Alkali metals (lithium, sodium, and potassium) are promising as anodes in emerging rechargeable batteries, ascribed to their high capacity or abundance. They are currently inhibited from public use by issues with interfacial reactivity, volume change, and dendrite growth. Surface coatings are among the most promising of techniques to address all of these issues. Atomic and molecular layer deposition (ALD and MLD) enable a variety of inorganic, organic, and even inorganic-organic hybrid materials, featuring accurate nanoscale controllability, low process temperature, and extremely uniform and conformal coverage. Coatings applied to alkali metals via ALD and MLD may offer some of the most scalable, tailorable, and effective surface coatings for safe and stable rechargeable anodes.
1. Introduction
Since commercialized in 1991 by Sony, lithium-ion batteries (LIBs) have dominated portable electronic devices and have even started to penetrate the market of electric vehicles
[1]. With their continuous development, LIBs now are approaching their theoretical energy limits
[2][3][4][5][6][2,3,4,5,6]. Thus, it is urgent to develop new battery technologies and materials for further boosting energy density, extending lifetime, improving safety, and reducing cost
[7][8][7,8].
In pursuing next-generation battery technologies with higher energy density, alkali metals are promising as anodes, due to their high capacity or abundance. Specifically, lithium (Li), sodium (Na), and potassium (K) have capacities of 3861, 1166, and 685 mAh/g, respectively. In addition, their potentials are −3.04, −2.73, and −2.936 V versus the standard hydrogen electrode (SHE), respectively. Li metal enables the highest theoretical specific capacity and the lowest electrochemical potential
[9][10][11][12][13][9,10,11,12,13]. Thus, Li metal has been considered as a “holy grail” for emerging lithium metal batteries (LMBs), such as lithium-sulfur (Li-S) and lithium-oxygen (Li-O
2) batteries
[11][14][15][11,14,15]. In comparison, Na metal is abundant and cost-effective. It can be used to constitute a variety of sodium metal batteries (NMBs), such as Na-S
[16], Na-O
2 [17], and Na-CO
2 batteries
[18][19][18,19]. K metal can enable a capacity about two times that of graphite anodes in state-of-the-art LIBs. Despite their great potentials as anodes in alkali metal batteries (AMBs), they are plagued by stability and safety issues, mainly due to two reasons: unstable solid electrolyte interphase (SEI) and dendrite growth
[20]. The ionically conductive but electronically insulating SEI layer was first coined by Pealed et al. in 1979
[21][22][21,22]. It originates from the spontaneous reactions between alkali metal anodes and organic liquid electrolytes
[23]. The SEI layer is generally non-uniform and heterogeneous in composition and property
[24]. During a plating process, furthermore, the generated alkali metal dendrites can easily break the fragile and soft SEI, exposing new fresh alkali metal to the electrolyte and thereby leading to more SEI formation
[25]. In the subsequent stripping process, the gracile alkali dendrites could be trapped and electrically isolated within the SEI layer. This results in dead alkali metals
[26][27][28][26,27,28]. As the plating/stripping processes repeat, electrolyte and active alkali metals are continuously consumed. At the same time, cell impedance increases with inferior Coulombic efficiency (CE)
[9][29][9,29]. Particularly, the propagation of alkali metal dendrites can penetrate the cell’s separator, lead to electrical short of cells, and cause battery thermal runaway, fires, and even explosions, as illustrated in
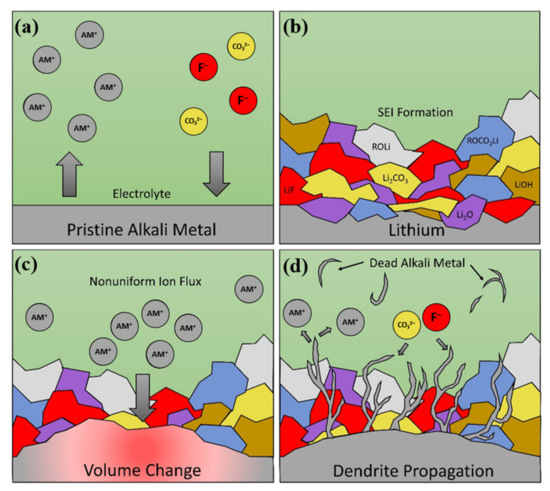
Scheme 1 [10][13][30] Scheme 1 [10,13,30].
Scheme 1. Alkali metal cell failure with respect to (a) Initial ion exchange between electrode and electrolyte, (b) Formation of mosaic SEI exemplified with Li metal (R indicates hydrocarbon groups), (c) Heterogeneous ion flux during cycling causing volume change and internal stress, and (d) Stress relief through dendrite propagation, dead active metal, and new SEI layer growth leading to continued consumption of electrolyte and active material.
To address these issues of AMBs, many strategies have been explored for Li
[31][32][31,32], Na
[33], and K
[34] metals, including solid-state electrolytes
[35][36][37][38][39][40][35,36,37,38,39,40], new electrolytes and additives
[41][42][43][41,42,43], ionic liquids
[44][45][46][44,45,46], and nanostructured electrode designs
[47][48][47,48]. Some comprehensive reviews of these techniques have been documented in literature
[33][49][50][51][52][53][33,49,50,51,52,53]. Recently, atomic and molecular layer deposition (ALD and MLD) have emerged as two tremendously powerful techniques for surface engineering of alkali metal anodes, featuring high accuracy over material growth, low process temperature, and extremely uniform and conformal coverage over substrates of any shape
[8][54][55][8,54,55]. Ascribed to their unique growth mechanisms, ALD and MLD could constitute the most intimate integration between the resultant coating and the substrate, thereby minimizing interfacial issues due to imperfect contacts. Thus, they remain as one of the most promising techniques for addressing the issues of alkali metal anodes. The effectiveness of ALD and MLD coatings depends on their resulting properties (e.g., electrical conductivity, ionic conductivity, and mechanical properties).
2. Mechanisms and Characteristics of ALD and MLD
Since its introduction in the 1970s
[56][57][58,59], ALD has now recognized as a powerful technique for surface and interface engineering in energy-related devices, as is well documented in literature
[2][8][54][57][58][59][60][61][62][63][64][65][2,8,54,59,60,61,62,63,64,65,66,67]. One of the most successful and prevalent ALD processes is growing Al
2O
3 films with the precursors of trimethylaluminum (TMA) and H
2O. Two half surface reactions are proceeded as follows
[63][66][65,68].
where “|” indicates the substrate surface, “-” indicates a chemical bond, and “↑” denotes the gas phase of the precursors (i.e., TMA and water) and the byproduct (i.e., CH
4). Typically, a four-step procedure is required to finish one ALD cycle: (i) pulsing the first precursor of TMA to react with the surface -OH functional groups and thereby produce a new layer of -Al(CH
3)
2 bounded to oxygen, releasing CH
4 as the byproduct, as shown in Equation (1); (ii) purging with an inert gas (e.g., Ar) to remove the oversupplied precursor TMA and the resultant byproduct CH
4; (iii) pulsing the second precursor H
2O to restore the substrate surface back to a full coverage of -OH with the release of CH
4 as the byproduct, as given in Equation (2); and (iv) purging with the inert gas to remove the oversupplied H
2O and the byproduct CH
4. A visual representation of this reaction and its analogous inorganic-organic hybrid aluminum ethylene glycol (AlEG) is presented in
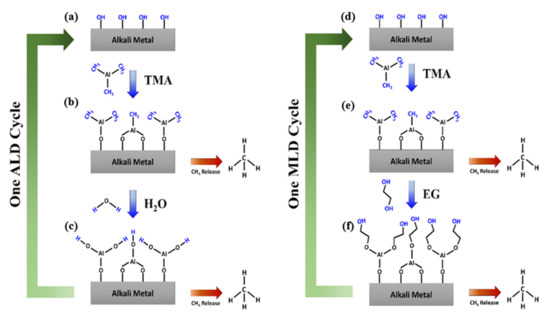
Scheme 2 Scheme 2. These two half surface reactions (i.e., Equations (1) and (2)) are self-limiting or self-terminating due to the finite amount of surface functional groups (e.g., -OH and -CH
3). The Al
2O
3 film thickness usually can be precisely controlled at the atomic level
[63][67][65,69], e.g., a growth per cycle (GPC) of ~1 Å
[68][70]. More details about the chemistry of ALD can be found in some previous excellent reviews
[69][70][71][72][71,72,73,74]. ALD is exclusively for growing inorganic thin films ranging from binary
[72][73][74][75][74,75,76,77], ternary
[76][78], and even more complex compounds
[77][78][79][80][79,80,81,82] MLD shares a highly similar working principle as that of ALD. It has been treated as a sister technique of ALD and is specifically used for depositing organic and inorganic-organic hybrid materials
[55]. MLD was first reported by Yoshimura et al. in 1991
[81][83] for growing a polyimide film. Thereafter, a variety of new polymeric films were reported, as recently reviewed by Meng
[54][63][54,65]. Compared to inorganic coatings, polymeric films have benefits in their flexibility and elasticity due to their long chains of primary covalent bonds and secondary van der Waals bonds. In many cases, these reactions proceed without the need for nucleation enhancers, though organometallic compounds have also been used to improve reaction spontaneity and reduce nucleation delays
[82][84]. ALD and MLD both recently have received increasing attention for their applications in new battery systems due to their distinguished capabilities for novel materials, and for the advancement of alkali metal anodes, as reviewed in this report.
Scheme 2. Illustration of one Al2O3 ALD growth cycle and one MLD AlEG growth cycle, featuring (a) Pristine alkali metal surface terminated with hydroxyl groups, (b) Surface reaction between hydroxyl termination and TMA molecule with CH4 byproduct release, and (c) Surface reaction between methyl termination and H2O molecule with CH4 byproduct release, (d) Pristine alkali metal surface terminated with hydroxyl groups, (e) Surface reaction between hydroxyl termination and TMA molecule with CH4 byproduct release, (f) Surface reaction between methyl termination and EG molecule with CH4 byproduct release. Surface termination groups that serve as reaction sites for the next step are colored in blue.