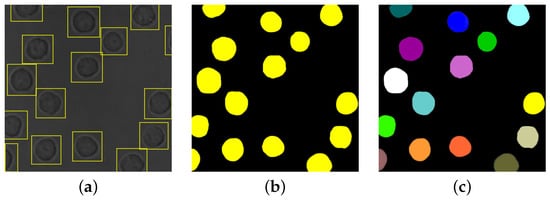
3. Event Detection and Classification
Even though segmentation remains the core of the subsequent imaging tasks, automated analysis of microscopy image sequences often bypasses the segmentation task. Usually, the detection of cell events under investigation is performed directly from heuristically generated ROIs. Detecting changes in cellular behavior plays a central role in different studies, where the focus is on identifying the changes in cellular growth, mitosis, and death. Such changes may be related to cell shape, division, and movement. They cannot be detected in a single image but require the analysis of video or time-lapse sequences. The difficulty primarily relies on the wide spatial-temporal variability of such phenomena, which requires suitable methods to handle time-varying phenomena. Nevertheless, the infinite spectrum of possible events faces an inherent shortage of labeled data. Automatic and robust approaches to detecting the time and location of cell events from image sequences often make use of the classification task. In microscopy, classification refers to identifying and distinguishing different cell types or states. Classification between other cells, types of tumors (benign or malignant), types of cell states (mitosis detection, alive-dead classification), and types of CIC (Cell-In-Cell) structures [47][33] are some typical applications.4. Cell Tracking
Object tracking consists in locating and monitoring one or more objects of interest and their behavior over time [21][34]. The image sequence containing cells can be acquired at specific time intervals using the time-lapse technique. When discussing cell tracking, it is generally assumed that segmentation or detection and classification have been performed.5. Software
In Table 1, we provide links to existing publicly available software.
6. Data
In Tables 2, 3, and 4, we provide details of available annotated data for cell segmentation, event detection, and tracking in traditional label-free images.
References
- Sebestyén, E.; Marullo, F.; Lucini, F.; Petrini, C.; Bianchi, A.; Valsoni, S.; Olivieri, I.; Antonelli, L.; Gregoretti, F.; Oliva, G.; et al. SAMMY-seq reveals early alteration of heterochromatin and deregulation of bivalent genes in Hutchinson-Gilford Progeria Syndrome. Nat. Commun. 2020, 11, 1–16.
- Marullo, F.; Cesarini, E.; Antonelli, L.; Gregoretti, F.; Oliva, G.; Lanzuolo, C. Nucleoplasmic Lamin A/C and Polycomb group of proteins: An evolutionarily conserved interplay. Nucleus 2016, 7, 103–111.
- Song, L.; Hennink, E.; Young, I.; Tanke, H. Photobleaching kinetics of fluoresce in quantitative fluorescence microscopy. Biophys J. 1995, 68, 2588–2600.
- Mir, M.; Bhaduri, B.; Wang, R.; Zhu, R.; Popescu, G. Quantitative Phase Imaging. Prog. Opt. 2012, 57, 133–217.
- Zernike, F. How I Discovered Phase Contrast. Science 1955, 121, 345–349.
- Nomarski, G. Differential microinterferometer with polarized waves. J. Phys. Radium Paris 1955, 16, 9S.
- Hoffman, R.; Gross, L. Modulation Contrast Microscope. Appl. Opt. 1975, 14, 1169–1176.
- Yin, Z.; Kanade, T.; Chen, M. Understanding the phase contrast optics to restore artifact-free microscopy images for segmentation. Med. Image Anal. 2012, 16, 1047–1062.
- Gregoretti, F.; Lucini, F.; Cesarini, E.; Oliva, G.; Lanzuolo, C.; Antonelli, L. Segmentation, 3D reconstruction and analysis of PcG proteins in fluorescence microscopy images in different cell culture conditions. In Methods in Molecular Biology; Springer: New York, NY, USA, 2022.
- Popescu, G. Quantitative Phase Imaging of Cells and Tissues; Mc-Graw-Hill: New York, NY, USA, 2011.
- Helgadottir, S.; Midtvedt, B.; Pineda, J.; Sabirsh, A.; Adiels, C.B.; Romeo, S.; Midtvedt, D.; Volpe, G. Extracting quantitative biological information from bright-field cell images using deep learning. Biophys. Rev. 2021, 2, 031401.
- Buggenthin, F.; Marr, C.; Schwarzfischer, M.; Hoppe, P.S.; Hilsenbeck, O.; Schroeder, T.; Theis, F.J. An automatic method for robust and fast cell detection in bright field images from high-throughput microscopy. BMC Bioinform. 2013, 14, 297.
- Selinummi, J.; Ruusuvuori, P.; Podolsky, I.; Ozinsky, A.; Gold, E.; Yli-Harja, O.; Aderem, A.; Shmulevich, I. Bright Field Microscopy as an Alternative to Whole Cell Fluorescence in Automated Analysis of Macrophage Images. PLoS ONE 2009, 4, 1–9.
- Naso, F.D.; Sterbini, V.; Crecca, E.; Asteriti, I.A.; Russo, A.D.; Giubettini, M.; Cundari, E.; Lindon, C.; Rosa, A.; Guarguaglini, G. Excess TPX2 interferes with microtubule disassembly and nuclei reformation at mitotic exit. Cells 2020, 9, 374.
- Jiang, Q.; Sudalagunta, P.; Meads, M.B.; Ahmed, K.T.; Rutkowski, T.; Shain, K.; Silva, A.S.; Zhang, W. An Advanced Framework for Time-lapse Microscopy Image Analysis. bioRxiv 2020.
- Caldon, C.E.; Burgess, A. Label free, quantitative single-cell fate tracking of time-lapse movies. MethodsX 2019, 6, 2468–2475.
- Janiesch, C.; Zschech, P.; Heinrich, K. Machine learning and deep learning. Electron. Mark. 2021, 31, 685–695.
- Gupta, A.; Harrison, P.J.; Wieslander, H.; Pielawski, N.; Kartasalo, K.; Partel, G.; Solorzano, L.; Suveer, A.; Klemm, A.H.; Spjuth, O.; et al. Deep Learning in Image Cytometry: A Review. Cytometry Part A 2019, 95, 366–380.
- Van Valen, D.A.; Kudo, T.; Lane, K.M.; Macklin, D.N.; Quach, N.T.; DeFelice, M.M.; Maayan, I.; Tanouchi, Y.; Ashley, E.A.; Covert, M.W. Deep Learning Automates the Quantitative Analysis of Individual Cells in Live-Cell Imaging Experiments. PLoS Comput. Biol. 2016, 12, e1005177.
- Lux, F.; Matula, P. Cell segmentation by combining marker-controlled watershed and deep learning. arXiv 2020, arXiv:2004.01607.
- Hilsenbeck, O.; Schwarzfischer, M.; Loeffler, D.; Dimopoulos, S.; Hastreiter, S.; Marr, C.; Theis, F.J.; Schroeder, T. fastER: A user-friendly tool for ultrafast and robust cell segmentation in large-scale microscopy. Bioinformatics 2017, 33, 2020–2028.
- Edlund, C.; Jackson, T.R.; Khalid, N.; Bevan, N.; Dale, T.; Dengel, A.; Ahmed, S.; Trygg, J.; Sjögren, R. LIVECell: A large-scale dataset for label-free live cell segmentation. Nat. Methods 2021, 18, 1038–1045.
- Caicedo, J.; Goodman, A.; Karhohs, K.; Cimini, B.; Ackerman, J.; Haghighi, M.; Heng, C.; Becker, T.; Doan, M.; McQuin, C.; et al. Nucleus segmentation across imaging experiments: The 2018 Data Science Bowl. Nat. Methods 2019, 16, 1247–1253.
- Casalino, L.; D’Ambra, P.; Guarracino, M.R.; Irpino, A.; Maddalena, L.; Maiorano, F.; Minchiotti, G.; Jorge Patriarca, E. Image Analysis and Classification for High-Throughput Screening of Embryonic Stem Cells. In Proceedings of the Mathematical Models in Biology: Bringing Mathematics to Life; Zazzu, V., Ferraro, M.B., Guarracino, M.R., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 17–31.
- Casalino, L.; Guarracino, M.R.; Maddalena, L. Imaging for High-Throughput Screening of Pluripotent Stem Cells, SIAM Conference on Imaging Science—IS18. 2018. Available online: https://www.siam-is18.dm.unibo.it/presentations/811.html (accessed on 3 August 2022).
- Vicar, T.; Balvan, J.; Jaros, J.; Jug, F.; Kolar, R.; Masarik, M.; Gumulec, J. Cell segmentation methods for label-free contrast microscopy: Review and comprehensive comparison. BMC Bioinform. 2019, 20, 1–25.
- de Haan, K.; Rivenson, Y.; Wu, Y.; Ozcan, A. Deep-Learning-Based Image Reconstruction and Enhancement in Optical Microscopy. Proc. IEEE 2020, 108, 30–50.
- Ulman, V.; Maška, M.; Magnusson, K.E.G.; Ronneberger, O.; Haubold, C.; Harder, N.; Matula, P.; Matula, P.; Svoboda, D.; Radojevic, M.; et al. An objective comparison of cell-tracking algorithms. Nat. Methods 2017, 14, 1141–1152.
- Gregoretti, F.; Cesarini, E.; Lanzuolo, C.; Oliva, G.; Antonelli, L. An Automatic Segmentation Method Combining an Active Contour Model and a Classification Technique for Detecting Polycomb-group Proteinsin High-Throughput Microscopy Images. In Polycomb Group Proteins: Methods and Protocols; Lanzuolo, C., Bodega, B., Eds.; Springer New York: New York, NY, USA, 2016; pp. 181–197.
- Yi, J.; Wu, P.; Jiang, M.; Huang, Q.; Hoeppner, D.J.; Metaxas, D.N. Attentive neural cell instance segmentation. Med. Image Anal. 2019, 55, 228–240.
- Gregoretti, F.; Cortesi, A.; Oliva, G.; Bodega, B.; Antonelli, L. An Algorithm for the Analysis of the 3D Spatial Organization of the Genome. In Capturing Chromosome Conformation: Methods and Protocols; Bodega, B., Lanzuolo, C., Eds.; Springer US: New York, NY, USA, 2021; pp. 299–320.
- Antonelli, L.; De Simone, V.; di Serafino, D. A view of computational models for image segmentation. In Annali dell’Universitá di Ferrara; Springer: Cham, Switzerland, 2022.
- Borensztejn, K.; Tyrna, P.; Gaweł, A.M.; Dziuba, I.; Wojcik, C.; Bialy, L.P.; Mlynarczuk-Bialy, I. Classification of Cell-in-Cell Structures: Different Phenomena with Similar Appearance. Cells 2021, 10, 2569.
- Emami, N.; Sedaei, Z.; Ferdousi, R. Computerized cell tracking: Current methods, tools and challenges. Visual Inform. 2021, 5, 1–13.
