Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Catherine Yang and Version 3 by Catherine Yang.
Fatty acid binding proteins (FABPs) are proteins found in the cytosol that contribute to disorders related to the cardiovascular system, including atherosclerosis and metabolic syndrome. Functionally, FABPs serve as intracellular lipid chaperones, interacting with hydrophobic ligands and mediating their transportation to sites of lipid metabolism.
- fatty acid binding protein
- FABP3
- cardiovascular diseases
1. Introduction
Cardiovascular disease (CVD) is a major contributor to mortality rates globally, leading to ~17.5 million deaths annually [1]. Atherosclerosis, endothelial dysfunction, and dyslipidemia remain significant drivers of CVD [2]. Clinically, complications of blood vessels leading to coronary, cerebrovascular, and peripheral artery disease (PAD) account for most CVD-related morbidity and mortality [3].
PAD is a devastating CVD that involves lower extremity arterial atherosclerosis, affecting over 200,000,000 patients globally [4]. Although this condition significantly increases the risk of amputation and death, PAD remains poorly diagnosed and treated [5]. Compared to patients with coronary artery disease (CAD), PAD patients generally have an inferior prognosis over the long term [6]. One reason is the lack of a biomarker for PAD diagnosis and prognosis, as there is no PAD equivalent for cardiac troponin, a widely used biomarker in CAD [7]. Consequently, PAD patients are often misdiagnosed and receive delayed care [8].
Fatty acid binding proteins (FABP) are intracellular proteins responsible for the transportation of lipids [9]. To date, researchers have identified nine unique isoforms of this protein that share the mechanism of interacting with lipid ligands and escorting them to metabolic sites [9]. The unique function of each isoform remains an area of heavy investigation [10]. Nevertheless, the role of FABPs in lipid processing within cells involved in atherosclerosis, including adipocytes, macrophages, and endothelial cells, suggests their importance in CVD development [11]. FABP3 has been shown to be associated with an assortment of CVDs.
2. Fatty Acid Binding Proteins
FABPs are 12–15 kDa cytosolic proteins primarily found in tissues with high levels of lipid metabolism, such as the heart, skeletal muscle, and liver [12]. They are also found in cell types specialized for lipid storage, transportation, and signaling, such as macrophages and adipocytes [12]. FABPs have been demonstrated to be important in various metabolic and cardiovascular disorders, including atherosclerosis, obesity, and hyperglycemia [12]. All FABP members share a β-barrel signature containing a cavity filled with water and a lipid ligand binding site [12]. FABP 1–9 are nine members of the FABP3 family identified to date, each with 20–70% sequence homology [13]. Members are named based on the tissue that most abundantly expresses the protein [13]. As an example, FABP3 is also known as heart-type FABP as it is found primarily in cardiomyocytes, while FABP4/5 is expressed most prominently in adipocytes and epidermal cells [13]. Functionally, FABPs serve as lipid chaperones by reversibly interacting with hydrophobic ligands and mediating their escort to sites of lipid metabolism [14]. Target sites include the plasma membrane (lipid transport), mitochondria (lipid metabolism), and endoplasmic reticulum (lipid biosynthesis) [14]. Although lipid signaling is related to FABP expression, the specific function of each member of the FABP family is poorly understood [9]. This is due to the fact that their mechanisms relate to many complex regulatory pathways [9].3. FABP3
FABP3 is most abundantly expressed in skeletal and heart muscle [15]. As a chaperone for lipids, FABP3 is critical for maintaining the homeostatic function of skeletal and cardiac muscle [15]. Seventy percent of muscle cell energy is derived from fatty acid oxidation in mitochondria, which requires lipid transportation [16]. Studies of FABP3-deficient mice show compromised uptake of fatty acids, reduced capacity for exercise, and rapid glucose consumption, ultimately leading to cardiac dysfunction [17]. FABP3 has been demonstrated to be a biomarker for heart injury, particularly myocardial infarction (MI) [18]. During cardiomyocyte injury, cellular components are broken down and released into the circulation [19]. Myocardial protein leakage serves as a biomarker of pathology that can be measured to allow early detection of cardiac injury [20]. Currently, troponin is a widely used biomarker for MI, which becomes elevated 2–4 h after the development of chest pain [21]. In contrast, serum FABP3 levels begin to rise 30 min after chest pain onset and peak in a few hours [22]. Early FABP3 release from injured myocardium has been demonstrated in animal models and humans [23][24]. Therefore, FABP3 has the potential to be an important biomarker for coronary artery disease [25]. Of recent interest, FABP3 has been linked to atherosclerosis in other blood vessels owing to its contribution to endothelial dysfunction [26]. Recently, the research group has conducted several studies demonstrating the utility of FABP3 as a biomarker for PAD [27][28][29]. PAD patients essentially have an acquired myopathy secondary to repetitive tissue ischemia and reperfusion [30]. This ultimately leads to abnormal changes in mitochondrial enzyme expression, increased oxidative stress, and apoptosis [30]. This process can contribute to the release of FABP3 into the circulation and urine.4. Pathophysiological Underpinnings of the Relationship between FABP3 Levels and PAD
While the exact pathophysiology delineating the relationship between FABP3 levels and PAD is yet to be understood, plausible insights, explanations, and hypotheses could be generated from reviewing the literature. Patients experiencing myopathies have been shown to release FABP3 after incurring injury to the skeletal muscle (exercise-induced or otherwise) [31]. PAD can be considered a form of myopathy following skeletal muscle injury, primarily due to tissue ischemia and reperfusion [32]. These often translate to detrimental changes in mitochondrial oxidative stress, enzymes, and respiration, ultimately leading to muscle apoptosis [30]. As a byproduct of these cellular processes and changes, FABP3 is potentially released into the circulation [33]. Furthermore, the extent of skeletal muscle injury could result in more/less release of FABP3 into circulation—potentially explaining the researchers' observed finding of a relationship between increased PAD severity and elevated FABP3 levels [34]. This could be a plausible mechanism explaining the pathophysiology of the findings but requires further investigation and confirmation. Figure 1 highlights the clinical workflow for the potential use of FABP3 as a diagnostic and prognostic biomarker for PAD.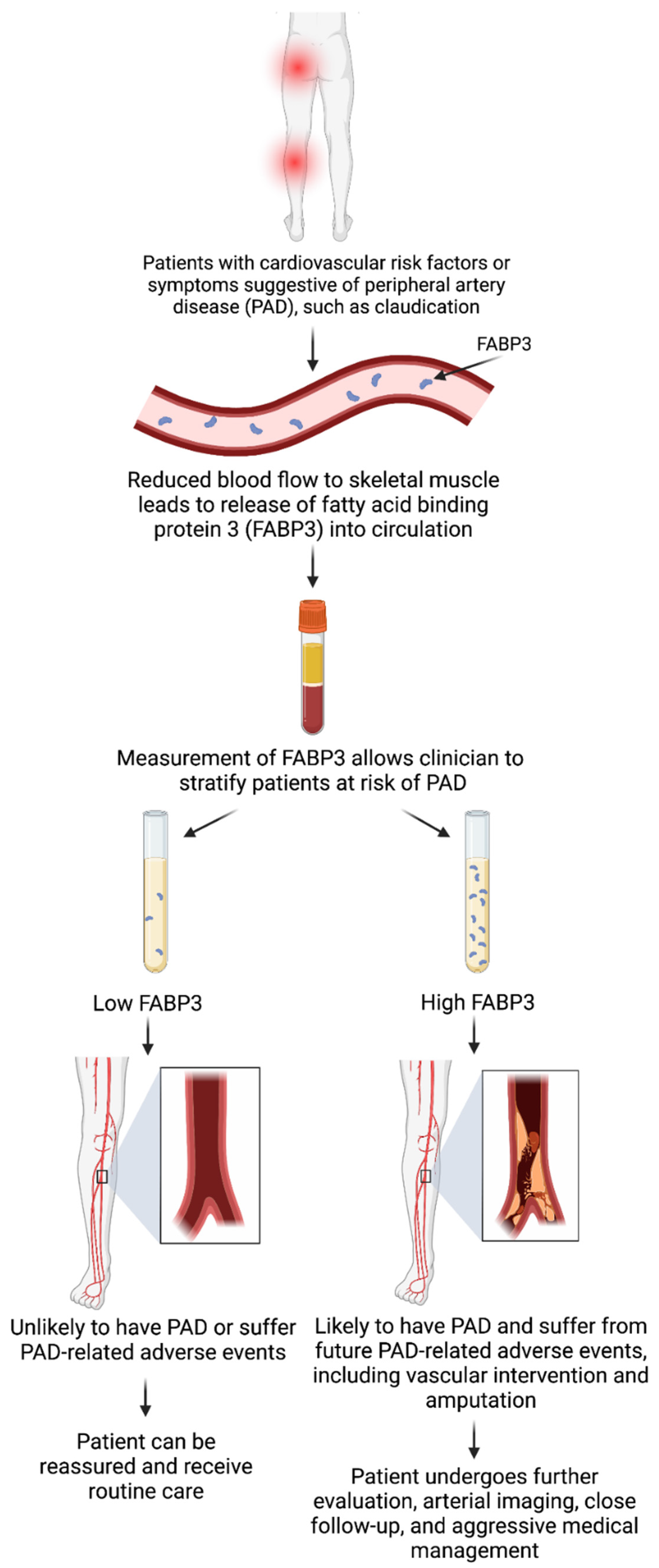
Figure 1. Clinical workflow for the use of fatty acid binding protein 3 (FABP3) as a diagnostic and prognostic biomarker for peripheral artery disease (PAD). Created using BioRender with permission.
56. FABP3 as a Biomarker for Various Disease States
A growing body of literature suggests that FABP3 may be used as a biomarker for other disease states. For instance, Tanaka and colleagues showed an elevation of uFABP3 in patients with MI [35]. Other groups, including Nayashida et al., studied the impact of kidney function on uFABP3 levels in patients undergoing coronary artery bypass grafting and showed that uFABP3 was a marker for myocardial injury [36]. The literature suggests that following injury to the myocardium, kidney filtration of FABP3 can be impaired from acute kidney injury due to reduced creatinine clearance, resulting in lower levels of uFABP3 [37]. Therefore, urinary FABPs may also help in expediting the diagnosis of acute renal failure [38][39]. Most recently, it was demonstrated that uFABP3 is associated not only with PAD diagnosis but major adverse limb events related to PAD [29]. This demonstrates that uFABP3 has both diagnostic and prognostic value in identifying PAD patients with a high risk of complications and who may require more intensive medical and surgical therapy [29].
References
- Shi, A.; Tao, Z.; Wei, P.; Zhao, J. Epidemiological Aspects of Heart Diseases. Exp. Ther. Med. 2016, 12, 1645–1650.
- Shimokawa, H. Primary Endothelial Dysfunction: Atherosclerosis. J. Mol. Cell Cardiol. 1999, 31, 23–37.
- Shah, A.M.; Banerjee, T.; Mukherjee, D. Coronary, Peripheral and Cerebrovascular Disease: A Complex Relationship. Herz Kardiovaskuläre Erkrank. 2008, 33, 475–480.
- Zemaitis, M.R.; Boll, J.M.; Dreyer, M.A. Peripheral Arterial Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021.
- Olin, J.W.; Sealove, B.A. Peripheral Artery Disease: Current Insight into the Disease and Its Diagnosis and Management. Mayo Clin. Proc. 2010, 85, 678–692.
- Welten, G.M.J.M.; Schouten, O.; Hoeks, S.E.; Chonchol, M.; Vidakovic, R.; van Domburg, R.T.; Bax, J.J.; van Sambeek, M.R.H.M.; Poldermans, D. Long-Term Prognosis of Patients with Peripheral Arterial Disease: A Comparison in Patients with Coronary Artery Disease. J. Am. Coll. Cardiol. 2008, 51, 1588–1596.
- Cooke, J.P.; Wilson, A.M. Biomarkers of Peripheral Arterial Disease. J. Am. Coll. Cardiol. 2010, 55, 2017–2023.
- Lecouturier, J.; Scott, J.; Rousseau, N.; Stansby, G.; Sims, A.; Allen, J. Peripheral Arterial Disease Diagnosis and Management in Primary Care: A Qualitative Study. BJGP Open 2019, 3, bjgpopen19X101659.
- Furuhashi, M.; Hotamisligil, G.S. Fatty Acid-Binding Proteins: Role in Metabolic Diseases and Potential as Drug Targets. Nat. Rev. Drug Discov. 2008, 7, 489.
- Storch, J.; McDermott, L. Structural and Functional Analysis of Fatty Acid-Binding Proteins. J. Lipid Res. 2009, 50, S126–S131.
- Furuhashi, M.; Saitoh, S.; Shimamoto, K.; Miura, T. Fatty Acid-Binding Protein 4 (FABP4): Pathophysiological Insights and Potent Clinical Biomarker of Metabolic and Cardiovascular Diseases. Clin. Med. Insights Cardiol. 2015, 8, 23–33.
- Chmurzyńska, A. The Multigene Family of Fatty Acid-Binding Proteins (FABPs): Function, Structure and Polymorphism. J. Appl. Genet. 2006, 47, 39–48.
- Smathers, R.L.; Petersen, D.R. The Human Fatty Acid-Binding Protein Family: Evolutionary Divergences and Functions. Hum. Genom. 2011, 5, 170.
- Makowski, L.; Hotamisligil, G.S. Fatty Acid Binding Proteins--the Evolutionary Crossroads of Inflammatory and Metabolic Responses. J. Nutr. 2004, 134, 2464S–2468S.
- Vergnes, L.; Chin, R.; Young, S.G.; Reue, K. Heart-Type Fatty Acid-Binding Protein Is Essential for Efficient Brown Adipose Tissue Fatty Acid Oxidation and Cold Tolerance. J. Biol. Chem. 2011, 286, 380–390.
- Talley, J.T.; Mohiuddin, S.S. Biochemistry, Fatty Acid Oxidation. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022.
- Zhuang, L.; Mao, Y.; Liu, Z.; Li, C.; Jin, Q.; Lu, L.; Tao, R.; Yan, X.; Chen, K. FABP3 Deficiency Exacerbates Metabolic Derangement in Cardiac Hypertrophy and Heart Failure via PPARα Pathway. Front. Cardiovasc. Med. 2021, 8, 722908.
- Moon, M.G.; Yoon, C.H.; Lee, K.; Kang, S.H.; Youn, T.J.; Chae, I.H. Evaluation of Heart-Type Fatty Acid-Binding Protein in Early Diagnosis of Acute Myocardial Infarction. J. Korean Med. Sci. 2021, 36, e61.
- Palasubramaniam, J.; Wang, X.; Peter, K. Myocardial Infarction—From Atherosclerosis to Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2019, 39, e176–e185.
- Knowlton, A.A.; Apstein, C.S.; Saouf, R.; Brecher, P. Leakage of Heart Fatty Acid Binding Protein with Ischemia and Reperfusion in the Rat. J. Mol. Cell Cardiol. 1989, 21, 577–583.
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in Acute Myocardial Infarction: Current Perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10.
- Gururajan, P.; Gurumurthy, P.; Nayar, P.; Srinivasa Nageswara Rao, G.; Babu, S.; Cherian, K.M. Heart Fatty Acid Binding Protein (H-FABP) as a Diagnostic Biomarker in Patients with Acute Coronary Syndrome. Heart Lung Circ. 2010, 19, 660–664.
- Huang, C.; Xiao, S.; Xia, Z.; Cheng, Y.; Li, Y.; Tang, W.; Shi, B.; Qin, C.; Xu, H. The Diagnostic Value of Plasma MiRNA-497, CTnI, FABP3 and GPBB in Pediatric Sepsis Complicated with Myocardial Injury. Ther. Clin. Risk Manag. 2021, 17, 563–570.
- Rezar, R.; Jirak, P.; Gschwandtner, M.; Derler, R.; Felder, T.K.; Haslinger, M.; Kopp, K.; Seelmaier, C.; Granitz, C.; Hoppe, U.C.; et al. Heart-Type Fatty Acid-Binding Protein (H-FABP) and Its Role as a Biomarker in Heart Failure: What Do We Know So Far? J. Clin. Med. 2020, 9, 164.
- Zhuang, L.; Li, C.; Chen, Q.; Jin, Q.; Wu, L.; Lu, L.; Yan, X.; Chen, K. Fatty Acid-Binding Protein 3 Contributes to Ischemic Heart Injury by Regulating Cardiac Myocyte Apoptosis and MAPK Pathways. Am. J. Physiol. Heart Circ. Physiol. 2019, 316, H971–H984.
- Tan, L.; Lu, J.; Liu, L.; Li, L. Fatty Acid Binding Protein 3 Deficiency Limits Atherosclerosis Development via Macrophage Foam Cell Formation Inhibition. Exp. Cell Res. 2021, 407, 112768.
- Syed, M.H.; Zamzam, A.; Khan, H.; Singh, K.; Forbes, T.L.; Rotstein, O.; Abdin, R.; Eikelboom, J.; Qadura, M. Fatty Acid Binding Protein 3 Is Associated with Peripheral Arterial Disease. JVS Vasc. Sci. 2020, 1, 168–175.
- Zamzam, A.; Syed, M.H.; Harlock, J.; Eikelboom, J.; Singh, K.K.; Abdin, R.; Qadura, M. Urinary Fatty Acid Binding Protein 3 (UFABP3) Is a Potential Biomarker for Peripheral Arterial Disease. Sci. Rep. 2021, 11, 11061.
- Li, B.; Zamzam, A.; Syed, M.H.; Jahanpour, N.; Jain, S.; Abdin, R.; Qadura, M. Urinary Fatty Acid Binding Protein 3 (UFABP3) Has Diagnostic and Prognostic Value in Peripheral Artery Disease. J. Vasc. Surg. 2022, 75, e325.
- Brass, E.P.; Hiatt, W.R. Acquired Skeletal Muscle Metabolic Myopathy in Atherosclerotic Peripheral Arterial Disease. Vasc. Med. 2000, 5, 55–59.
- Sorichter, S.; Mair, J.; Koller, A.; Pelsers, M.M.; Puschendorf, B.; Glatz, J.F. Early Assessment of Exercise Induced Skeletal Muscle Injury Using Plasma Fatty Acid Binding Protein. Br. J. Sports Med. 1998, 32, 121–124.
- Burch, P.M.; Greg Hall, D.; Walker, E.G.; Bracken, W.; Giovanelli, R.; Goldstein, R.; Higgs, R.E.; King, N.M.P.; Lane, P.; Sauer, J.-M.; et al. Evaluation of the Relative Performance of Drug-Induced Skeletal Muscle Injury Biomarkers in Rats. Toxicol. Sci. 2016, 150, 247–256.
- Zhen, E.Y.; Berna, M.J.; Jin, Z.; Pritt, M.L.; Watson, D.E.; Ackermann, B.L.; Hale, J.E. Quantification of Heart Fatty Acid Binding Protein as a Biomarker for Drug-Induced Cardiac and Musculoskeletal Necroses. Proteom. Clin. Appl 2007, 1, 661–671.
- Pritt, M.L.; Hall, D.G.; Recknor, J.; Credille, K.M.; Brown, D.D.; Yumibe, N.P.; Schultze, A.E.; Watson, D.E. Fabp3 as a Biomarker of Skeletal Muscle Toxicity in the Rat: Comparison with Conventional Biomarkers. Toxicol. Sci. 2008, 103, 382–396.
- Tanaka, T.; Hirota, Y.; Sohmiya, K.-I.; Nishimura, S.; Kawamura, K. Serum and Urinary Human Heart Fatty Acid-Binding Protein in Acute Myocardial Infarction. Clin. Biochem. 1991, 24, 195–201.
- Nayashida, N.; Chihara, S.; Tayama, E.; Akasu, K.; Kai, E.; Kawara, T.; Aoyagi, S. Influence of Renal Function on Serum and Urinary Heart Fatty Acid-Binding Protein Levels. J. Cardiovasc. Surg. 2001, 42, 735–740.
- Hayashida, N.; Chihara, S.; Akasu, K.; Oda, T.; Tayama, E.; Kai, E.; Kawara, T.; Aoyagi, S. Plasma and Urinary Levels of Heart Fatty Acid-Binding Protein in Patients Undergoing Cardiac Surgery. Jpn. Circ. J. 2000, 64, 18–22.
- Noiri, E.; Doi, K.; Negishi, K.; Tanaka, T.; Hamasaki, Y.; Fujita, T.; Portilla, D.; Sugaya, T. Urinary Fatty Acid-Binding Protein 1: An Early Predictive Biomarker of Kidney Injury. Am. J. Physiol. Ren. Physiol. 2009, 296, F669–F679.
- Doi, K.; Noiri, E.; Maeda-Mamiya, R.; Ishii, T.; Negishi, K.; Hamasaki, Y.; Fujita, T.; Yahagi, N.; Koide, H.; Sugaya, T.; et al. Urinary L-Type Fatty Acid-Binding Protein as a New Biomarker of Sepsis Complicated with Acute Kidney Injury. Crit. Care Med. 2010, 38, 2037–2042.
More
