Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Vivi Li and Version 1 by Kasey Leung.
EVs are cell-derived membranous structures and are involved in many physiological processes. Naïve and engineered EVs have much therapeutic potential, but proper delivery systems are required to prevent non-specific and off-target effects. Targeted and site-specific delivery using polymeric scaffolds can address these limitations. EV delivery with scaffolds has shown improvements in tissue remodeling, wound healing, bone healing, immunomodulation, and vascular performance. Thus, EV delivery via biopolymeric scaffolds is becoming an increasingly popular approach to tissue engineering.
- mesenchymal stem cells
- extracellular vesicles
- biomaterials
- tissue repair
- scaffolds
- biopolymers
1. Introduction
The use of extracellular vesicles (EVs) in lieu of stems cells in scaffolds has become increasingly popular in recent years. Stem cells such as mesenchymal stem cells (MSCs) have immunomodulatory and differentiation effects, however, they have been found to cause abnormal differentiation and tumor formation [1]. MSCs exert their therapeutic functions via their secretome, including EVs [2]. For tissue engineering, EVs are an attractive alternative to stem cell transplantation as they have emerged as important mediators of cellular communication and can directly affect a number of biological processes in target cells [3]. Thus, regenerative research is shifting from the use of stem cells to the use of EVs.
Scaffolds serve as an approach to restore form and function to diseased, damaged, and lost tissue by acting as the ECM that supports the cells and their fate and function [4]. Various natural and synthetic biopolymers can be used to create such scaffolds. Incorporating EVs into the synthesis of scaffolds provides a system that supports host regeneration through structural and physiological means.
2. Extracellular Vesicles (EVs)
Extracellular vesicles are nanosized cell-derived membranous structures and can be categorized into several subclasses including exosomes (40–160 nm), microvesicles (150–1000 nm), and apoptotic bodies (>1000 nm) [5] (Figure 1). Exosomes are formed by the inward budding of endosomes and results in the generation of intraluminal vesicles within multivesicular bodies (MVBs) [6]. When MVBs fuse with the plasma membrane, the intraluminal vesicles are released into the extracellular space and are then referred to as exosomes [6]. It is unlikely that researchers will be able to capture live images of EV release in order to assign EVs to subclasses [7]. Thus, the authors often refer to EV subclasses based on the physical characteristics (e.g., small EVs (sEVs)), biochemical composition (e.g., Annexin A5-stained EVs), conditions (e.g., hypoxic EVs), or cell of origin (e.g., hMSC EVs) [7]. Ultimately, it is important to continue studying specific and reliable markers of EV subtypes, so that a consensus regarding nomenclature can be established.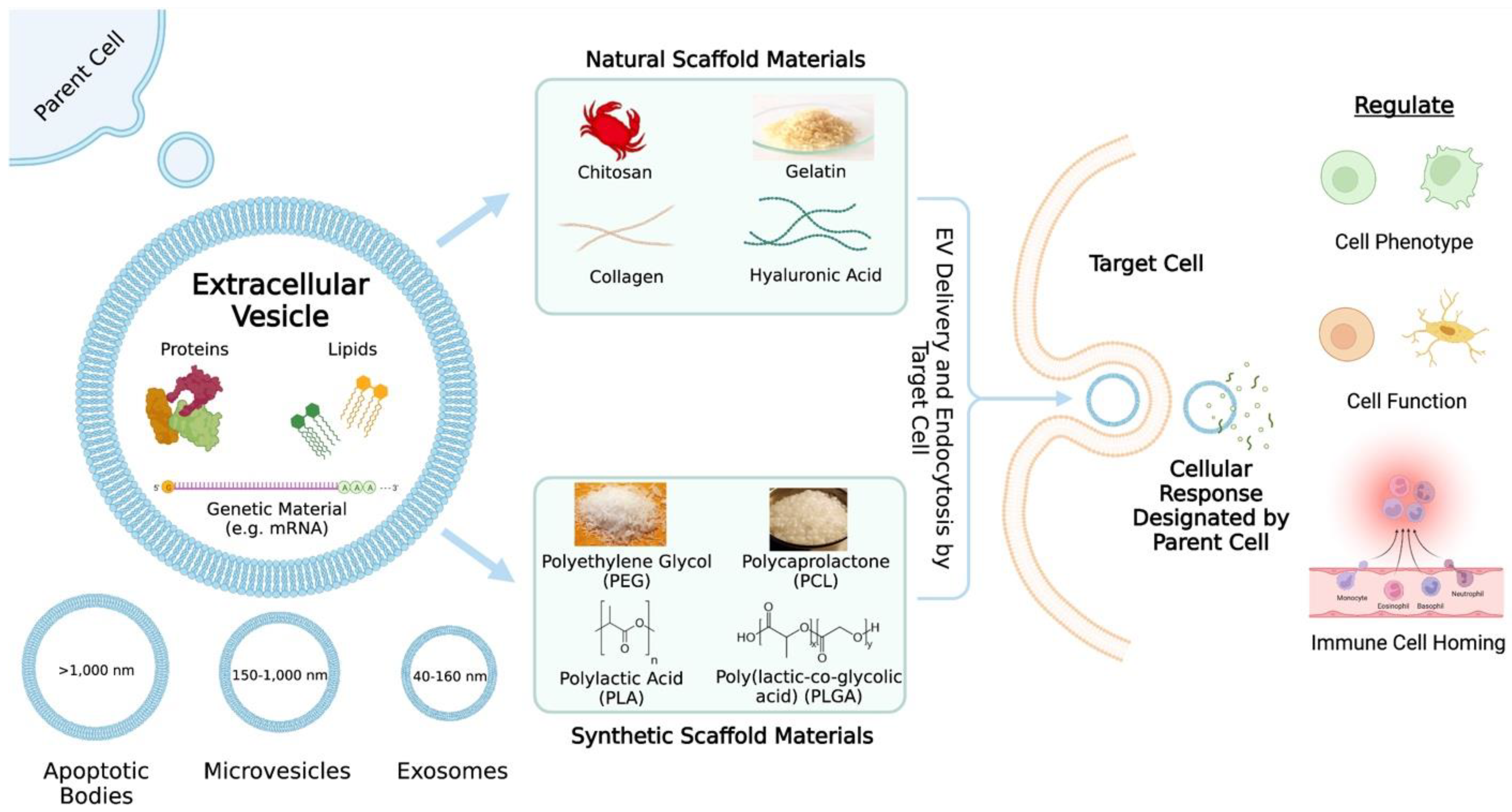
Figure 1. An overview of extracellular vesicle (EV) delivery via scaffolds. The contents and categories of the extracellular vesicles as well as what the extracellular vesicles may regulate are described. Common natural and synthetic biomaterials for scaffold fabrication are highlighted.
3. Natural Biopolymer Scaffolds for Therapeutic EV Delivery
3.1. Sodium Alginate
Sodium alginate (SA) is a linear polysaccharide derived from brown seaweed [55][8]. More specifically, SA is a derivative of alginic acid and is composed of α-1-guluornic (G) and 1,4-linked-β-D-mannuronic (M) monomers [55][8]. Alginate has been used to deliver various classes of drugs including NSAIDs [56][9], chemotherapeutics [57[10][11],58], and anesthetics [59][12]. Hormones such as insulin [60][13] and salmon calcitonin [61][14] have also been delivered via alginate. Furthermore, neuropeptides [62][15], genetic material [63][16], and probiotics [64][17] have been delivered using alginate. EV-laden alginate-based hydrogels have been studied for a wide range of therapeutic applications including diabetic wound healing [37][18], peripheral nerve regeneration [38][19], and myocardial infarction (MI) [26][20]. Lv et al. delivered bone marrow mesenchymal stem cell (MSC)-derived small EVs (sEVs) to the heart using a natural sodium alginate hydrogel as a therapy for myocardial infarction. The hydrogels were embedded with sEVs by simply mixing the sEV solution with the sodium alginate solution prior to hydrogel formation with calcium chloride solution [26][20]. Additionally, the authors found that hydrogels formed with 0.5% or 1% calcium chloride solutions resulted in nearly all of the sEVs being released by day 10 with a quick burst of sEV release during the first couple of days compared to a 2% calcium chloride solution [26][20]. Lv et al. reasoned that hydrogels with a quicker sEV release profile would better suit a myocardial infarction model [26][20]. The authors labeled sEVs and found through ex vivo imaging that sEVs embedded in the hydrogel (sEV-gel) were retained in the heart compared to freely injected sEVs [26][20]. Lv et al. examined the expression levels of miRNAs (miR) related to anti-apoptosis and pro-angiogenesis and performed TUNEL staining; they found that treatment using the sEV-gel showed decreased cardiac cell apoptosis [26][20]. Additionally, Lv et al. looked at the number of CD68+ macrophages as well as the ratio of CD206+ to CD68+ macrophages and found that the sEV-gel treatment promoted M1 to M2 macrophage polarization only a couple days after myocardial infarction [26][20]. Furthermore, Lv et al. used CD31+ staining, α-SMA staining, and Western blotting to show that sEV-gel treatment promoted angiogenesis [26][20]. Using echocardiography and histology, Lv et al. saw that the sEV-gel treatment resulted in improved cardiac function and enhanced scar thickness compared to only using sEVs [26][20]. Overall, the authors demonstrated that the sEV-gel treatment promoted angiogenesis, reduced cardiac apoptosis and fibrosis, and improved cardiac function after MI [26][20]. This sentudry highlights the importance of using a scaffold to allow for an appropriate, efficient, and locally concentrated delivery of EVs, for without some type of retention system, administered EVs will be quickly cleared by the body and will have a minimal therapeutic effect.3.2. Silk Fibroin
Silk fibroin (SF) is a hydrophobic protein from the Bombyx mori silkworm that self-assembles into strong and resilient materials [65,66][21][22]. Silk fibroin on its own is biocompatible, has controllable biodegradability, and has tunable mechanical properties [67][23]. It also causes minimal inflammation of host tissue, is low-cost, and easy to use [67][23]. Anti-proliferative [68][24], anti-inflammatory [69,70][25][26], anabolic [70][26], and anti-retroviral drugs [71][27] have been delivered using silk fibroin. Additional drugs include anti-inflammatory compounds such as curcumin [72,73][28][29] and chemotherapeutics [74,75][30][31]. Aside from drug delivery, SF has been utilized for the delivery of antibodies [76][32], proteins [76[32][33],77], hormones (e.g., insulin) [78][34], genetic material [79][35], and cells (e.g., mesenchymal stem cells) [80][36]. Cunnane et al. examined the effect of human adipose-derived mesenchymal stem cell EVs (hADMSC EVs) on vascular cells in vitro [33][37]. They found that the application of these EVs on smooth muscle cells and endothelial cells increased proliferation as well as migration in a dose-dependent manner [33][37]. Cunnane et al. then vacuum-seeded EVs into porous silk-based tubular scaffolds by turning mounted scaffolds within a vacuum chamber and infusing the scaffolds with an EV isolate. Using a micro bicinchoninic acid protein assay and fluorescent imaging, they found that this method of seeding retained a greater amount of protein and increased EV coverage, respectively, within the scaffold compared to the soak-loading method [33][37]. Cunnane et al. implanted the silk-based scaffolds into rat aortas to study the remodeling capacity of the EV-doped scaffolds. After 8 weeks, the explants were stained for cell, collagen, and elastin distribution [33][37]. Additionally, elastin and collagen content assays were performed to quantify protein deposition within each explant [33][37]. Their in vivo findings showed that the inclusion of EVs in the scaffold wall improved patency and matrix deposition, including more elastin and collagen production, which is crucial for neo-tissue formation [33][37]. This sentudry demonstrates that EVs play an effective bioinstructive role when incorporated into and delivered by SF-based vascular grafts.3.3. Chitosan
Chitosan is a cationic polysaccharide derived from chitin and is made up of diglucose amine and N-acetyl glucose amine groups [81,82,83][38][39][40]. Antibiotics [81[38][40],83], antivirals [84][41], and immunosuppressants [85][42] have been delivered using chitosan. Additionally, chitosan has been utilized to deliver insulin [82][39] and genetic material [86,87,88][43][44][45]. Chitosan-based scaffolds have been used to deliver EVs to improve bone defect repair [41][46], corneal diseases [42][47], skin wound healing [32][48], and articular cartilage injuries [43][49]. Wu et al. developed chitosan-based thermosensitive hydrogels laden with bone mesenchymal stem cell (BMSC)-derived sEVs to accelerate osteogenesis and angiogenesis. After isolating the sEVs, Wu et al. characterized the sEVs through analyzing the size distribution and morphology of the sEVs as well as through Western blotting to detect sEV-specific surface markers [41][46]. The authors also ensured that the sEVs could be internalized by BMSCs and HUVECs [41][46]. In vitro experimentation included examining the ALP activity of BMSCs when exposed to sEVs. The results indicated the early-stage osteogenic differentiation of BMSCs when exposed to sEVs, shown by elevated ALP activity in the sEV groups [41][46]. Furthermore, Wu et al. found through examining the mRNA (OCN, OPN, and Runx2) and protein (OCN, OPN, and RUNX2) levels that BMSC-sEVs can upregulate osteogenic gene expression [41][46]. The authors also studied the migratory capability of HUVECs exposed to sEVs and found that the proliferation of HUVECs exposed to sEVs increased compared to the control group [41][46]. Furthermore, mRNA and protein levels relevant to angiogenesis increased in cells exposed to sEVs [41][46]. Wu et al. added β-glycerphosphate to chitosan to formulate a thermosensitive injectable hydrogel, and they found that sEVs embedded in this hydrogel showed a good slow-release performance of 80% sEV release on day 8, with a slowed release rate thereafter [41][46]. The in vivo micro-CT results of a calvarial defect model showed that hydrogels embedded with sEVs resulted in a greater area of newly formed bone compared to other groups [41][46]. Histological staining showed that there was newly formed bone in the sEV-hydrogel and hydrogel-only groups compared to the control group, which resulted in a defect mainly filled with fibrotic connective tissue [41][46]. Immunohistochemical staining of bone defect sections indicated more CD31+ in the sEV-hydrogel group compared to the hydrogel-only group, which indicated new vessel formation within the bone defect [41][46]. Overall, the in vivo experimentation demonstrated that sEVs promote calvarial defect repair and enhanced osteogenesis and angiogenesis [41][46]. Wu et al. examined the potential cause of their observed results by studying the relationship between miR-21 and SPRY2. After performing the reporter assays and rescue experiments, they found that the angiogenic protein levels in cells transfected with miR-21 mimics were higher than the control cells [41][46]. These experiments indicated that exosomal miR-21 may promote HUVEC migration and angiogenesis by targeting SPRY2 [41][46]. Overall, Wu et al. successfully developed a thermosensitive injectable chitosan-based hydrogel laden with BMSC-derived sEVs. The hydrogel promoted bone healing and served as a scaffold for sEVs [41][46]. The sEV-loaded hydrogel promoted bone healing in vivo by enhancing angiogenesis, which may be mediated by miR-21 expression upregulation in sEVs and the regulation of SPRY2 by miR-21 [41][46]. This sentudry highlights the potential of delivering EVs via scaffolds to promote bone regeneration and the importance of understanding the mechanism behind positive results to continue improving the therapeutic outcomes.3.4. Collagen
Collagen is the most abundant protein in mammals [89][50] and is composed of three intertwined α-chains [90][51]. Some of the functions of collagen include cell adhesion and migration, tissue repair, and scaffolding [91][52]. Collagen has proven to be useful in medical applications as a delivery tool. Similar to the natural biopolymers discussed thus far, collagen has been utilized to deliver drugs [92[53][54],93], cells [94,95][55][56], and bioactive substances with antioxidant properties [96][57]. It is also possible to deliver growth factors using collagen [97,98][58][59]. Collagen-based scaffolds have been utilized for therapeutic purposes including bone regeneration [39][60] and endometrium regeneration [40][61]. Xin et al. designed a collagen scaffold containing umbilical cord-derived mesenchymal stem cell (UC-MSC)-derived exosomes for endometrial regeneration in a rat endometrium-damage model [40][61]. The authors confirmed successful exosome extraction through TEM, NTA, and Western blot [40][61]. Xin et al. added exosome suspension dropwise to the collagen scaffold, and they obtained a sustained release profile with a majority of exosomes being released within 14 days [40][61]. Xin et al. examined short-term and long-term outcomes from a rat endometrium-damage model involving exosome-collagen, collagen-only, exosome-only, and no treatment (control) groups [40][61]. Through H&E staining, Xin et al. found that transplantation of the exosome-collagen scaffold promoted endometrium regeneration and glandular reconstruction, which was related to rapid cell proliferation and re-epithelialization [40][61]. Additionally, Xin et al. performed immunostaining and Masson’s trichrome staining and found that transplantation of the exosome-collagen resulted in excellent neovascularization, reduced fibrosis formation, and promoted collagen remodeling [40][61]. Through immunohistochemical staining and subsequent software analysis, Xin et al. found that a high number of anti-estrogen receptor α (ERα) positive cells and anti-progesterone receptor (PR) positive cells were present 30 days after exosome-collagen treatment, which suggests the rapid functional recovery of the regenerated endometrium [40][61]. Furthermore, Xin et al. showed through Evans blue staining that the implantation of the exosome-collagen scaffold resulted in the structural and functional reconstruction of endometrium that could support implantation and the development of embryos in vivo [40][61]. Xin et al. investigated the potential mechanisms behind the promising outcomes observed with exosome-collagen treatment. The authors found that exosome-collagen treatment promoted the macrophage infiltration within 7 days, with high numbers of M1 macrophages in the exosome-collagen and collagen-only groups within 3 days, likely due to the immunogenicity of the implanted collagen scaffold [40][61]. Additionally, the exosome-collagen group showed the highest numbers of M2 macrophages within 7 days, indicating that exosome incorporation may have induced the polarization of macrophages to the M2 phenotype [40][61]. Furthermore, Xin et al. examined the M1 and M2 macrophage-related cytokines. When looking at the M1-related cytokines, they found that the inclusion of exosomes in the scaffold eased inflammation due to the foreign body reaction to collagen within 7 days [40][61]. Furthermore, there was an enhanced expression of M2-related cytokines with exosome-collagen transplantation. Altogether, transplantation of the exosome-collagen scaffold promoted M1 macrophage infiltration during the early stages of wound healing and induced macrophage transition from an M1 to a M2 phenotype during a later stage of healing [40][61]. Through analyzing their RNA-Seq data against a publicly-available database, Xin et al. identified a top candidate related to macrophage immunomodulation (miR-223-3p), which has been reported to promote macrophage polarization to an M2 phenotype, as the key cargo within exosomes [40][61]. Xin et al. believe that miR-223-3p may target Stmn1 within macrophages to confer functional benefits [40][61]. Overall, Xin et al. developed a local exosome chitosan-based delivery system that could promote endometrium regeneration and fertility restoration [40][61]. They found that this system involved a mechanism-of-action related to M2 macrophage polarization with miRNA-223-3p serving as a top key component within exosomes [40][61].3.5. Hyaluronic Acid
Hyaluronic acid (HA) is a linear polysaccharide with repeating units of D-glucuronic acid and N-acetyl-D-glucosamine [90][51]. HA is characterized by a strong water binding ability and is found in the extracellular matrix [90][51]. HA has been used to deliver anti-bacterial drugs [99][62], immunosuppressants [100][63], anabolic and anti-inflammatory compounds [70,101,102,103,104][26][64][65][66][67]. The delivery of chemotherapeutics using hyaluronic acid have been well-studied [105,106,107,108][68][69][70][71]. Examples of these delivered therapeutics include doxorubicin [109[72][73][74][75],110,111,112], cisplatin [113][76], and cantharidin [114][77]. Additionally, anti-oxidants [115][78], flavonols [116][79], anti-photoaging agents [117][80], and peptides [118][81] have been delivered using HA. Furthermore, HA has been used to deliver cells (including cell secretome) [119[82][83],120], genetic material [112][75], growth factors [102[65][84],121], and hormones [122][85]. Hyaluronic acid-based scaffolds have been used to deliver EVs as a therapy for tendon repair [45][86] and osteoarthritis cartilage injuries [46][87]. K. Song et al. isolated exosomes from tendon derived stem cells (TDSC-Exos), loaded a hyaluronic acid scaffold with the exosomes, and studied the therapeutic effects of this system for tendon repair. The authors examined exosome size distribution, morphology, and the presence of exosome-related markers (CD9, CD63, CD81, and TSG101) using Western blot [45][86]. They also used the protein concentration of exosomes as a representation of exosome concentration throughout the study [45][86]. The authors then studied the effects of TDSC-Exos on the proliferation and function of tenocytes in vitro and found that high concentrations of exosomes (100 μg/mL) could protect tenocytes from oxidative stress and serum deprivation [45][86]. Additionally, the treatment of tenocytes with TDSC-Exos increased type 1 collagen production and elevated tendon-specific marker (Scx, Col1a1, and Dcn) expression [45][86]. The authors then produced a scaffold by irradiation, containing a uniform exosome distribution that could serve as a sustained exosome-release system (50% of exosomes retained after 14 days) [45][86]. Song et al. then created a rat tendon defect model and used the hyaluronic scaffold (pHA) or the hyaluronic scaffold containing exosomes (pHA-TDSC-Exos) to fill the gap in the patellar tendons [45][86]. Not only did they see decreased wound visibility with the pHA-TDSC-Exos group, but the authors found through H&E staining that the wound healing outcomes of this group were significantly better than the pHA and control groups and could enhance tendon repair in this model [45][86]. Masson trichrome staining showed better collagen fiber arrangement with the pHA-TDSC-Exos group, and immunohistochemistry was used to show that this group promoted the early repair of the injured tendon with earlier type III collagen presence and reduction during the tendon healing process compared to the other groups [45][86]. Furthermore, pHA-TDSC-Exos facilitated the restoration of the biomechanical properties of the injured tendon [45][86]. With increasing evidence of the important roles miRNAs play during tissue repair, Song et al. performed RNA sequencing to find miRNAs that were expressed significantly higher in TDSC-Exos compared to tenocytes and chose to focus on miR-144-3p [45][86]. Song et al. showed that miR-144-3p enhanced cell proliferation and migration. They found that miR-144-3p from TDSC-Exos played an important role in tendon repair by targeting ARID1A in tenocytes [45][86]. ARID1A is a key component of the switch/sucrose non-fermentable ATP-dependent chromatin-remodeling complex, which plays a critical role in cell cycle modulation [45][86]. MiR-144-3p is only one potential mechanism by which TDSC-Exos may exert therapeutic effects [45][86]. Overall, Song et al. showed that TDSC-derived exosomes could promote tendon repair and that miR-144-3p transferred from these exosomes enhanced tenocyte proliferation and migration by targeting ARID1A.3.6. Gelatin
Gelatin is a widely used natural biopolymer in regenerative medicine and tissue engineering [123][88]. Similar to the natural biopolymers discussed above, gelatin has been utilized to deliver various types of drugs including anti-fungal/anti-yeast drugs [124][89], anti-inflammatory agents [125,126,127][90][91][92], antibiotics [128,129[93][94][95],130], and chemotherapeutics [131,132,133,134][96][97][98][99]. Other factors such as probiotics [135][100], vitamins [136][101], peptides [137][102], and ions [138,139][103][104] have been delivered using gelatin. Furthermore, researchers have used gelatin to deliver cells [140[105][106][107],141,142], signaling molecules [143[108][109],144], and genetic material [145,146][110][111]. Gelatin-based scaffolds have been utilized for bone regenerative purposes [44][112]. Man et al. epigenetically enhanced osteoblast-derived EVs with the histone deacetylase inhibitor Trichostatin A (TSA) to promote EV osteoinductive potency [44][112]. In this study, Man et al. used gelatin methacryloyl (GelMA) functionalized with synthetic nanoclay laponite (LAP) (GelMA-LAP), which binds, stabilizes, and improves biofactor retention [44][112]. They characterized these enhanced EVs (TSA-EVs) using TEM imaging, nano-flow cytometry, single-particle phenotyping, measuring EV protein content, and EV release kinetics from the GelMA-LAP hydrogel [44][112]. The authors also found that the TSA-EVs released from the GelMA-LAP hydrogel were internalized by hBMSCs, and these EVs promoted hBMSC proliferation and migration [44][112]. Furthermore, the authors found that these released TSA-EVs enhanced histone acetylation and mineralization of hBMSCs [44][112]. Man et al. went further to evaluate the effects of TSA-EVs on hBMSC extracellular matrix mineralization within the GelMA-LAP hydrogels by assessing the ALP activity, collagen production, and calcium deposition [44][112]. There was enhanced ALP activity in the hBMSCs within the TSA-EV hydrogels compared to the untreated osteoblast-EVs (MO-EV) and EV-free groups [44][112]. hBMSC collagen production within the hydrogel was evaluated through picrosirius red staining, and TSA-EV treatment resulted in the greatest collagen content with a high dosage of TSA-EVs (50 µg/mL) (TSA-EV-50), resulting in the greatest collagen production [44][112]. Furthermore, TSA-EV-50 gels resulted in greater calcium deposition compared to other groups, as found through alizarin red staining [44][112]. Man et al. believe that the osteoinductive effect of TSA-EVs within the GelMA-LAP hydrogel is due to the 3D matrix (rather than a 2D matrix). More specifically, the 3D matrix elicits an altered cellular response to chemical and physical stimulation, so hBMSCs within the GelMA-LAP hydrogels may be more receptive to osteoinductive stimulation, which is induced by TSA-EVs compared to hBMSCs in 2D culture [44][112]. Additionally, the 3D microenvironment of the GelMA-LAP hydrogel may have altered the epigenetic landscape of encapsulated hBMSCs, which may have primed the cells with enhanced differentiation capacity compared to the 2D cultured cells [44][112]. Finally, the 3D microenvironment of the hydrogel and ECM produced by the hBMSCs likely influenced the sequestering of bioactive factors (e.g., EVs) within the secretome, which would further facilitate mineralization within the GelMA-LAP hydrogel [44][112]. Altogether, these findings demonstrate the improved therapeutic potential of epigenetically enhanced EVs delivered via a gelatin-based scaffold for bone regeneration.4. Synthetic Biopolymer Scaffolds for Therapeutic EV Delivery
4.1. Polyethylene Glycol (PEG)
PEG is an FDA-approved, hydrophilic, and flexible polymer that has been proven to be safe for use in biomedical applications [150][113]. PEG has been used to deliver chemotherapeutics [150[113][114][115][116][117][118][119][120][121][122],151,152,153,154,155,156,157,158,159], anti-inflammatory drugs [160][123], antibiotics [161[124][125],162], and bisphosphonates [163][126]. Additionally, vitamins [164][127], phenols [165][128], and hormones [166][129] have been delivered using PEG. Furthermore, PEG has been utilized for cell [167][130], signaling molecule [143][108], and genetic material delivery [168,169,170][131][132][133]. Exosomes have been delivered via PEG hydrogels for cutaneous wound healing [47,171][134][135]. Additionally, macrophages adopt polarization states in response to the local microenvironment [47][134]. Based on Kwak et al.’s miRNA-sequencing data, exosomes derived from M1 macrophages (M1-Exos) and M2 macrophages (M2-Exos) contain proteins and miRNAs that are capable of shifting macrophage polarity [47][134]. Thus, Kwak et al. utilized M2-Exos to induce the reprogramming of nearby proinflammatory M1 macrophages toward an anti-inflammatory M2 phenotype [47][134]. They then encapsulated the M2-Exos in hydrolytically degradable PEG hydrogels (M2-Exogel) and found that the degradation time was adjustable from 6 to 27 days through controlling the crosslinking density and tightness [47][134]. Kwak et al. used a full-thickness excisional wound model to assess the therapeutic effects of the M2-Exogel in vivo. They treated the wounds with saline, hydrogel alone, free exosomes, or M2-Exogel. Using immunohistochemistry and cytokine expression analyses, Kwak et al. showed the successful local transition of M1 macrophages to M2 macrophages within the lesion for more than 6 days [47][134]. The M2-Exogel served as a long-term supply of the critical concentration of exosomes needed to initiate and sustain the reprogramming of M1 to M2 macrophages, ultimately contributing to improved wound healing [47][134]. Kwak et al. found that localizing M2-Exos in the hydrogel led to rapid wound closure and increased healing quality compared to other groups [47][134]. More specifically, the wound size significantly decreased after day 8 in the M2-Exogel group compared to other groups [47][134]. Furthermore, they stained closed wound tissues with Masson’s trichrome and saw that the M2-Exogels produced superior results in the stable closure of full-thickness skin wounds as well as enhanced the dermal adipogenesis and hair follicle regeneration compared to freely injected exosomes [47][134]. Altogether, the PEG hydrogel-based exosome delivery system serves as a method to locally regulate the polarization state of macrophages, which is critical for tissue homeostasis and proper wound repair [47][134].4.2. Polycaprolactone (PCL)
PCL is a linear, hydrophobic, aliphatic polyester with high mechanical strength and is biocompatible as well as biodegradable [172,173][136][137]. Chemotherapeutics [154[117][120][122][138][139][140][141][142][143],157,159,174,175,176,177,178,179], antimicrobials [162[125][144][145],180,181], and anti-inflammatory drugs [160,182][123][146] have been delivered using PCL. Furthermore, PCL has been used for the delivery of hypotensive agents [183][147], protease inhibitors [184][148], anticonvulsants [172][136], and sulfonylureas [185][149]. In addition to drug delivery, PCL has been used to deliver polyphenols [186][150], antiretrovirals, and hormones [187][151]. Similar to the previously discussed biopolymers, the delivery of genetic material [169,188[132][152][153],189], growth factors [190][154], and cells [191][155] using PCL has been studied. Synthetic biopolymer-based scaffolds containing EVs have been used to improve vascular performance and functionality (Table 1). Wei et al. were interested in using heparin-functionalized vascular PCL grafts to enhance anti-thrombogenicity. The authors fabricated the tubular PCL grafts using electrospinning, modified the grafts with heparin, and loaded MSC-derived small EVs (MSC-sEVs) by soaking the scaffolds in sEV solution [49][156]. The authors observed a uniform distribution of sEVs on the grafts using a confocal laser scanning microscope [49][156]. Wei et al. examined the in vivo stability of the grafts by in vivo imaging using labeled sEVs and found that the bioluminescence intensities were higher in the heparinized scaffold group [49][156]. The authors then studied the performance of the PCL vascular grafts modified with heparin and loaded with sEVs in a hyperlipidemia rat model [49][156]. sEVs play many roles in this model. Wei et al. found that heparin enhanced anti-thrombogenicity while the addition of immunomodulatory sEVs inhibited thrombosis and calcification, which therefore improved the patency of the graft [49][156]. The patency rate was measured using color doppler ultrasound, and H&E and von Kossa staining was used for calcification detection [49][156]. Additionally, bioactive molecules (e.g., VEGF and miRNA 126) from the MSC-sEVs enhanced endothelium and vascular smooth muscle regeneration, shown through H&E staining and immunofluorescence staining with the CD31 antibody, α-SMA antibody, and myosin heavy chain [49][156]. Furthermore, flow cytometric analysis revealed that sEVs induced polarization from pro-inflammatory and atherogenic M1 macrophages to anti-inflammatory and anti-osteogenic M2c macrophages [49][156]. This sentudry further highlights how synthetic biomaterials can be enhanced using EVs to create translational scaffolds for regenerative medicine.4.3. Poly(Lactic-Co-Glycolic Acid) (PLGA)
PLGA is a copolymer that is similar to PCL in that it is a biocompatible, biodegradable, and flexible biopolymer [192,193][157][158]. The delivery of antibiotics [194,195,196,197,198][159][160][161][162][163] and chemotherapeutics [199,200,201,202,203,204][164][165][166][167][168][169] using PLGA has been well-studied. Dopamine agonists [205][170], anticonvulsants [206][171], statins [207][172], and immunosuppressants [208][173] have also been delivered using PLGA. Furthermore, growth factors [209][174], various proteins [210[175][176][177],211,212], hormones [213,214][178][179], genetic material [215][180], and vaccines [216][181] have been delivered using PLGA. PLGA scaffolds containing EVs have been studied to improve bone defects [52][182] and chronic kidney disease [51][183]. Ko et al. designed a PLGA-based scaffold to deliver stem cell-derived EVs for kidney regeneration. The composite scaffold was composed of PLGA, magnesium hydroxide, and decellularized porcine kidney extracellular matrix, and polydeoxyribonucleotide (PDRN) and was fabricated using ice particle leaching [51][183]. The scaffold was enhanced with EVs that were derived from TNF-α/IFN-γ-primed UC-MSCs (TI-EVs) [51][183]. Ko et al. characterized the EVs by shape and size. They then studied the effector molecules within the EVs; more specifically, Ko et al. focused on proteins within TI-EVs and unprimed EVs (UC-EVs). The results indicated that treating UC-MSCs with TNF-α/IFN-γ enhanced the cellular uptake capabilities of secreted EVs and induced changes in protein cargo, which is indicative of kidney tissue regeneration [51][183]. In a partial nephrectomy mouse model, the scaffold containing PDRN and EVs induced glomerular regeneration and the restoration of kidney function [51][183]. More specifically, the kidney developmental factors (Pax2, Wt1, and Emx2) increased in expression with PME/PDRN/TI-EV scaffold treatment [51][183]. Additionally, there was an increase in the population of Pax2-expressing host cells, which indicates that the scaffold can facilitate host renal stem/progenitor cell infiltration [51][183]. Furthermore, there was an increased expression of pro-angiogenic growth factors (FGF2, HGF, and VEGF) in the PME/PDRN/TI-EV scaffold group [51][183]. Ko et al. determined the total number of functional glomeruli using H&E staining, with the PME/PDRN/TI-EV group showing the best results [51][183]. They then examined renal function recovery by evaluating the serum creatinine and blood urea nitrogen (BUN) levels. They found significantly better metabolic function in the PME/PDRN/TI-EV group [51][183]. Furthermore, the glomerular filtration rate (GFR) was restored in the PME/PDRN/TI-EV group (227.2 μL/min) to a level similar to that of native mice (232.5 μL/min) [51][183]. These results show structural and functional kidney tissue regeneration with the use of the PME/PDRN/TI-EV scaffold. Overall, the biochemical cues from TI-EVs and PDRN as well as the biophysical cues from the PLGA scaffold serve as potential tissue engineering platforms for kidney tissue regeneration [51][183].4.4. Poly(L-Lactide) (PLLA)
PLLA degrades by nonenzymatic hydrolysis and its by-products are eliminated via normal cell metabolism [217][184]. Thus, it is biodegradable and biocompatible. The delivery of antibiotics [218,219,220,221][185][186][187][188] and chemotherapeutics [222,223,224,225,226][189][190][191][192][193] using PLLA has been frequently studied as well as anti-inflammatory drugs [227][194], anti-psychotics [228][195], acetylcholinesterase inhibitors [229][196], and ocular disease therapeutics [230,231][197][198]. In addition to drug delivery, cells [232[199][200],233], growth factors [234[201][202][203][204],235,236,237], and genetic material [238][205] have been delivered using PLLA. Furthermore, PLLA has been utilized to deliver hormones and fertilizer [239][206]. Swanson et al. engineered a biodegradable PLLA-based delivery platform to control the release of exosomes from microspheres to promote craniofacial bone healing. More specifically, they used PLGA and PEG triblock copolymer microspheres to encapsulate and control the timed release of human dental pulp stem cell (hDPSC)-derived exosomes [54][207]. This delivery platform was integrated with a 3D tissue engineered PLLA scaffold. They found that microspheres containing exosomes demonstrated a linear and consistent release profile over a longer period of time when attached to a PLLA scaffold compared to freely suspended microspheres [54][207]. They also found through NTA and TEM that the exosomes maintained their characteristic diameter and morphology throughout their encapsulation and release [54][207]. Furthermore, Swanson et al. confirmed the in vitro bioactivity of the exosome-containing microspheres by culturing mouse BMSCs on nanofibrous scaffolds functionalized with the microspheres. They used the colorimetric calcium assay to examine hydroxyapatite mineralization and energy dispersive X-ray spectroscopy (EDX) to determine the spatial distribution of elements in the construct [54][207]. It was found that microsphere-functionalized constructs and groups treated with exogenous exosomes showed an increased calcium phosphate content and a decreased proportion of organic components as minerals was deposited [54][207]. Furthermore, the authors demonstrated exosome functionality in vivo by implanting constructs in mice and subsequent staining (Masson’s Trichrome and von Kossa staining). This revealed that constructs with exosome-containing microspheres increased hECM deposition and promoted early mineralization compared to blank microsphere constructs and blank scaffolds, which attributes the function to the exosomes [54][207]. Furthermore, the constructs were used in a calvarial bone defect model. After 8 weeks, the functionalized constructs were laden with cells, collagen-rich matrix, marrow-containing bone tissue, and were integrated with the host at 8 weeks [54][207]. Additionally, the µCT of the skulls showed that localized delivery of the exosomes via microspheres in the scaffold resulted in the best regenerative outcome among the treatment groups [54][207]. Overall, the functionalized scaffold system was able to recruit endogenous cells and stimulate bone tissue neogenesis in vivo [54][207]. The exosomes used in this sentudry provided pro-mineralization cues that guided local progenitor cells toward osteogenic differentiation both in vitro and in vivo [54][207]. By incorporating exosomes into a synthetic biopolymer-based scaffold, researchers can work toward overcoming the inherent lack of biochemical cues in synthetic biomaterials to achieve therapeutic effects such as bone healing.References
- Liu, H.; Li, R.; Liu, T.; Yang, L.; Yin, G.; Xie, Q. Immunomodulatory Effects of Mesenchymal Stem Cells and Mesenchymal Stem Cell-Derived Extracellular Vesicles in Rheumatoid Arthritis. Front. Immunol. 2020, 11, 1912.
- Zhao, M.; Liu, S.; Wang, C.; Wang, Y.; Wan, M.; Liu, F.; Gong, M.; Yuan, Y.; Chen, Y.; Cheng, J.; et al. Mesenchymal Stem Cell-Derived Extracellular Vesicles Attenuate Mitochondrial Damage and Inflammation by Stabilizing Mitochondrial DNA. ACS Nano 2021, 15, 1519–1538.
- Branscome, H.; Paul, S.; Khatkar, P.; Kim, Y.; Barclay, R.A.; Pinto, D.O.; Yin, D.; Zhou, W.; Liotta, L.A.; El-Hage, N.; et al. Stem Cell Extracellular Vesicles and Their Potential to Contribute to the Repair of Damaged CNS Cells. J. Neuroimmune Pharmacol. 2020, 15, 520–537.
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A Perspective on the Clinical Translation of Scaffolds for Tissue Engineering. Ann. Biomed. Eng. 2015, 43, 641–656.
- Shahin, H.I.; Radnaa, E.; Tantengco, O.A.G.; Kechichian, T.; Kammala, A.K.; Sheller-Miller, S.; Taylor, B.D.; Menon, R. Microvesicles and Exosomes Released by Amnion Epithelial Cells under Oxidative Stress Cause Inflammatory Changes in Uterine Cells. Biol. Reprod. 2021, 105, 464–480.
- Fonseka, P.; Marzan, A.L.; Mathivanan, S. Introduction to the Community of Extracellular Vesicles. In New Frontiers: Extracellular Vesicles; Mathivanan, S., Fonseka, P., Nedeva, C., Atukorala, I., Eds.; Subcellular Biochemistry; Springer International Publishing: Cham, Switzerland, 2021; pp. 3–18.
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles 2018, 7, 1535750.
- Homem, N.C.; Tavares, T.D.; Miranda, C.S.; Antunes, J.C.; Amorim, M.T.P.; Felgueiras, H.P. Functionalization of Crosslinked Sodium Alginate/Gelatin Wet-Spun Porous Fibers with Nisin Z for the Inhibition of Staphylococcus aureus-Induced Infections. Int. J. Mol. Sci. 2021, 22, 1930.
- Athamneh, T.; Amin, A.; Benke, E.; Ambrus, R.; Gurikov, P.; Smirnova, I.; Leopold, C.S. Pulmonary Drug Delivery with Aerogels: Engineering of Alginate and Alginate-Hyaluronic Acid Microspheres. Pharm. Dev. Technol. 2021, 26, 509–521.
- Elbialy, N.S.; Mohamed, N. Alginate-Coated Caseinate Nanoparticles for Doxorubicin Delivery: Preparation, Characterisation, and in Vivo Assessment. Int. J. Biol. Macromol. 2020, 154, 114–122.
- Sheng, Y.; Gao, J.; Yin, Z.-Z.; Kang, J.; Kong, Y. Dual-Drug Delivery System Based on the Hydrogels of Alginate and Sodium Carboxymethyl Cellulose for Colorectal Cancer Treatment. Carbohydr. Polym. 2021, 269, 118325.
- Sarheed, O.; Dibi, M.; Ramesh, K.V.R.N.S.; Drechsler, M. Fabrication of Alginate-Based O/W Nanoemulsions for Transdermal Drug Delivery of Lidocaine: Influence of the Oil Phase and Surfactant. Molecules 2021, 26, 2556.
- Rajalekshmy, G.; Rekha, M. Synthesis and Evaluation of an Alginate-Methacrylate Xerogel for Insulin Delivery towards Wound Healing Applications. Ther. Deliv. 2021, 12, 215–234.
- Chen, Z.; Yu, P.; Miao, Z.; Zhang, H.; Xiao, H.; Xie, J.; Ding, C.; Li, J. Sulfated Alginate Based Complex for Sustained Calcitonin Delivery and Enhanced Osteogenesis. Biomed. Mater. 2021, 16, 035022.
- Kumar, A.; Belhaj, M.; DiPette, D.J.; Potts, J.D. A Novel Alginate-Based Delivery System for the Prevention and Treatment of Pressure-Overload Induced Heart Failure. Front. Pharmacol. 2020, 11, 602952.
- Alallam, B.; Altahhan, S.; Taher, M.; Mohd Nasir, M.H.; Doolaanea, A.A. Electrosprayed Alginate Nanoparticles as CRISPR Plasmid DNA Delivery Carrier: Preparation, Optimization, and Characterization. Pharmaceuticals 2020, 13, 158.
- Han, C.; Xiao, Y.; Liu, E.; Su, Z.; Meng, X.; Liu, B. Preparation of Ca-Alginate-Whey Protein Isolate Microcapsules for Protection and Delivery of L. Bulgaricus and L. Paracasei. Int. J. Biol. Macromol. 2020, 163, 1361–1368.
- Zhang, Y.; Zhang, P.; Gao, X.; Chang, L.; Chen, Z.; Mei, X. Preparation of Exosomes Encapsulated Nanohydrogel for Accelerating Wound Healing of Diabetic Rats by Promoting Angiogenesis. Mater. Sci. Eng. C 2021, 120, 111671.
- Yang, Z.; Yang, Y.; Xu, Y.; Jiang, W.; Shao, Y.; Xing, J.; Chen, Y.; Han, Y. Biomimetic Nerve Guidance Conduit Containing Engineered Exosomes of Adipose-Derived Stem Cells Promotes Peripheral Nerve Regeneration. Stem Cell Res. Ther. 2021, 12, 442.
- Lv, K.; Li, Q.; Zhang, L.; Wang, Y.; Zhong, Z.; Zhao, J.; Lin, X.; Wang, J.; Zhu, K.; Xiao, C.; et al. Incorporation of Small Extracellular Vesicles in Sodium Alginate Hydrogel as a Novel Therapeutic Strategy for Myocardial Infarction. Theranostics 2019, 9, 7403–7416.
- Bossi, A.M.; Bucciarelli, A.; Maniglio, D. Molecularly Imprinted Silk Fibroin Nanoparticles. ACS Appl. Mater. Interfaces 2021, 13, 31431–31439.
- Rockwood, D.N.; Preda, R.C.; Yücel, T.; Wang, X.; Lovett, M.L.; Kaplan, D.L. Materials Fabrication from Bombyx Mori Silk Fibroin. Nat. Protoc. 2011, 6, 1612–1631.
- Guan, Y.; You, H.; Cai, J.; Zhang, Q.; Yan, S.; You, R. Physically Crosslinked Silk Fibroin/Hyaluronic Acid Scaffolds. Carbohydr. Polym. 2020, 239, 116232.
- Lee, J.; Jang, E.H.; Kim, J.H.; Park, S.; Kang, Y.; Park, S.; Lee, K.; Kim, J.-H.; Youn, Y.-N.; Ryu, W. Highly Flexible and Porous Silk Fibroin Microneedle Wraps for Perivascular Drug Delivery. J. Control. Release 2021, 340, 125–135.
- Opálková Šišková, A.; Kozma, E.; Opálek, A.; Kroneková, Z.; Kleinová, A.; Nagy, Š.; Kronek, J.; Rydz, J.; Eckstein Andicsová, A. Diclofenac Embedded in Silk Fibroin Fibers as a Drug Delivery System. Materials 2020, 13, 3580.
- Ziadlou, R.; Rotman, S.; Teuschl, A.; Salzer, E.; Barbero, A.; Martin, I.; Alini, M.; Eglin, D.; Grad, S. Optimization of Hyaluronic Acid-Tyramine/Silk-Fibroin Composite Hydrogels for Cartilage Tissue Engineering and Delivery of Anti-Inflammatory and Anabolic Drugs. Mater. Sci. Eng. C 2021, 120, 111701.
- Crakes, K.R.; Herrera, C.; Morgan, J.L.; Olstad, K.; Hessell, A.J.; Ziprin, P.; LiWang, P.J.; Dandekar, S. Efficacy of Silk Fibroin Biomaterial Vehicle for in Vivo Mucosal Delivery of Griffithsin and Protection against HIV and SHIV Infection Ex Vivo. J. Int. AIDS Soc. 2020, 23, e25628.
- Crivelli, B.; Bari, E.; Perteghella, S.; Catenacci, L.; Sorrenti, M.; Mocchi, M.; Faragò, S.; Tripodo, G.; Prina-Mello, A.; Torre, M.L. Silk Fibroin Nanoparticles for Celecoxib and Curcumin Delivery: ROS-Scavenging and Anti-Inflammatory Activities in an in Vitro Model of Osteoarthritis. Eur. J. Pharm. Biopharm. 2019, 137, 37–45.
- Laomeephol, C.; Ferreira, H.; Kanokpanont, S.; Neves, N.M.; Kobayashi, H.; Damrongsakkul, S. Dual-Functional Liposomes for Curcumin Delivery and Accelerating Silk Fibroin Hydrogel Formation. Int. J. Pharm. 2020, 589, 119844.
- Pérez Quiñones, J.; Roschger, C.; Zierer, A.; Peniche-Covas, C.; Brüggemann, O. Self-Assembled Silk Fibroin-Based Aggregates for Delivery of Camptothecin. Polymers 2021, 13, 3804.
- Rahmani, H.; Fattahi, A.; Sadrjavadi, K.; Khaledian, S.; Shokoohinia, Y. Preparation and Characterization of Silk Fibroin Nanoparticles as a Potential Drug Delivery System for 5-Fluorouracil. Adv. Pharm. Bull. 2019, 9, 601–608.
- Yavuz, B.; Morgan, J.L.; Herrera, C.; Harrington, K.; Perez-Ramirez, B.; LiWang, P.J.; Kaplan, D.L. Sustained Release Silk Fibroin Discs: Antibody and Protein Delivery for HIV Prevention. J. Control. Release 2019, 301, 1–12.
- Teuschl, A.H.; Zipperle, J.; Huber-Gries, C.; Kaplan, D.L. Silk Fibroin Based Carrier System for Delivery of Fibrinogen and Thrombin as Coagulant Supplements. J. Biomed. Mater. Res. A 2017, 105, 687–696.
- Zhu, M.; Liu, Y.; Jiang, F.; Cao, J.; Kundu, S.C.; Lu, S. Combined Silk Fibroin Microneedles for Insulin Delivery. ACS Biomater. Sci. Eng. 2020, 6, 3422–3429.
- Zhang, H.; Lai, L.; Wang, Y.; Ye, B.; Deng, S.; Ding, A.; Teng, L.; Qiu, L.; Chen, J. Silk Fibroin for CpG Oligodeoxynucleotide Delivery. ACS Biomater. Sci. Eng. 2019, 5, 6082–6088.
- Li, T.; Song, X.; Weng, C.; Wang, X.; Wu, J.; Sun, L.; Gong, X.; Zeng, W.-N.; Yang, L.; Chen, C. Enzymatically Crosslinked and Mechanically Tunable Silk Fibroin/Pullulan Hydrogels for Mesenchymal Stem Cells Delivery. Int. J. Biol. Macromol. 2018, 115, 300–307.
- Cunnane, E.M.; Lorentz, K.L.; Ramaswamy, A.K.; Gupta, P.; Mandal, B.B.; O’Brien, F.J.; Weinbaum, J.S.; Vorp, D.A. Extracellular Vesicles Enhance the Remodeling of Cell-Free Silk Vascular Scaffolds in Rat Aortae. ACS Appl. Mater. Interfaces 2020, 12, 26955–26965.
- Amiri, N.; Ajami, S.; Shahroodi, A.; Jannatabadi, N.; Amiri Darban, S.; Fazly Bazzaz, B.S.; Pishavar, E.; Kalalinia, F.; Movaffagh, J. Teicoplanin-Loaded Chitosan-PEO Nanofibers for Local Antibiotic Delivery and Wound Healing. Int. J. Biol. Macromol. 2020, 162, 645–656.
- Bahmanpour, A.; Ghaffari, M.; Milan, P.B.; Moztarzadeh, F.; Mozafari, M. Synthesis and Characterization of Thermosensitive Hydrogel Based on Quaternized Chitosan for Intranasal Delivery of Insulin. Biotechnol. Appl. Biochem. 2020, 68, 247–256.
- Esfandiari, F.; Motazedian, M.H.; Asgari, Q.; Morowvat, M.H.; Molaei, M.; Heli, H. Paromomycin-Loaded Mannosylated Chitosan Nanoparticles: Synthesis, Characterization and Targeted Drug Delivery against Leishmaniasis. Acta Trop. 2019, 197, 105072.
- Deshkar, S.; Sikchi, S.; Thakre, A.; Kale, R. Poloxamer Modified Chitosan Nanoparticles for Vaginal Delivery of Acyclovir. Pharm. Nanotechnol. 2021, 9, 141–156.
- Zaman, M.; Iqbal, A.; Haider Rizvi, S.F.; Hussain, M.A.; Jamshaid, T.; Jamshaid, M. Chitosan Based Controlled Release Drug Delivery of Mycophenolate Mofetil Loaded in Nanocarriers System: Synthesis and in-Vitro Evaluation. Drug Dev. Ind. Pharm. 2021, 47, 477–483.
- Panão Costa, J.; Carvalho, S.; Jesus, S.; Soares, E.; Marques, A.P.; Borges, O. Optimization of Chitosan-α-Casein Nanoparticles for Improved Gene Delivery: Characterization, Stability, and Transfection Efficiency. AAPS PharmSciTech 2019, 20, 132.
- Soliman, O.Y.; Alameh, M.G.; De Cresenzo, G.; Buschmann, M.D.; Lavertu, M. Efficiency of Chitosan/Hyaluronan-Based MRNA Delivery Systems In Vitro: Influence of Composition and Structure. J. Pharm. Sci. 2020, 109, 1581–1593.
- Babii, O.; Wang, Z.; Liu, G.; Martinez, E.C.; van Drunen Littel-van den Hurk, S.; Chen, L. Low Molecular Weight Chitosan Nanoparticles for CpG Oligodeoxynucleotides Delivery: Impact of Molecular Weight, Degree of Deacetylation, and Mannosylation on Intracellular Uptake and Cytokine Induction. Int. J. Biol. Macromol. 2020, 159, 46–56.
- Wu, D.; Qin, H.; Wang, Z.; Yu, M.; Liu, Z.; Peng, H.; Liang, L.; Zhang, C.; Wei, X. Bone Mesenchymal Stem Cell-Derived SEV-Encapsulated Thermosensitive Hydrogels Accelerate Osteogenesis and Angiogenesis by Release of Exosomal MiR-21. Front. Bioeng. Biotechnol. 2022, 9, 829136.
- Tang, Q.; Lu, B.; He, J.; Chen, X.; Fu, Q.; Han, H.; Luo, C.; Yin, H.; Qin, Z.; Lyu, D.; et al. Exosomes-Loaded Thermosensitive Hydrogels for Corneal Epithelium and Stroma Regeneration. Biomaterials 2022, 280, 121320.
- Abolgheit, S.; Abdelkader, S.; Aboushelib, M.; Omar, E.; Mehanna, R. Bone Marrow-Derived Mesenchymal Stem Cells and Extracellular Vesicles Enriched Collagen Chitosan Scaffold in Skin Wound Healing (a Rat Model). J. Biomater. Appl. 2021, 36, 128–139.
- Nikhil, A.; Kumar, A. Evaluating Potential of Tissue-Engineered Cryogels and Chondrocyte Derived Exosomes in Articular Cartilage Repair. Biotechnol. Bioeng. 2022, 119, 605–625.
- Wang, X.; Ronsin, O.; Gravez, B.; Farman, N.; Baumberger, T.; Jaisser, F.; Coradin, T.; Hélary, C. Nanostructured Dense Collagen-Polyester Composite Hydrogels as Amphiphilic Platforms for Drug Delivery. Adv. Sci. 2021, 8, 2004213.
- Kupper, S.; Kłosowska-Chomiczewska, I.; Szumała, P. Collagen and Hyaluronic Acid Hydrogel in Water-in-Oil Microemulsion Delivery Systems. Carbohydr. Polym. 2017, 175, 347–354.
- Domene, C.; Jorgensen, C.; Abbasi, S.W. A Perspective on Structural and Computational Work on Collagen. Phys. Chem. Chem. Phys. 2016, 18, 24802–24811.
- Cheng, O.T.; Stein, A.P.; Babajanian, E.; Hoppe, K.R.; Li, S.; Jung, H.; Abrol, A.; Akkus, A.; Younesi, M.; Altawallbeh, G.; et al. Heparin-Mediated Antibiotic Delivery from an Electrochemically-Aligned Collagen Sheet. Biomed. Mater. Eng. 2021, 32, 159–170.
- Zeng, Y.; Zhou, M.; Mou, S.; Yang, J.; Yuan, Q.; Guo, L.; Zhong, A.; Wang, J.; Sun, J.; Wang, Z. Sustained Delivery of Alendronate by Engineered Collagen Scaffold for the Repair of Osteoporotic Bone Defects and Resistance to Bone Loss. J. Biomed. Mater. Res. A 2020, 108, 2460–2472.
- Zhang, S.; Huang, D.; Lin, H.; Xiao, Y.; Zhang, X. Cellulose Nanocrystal Reinforced Collagen-Based Nanocomposite Hydrogel with Self-Healing and Stress-Relaxation Properties for Cell Delivery. Biomacromolecules 2020, 21, 2400–2408.
- Zhou, X.; Wang, J.; Fang, W.; Tao, Y.; Zhao, T.; Xia, K.; Liang, C.; Hua, J.; Li, F.; Chen, Q. Genipin Cross-Linked Type II Collagen/Chondroitin Sulfate Composite Hydrogel-like Cell Delivery System Induces Differentiation of Adipose-Derived Stem Cells and Regenerates Degenerated Nucleus Pulposus. Acta Biomater. 2018, 71, 496–509.
- Anghel, N.; Dinu, M.V.; Zaltariov, M.; Pamfil, D.; Spiridon, I. New Cellulose-Collagen-Alginate Materials Incorporated with Quercetin, Anthocyanins and Lipoic Acid. Int. J. Biol. Macromol. 2021, 181, 30–40.
- Qu, Y.; Cao, C.; Wu, Q.; Huang, A.; Song, Y.; Li, H.; Zuo, Y.; Chu, C.; Li, J.; Man, Y. The Dual Delivery of KGF and BFGF by Collagen Membrane to Promote Skin Wound Healing. J. Tissue Eng. Regen. Med. 2018, 12, 1508–1518.
- Ruehle, M.A.; Li, M.-T.A.; Cheng, A.; Krishnan, L.; Willett, N.J.; Guldberg, R.E. Decorin-Supplemented Collagen Hydrogels for the Co-Delivery of Bone Morphogenetic Protein-2 and Microvascular Fragments to a Composite Bone-Muscle Injury Model with Impaired Vascularization. Acta Biomater. 2019, 93, 210–221.
- Imanishi, Y.; Hata, M.; Matsukawa, R.; Aoyagi, A.; Omi, M.; Mizutani, M.; Naruse, K.; Ozawa, S.; Honda, M.; Matsubara, T.; et al. Efficacy of Extracellular Vesicles from Dental Pulp Stem Cells for Bone Regeneration in Rat Calvarial Bone Defects. Inflamm Regen 2021, 41, 12.
- Xin, L.; Lin, X.; Zhou, F.; Li, C.; Wang, X.; Yu, H.; Pan, Y.; Fei, H.; Ma, L.; Zhang, S. A Scaffold Laden with Mesenchymal Stem Cell-Derived Exosomes for Promoting Endometrium Regeneration and Fertility Restoration through Macrophage Immunomodulation. Acta Biomater. 2020, 113, 252–266.
- Gerton, M.L.; Mann, B.K. Mucoadhesive Hyaluronic Acid-Based Films for Vaginal Delivery of Metronidazole. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109, 1706–1712.
- Du, H.; Liu, P.; Zhu, J.; Lan, J.; Li, Y.; Zhang, L.; Zhu, J.; Tao, J. Hyaluronic Acid-Based Dissolving Microneedle Patch Loaded with Methotrexate for Improved Treatment of Psoriasis. ACS Appl. Mater. Interfaces 2019, 11, 43588–43598.
- Camara, C.I.; Bertocchi, L.; Ricci, C.; Bassi, R.; Bianchera, A.; Cantu’, L.; Bettini, R.; Del Favero, E. Hyaluronic Acid-Dexamethasone Nanoparticles for Local Adjunct Therapy of Lung Inflammation. Int. J. Mol. Sci. 2021, 22, 10480.
- Cho, J.-A.; Kim, B.-J.; Hwang, Y.-J.; Woo, S.-W.; Noh, T.-S.; Suh, M.-W. Effect and Biocompatibility of a Cross-Linked Hyaluronic Acid and Polylactide-Co-Glycolide Microcapsule Vehicle in Intratympanic Drug Delivery for Treating Acute Acoustic Trauma. Int. J. Mol. Sci. 2021, 22, 5720.
- Lai, H.; Ding, X.; Ye, J.; Deng, J.; Cui, S. PH-Responsive Hyaluronic Acid-Based Nanoparticles for Targeted Curcumin Delivery and Enhanced Cancer Therapy. Colloids Surf. B Biointerfaces 2021, 198, 111455.
- Zhang, Y.; Zhang, K.; Wang, Z.; Hu, H.; Jing, Q.; Li, Y.; Guo, T.; Feng, N. Transcutol® P/Cremophor® EL/Ethyl Oleate-Formulated Microemulsion Loaded into Hyaluronic Acid-Based Hydrogel for Improved Transdermal Delivery and Biosafety of Ibuprofen. AAPS PharmSciTech 2019, 21, 22.
- Liu, D.; Zhang, Q.; Wang, J.; Guan, S.; Cai, D.; Liu, J. Inhibition of Growth and Metastasis of Breast Cancer by Targeted Delivery of 17-Hydroxy-Jolkinolide B via Hyaluronic Acid-Coated Liposomes. Carbohydr. Polym. 2021, 257, 117572.
- Rezaei, S.; Kashanian, S.; Bahrami, Y.; Cruz, L.J.; Motiei, M. Redox-Sensitive and Hyaluronic Acid-Functionalized Nanoparticles for Improving Breast Cancer Treatment by Cytoplasmic 17α-Methyltestosterone Delivery. Molecules 2020, 25, 1181.
- Sagbas Suner, S.; Ari, B.; Onder, F.C.; Ozpolat, B.; Ay, M.; Sahiner, N. Hyaluronic Acid and Hyaluronic Acid: Sucrose Nanogels for Hydrophobic Cancer Drug Delivery. Int. J. Biol. Macromol. 2019, 126, 1150–1157.
- Zhang, Q.; Wang, J.; Liu, D.; Zhu, W.; Guan, S.; Fan, L.; Cai, D. Targeted Delivery of Honokiol by Zein/Hyaluronic Acid Core-Shell Nanoparticles to Suppress Breast Cancer Growth and Metastasis. Carbohydr. Polym. 2020, 240, 116325.
- Lei, M.; Chen, G.; Zhang, M.; Lei, J.; Li, T.; Li, D.; Zheng, H. A PH-Sensitive Drug Delivery System Based on Hyaluronic Acid Co-Deliver Doxorubicin and Aminoferrocene for the Combined Application of Chemotherapy and Chemodynamic Therapy. Colloids Surf. B Biointerfaces 2021, 203, 111750.
- Li, M.; Li, J.-P.; Wang, Y.-S.; He, Q. . Sichuan Da Xue Xue Bao Yi Xue Ban 2021, 52, 577–584.
- Lu, B.; Xiao, F.; Wang, Z.; Wang, B.; Pan, Z.; Zhao, W.; Zhu, Z.; Zhang, J. Redox-Sensitive Hyaluronic Acid Polymer Prodrug Nanoparticles for Enhancing Intracellular Drug Self-Delivery and Targeted Cancer Therapy. ACS Biomater. Sci. Eng. 2020, 6, 4106–4115.
- Zhang, X.; Pan, J.; Yao, M.; Palmerston Mendes, L.; Sarisozen, C.; Mao, S.; Torchilin, V.P. Charge Reversible Hyaluronic Acid-Modified Dendrimer-Based Nanoparticles for SiMDR-1 and Doxorubicin Co-Delivery. Eur. J. Pharm. Biopharm. 2020, 154, 43–49.
- Serini, S.; Cassano, R.; Bruni, M.; Servidio, C.; Calviello, G.; Trombino, S. Characterization of a Hyaluronic Acid and Folic Acid-Based Hydrogel for Cisplatin Delivery: Antineoplastic Effect in Human Ovarian Cancer Cells in Vitro. Int. J. Pharm. 2021, 606, 120899.
- Sun, S.; Shang, E.; Ju, A.; Li, Y.; Wu, Q.; Li, Q.; Yang, Y.; Guo, Y.; Yang, D.; Lv, S. Tumor-Targeted Hyaluronic Acid-MPEG Modified Nanostructured Lipid Carriers for Cantharidin Delivery: An in Vivo and in Vitro Study. Fitoterapia 2021, 155, 105033.
- Kim, Y.; Bhattaccharjee, S.A.; Beck-Broichsitter, M.; Banga, A.K. Fabrication and Characterization of Hyaluronic Acid Microneedlesto Enhance Delivery of Magnesium Ascorbyl Phosphate into Skin. Biomed Microdevices 2019, 21, 104.
- Chen, S.; Han, Y.; Wang, Y.; Yang, X.; Sun, C.; Mao, L.; Gao, Y. Zein-Hyaluronic Acid Binary Complex as a Delivery Vehicle of Quercetagetin: Fabrication, Structural Characterization, Physicochemical Stability and in Vitro Release Property. Food Chem. 2019, 276, 322–332.
- De Oliveira, M.M.; Nakamura, C.V.; Auzély-Velty, R. Boronate-Ester Crosslinked Hyaluronic Acid Hydrogels for Dihydrocaffeic Acid Delivery and Fibroblasts Protection against UVB Irradiation. Carbohydr. Polym. 2020, 247, 116845.
- Liu, Y.; Wang, Y.; Yang, J.; Zhang, H.; Gan, L. Cationized Hyaluronic Acid Coated Spanlastics for Cyclosporine A Ocular Delivery: Prolonged Ocular Retention, Enhanced Corneal Permeation and Improved Tear Production. Int. J. Pharm. 2019, 565, 133–142.
- Hamilton, M.; Harrington, S.; Dhar, P.; Stehno-Bittel, L. Hyaluronic Acid Hydrogel Microspheres for Slow Release Stem Cell Delivery. ACS Biomater. Sci. Eng. 2021, 7, 3754–3763.
- Liu, F.; Hu, S.; Yang, H.; Li, Z.; Huang, K.; Su, T.; Wang, S.; Cheng, K. Hyaluronic Acid Hydrogel Integrated with Mesenchymal Stem Cell-Secretome to Treat Endometrial Injury in a Rat Model of Asherman’s Syndrome. Adv. Healthc. Mater. 2019, 8, e1900411.
- Jenjob, R.; Nguyen, H.-P.; Kim, M.-K.; Jiang, Y.; Kim, J.J.; Yang, S.-G. Bisphosphonate-Conjugated Photo-Crosslinking Polyanionic Hyaluronic Acid Microbeads for Controlled BMP2 Delivery and Enhanced Bone Formation Efficacy. Biomacromolecules 2021, 22, 4138–4145.
- Chen, Z.; Han, S.; Yang, X.; Xu, L.; Qi, H.; Hao, G.; Cao, J.; Liang, Y.; Ma, Q.; Zhang, G.; et al. Overcoming Multiple Absorption Barrier for Insulin Oral Delivery Using Multifunctional Nanoparticles Based on Chitosan Derivatives and Hyaluronic Acid. Int. J. Nanomed. 2020, 15, 4877–4898.
- Song, K.; Jiang, T.; Pan, P.; Yao, Y.; Jiang, Q. Exosomes from Tendon Derived Stem Cells Promote Tendon Repair through MiR-144-3p-Regulated Tenocyte Proliferation and Migration. Stem Cell Res. Ther. 2022, 13, 80.
- Heirani-Tabasi, A.; Hosseinzadeh, S.; Rabbani, S.; Tafti, S.H.A.; Jamshidi, K.; Soufizomorrod, M.; Soleimani, M. Cartilage Tissue Engineering by Co-Transplantation of Chondrocyte Extracellular Vesicles and Mesenchymal Stem Cells, Entrapped in Chitosan–Hyaluronic Acid Hydrogel. Biomed. Mater. 2021, 16, 055003.
- Derkach, S.R.; Kolotova, D.S.; Voron’ko, N.G.; Obluchinskaya, E.D.; Malkin, A.Y. Rheological Properties of Fish Gelatin Modified with Sodium Alginate. Polymers 2021, 13, 743.
- Dolci, L.S.; Albertini, B.; Di Filippo, M.F.; Bonvicini, F.; Passerini, N.; Panzavolta, S. Development and in Vitro Evaluation of Mucoadhesive Gelatin Films for the Vaginal Delivery of Econazole. Int. J. Pharm. 2020, 591, 119979.
- Oliveira, M.B.; da Silva, J.B.; Montanha, M.C.; Kimura, E.; Diniz, A.; Bruschi, M.L. Design and Characterization of Mucoadhesive Gelatin-Ethylcellulose Microparticles for the Delivery of Curcumin to the Bladder. Curr. Drug Deliv. 2018, 15, 1112–1122.
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Preparation and Optimization of Chitosan-Gelatin Films for Sustained Delivery of Lupeol for Wound Healing. Int. J. Biol. Macromol. 2018, 107, 1888–1897.
- Zhang, S.; Kang, L.; Hu, S.; Hu, J.; Fu, Y.; Hu, Y.; Yang, X. Carboxymethyl Chitosan Microspheres Loaded Hyaluronic Acid/Gelatin Hydrogels for Controlled Drug Delivery and the Treatment of Inflammatory Bowel Disease. Int. J. Biol. Macromol. 2021, 167, 1598–1612.
- Cao, S.; Li, L.; Du, Y.; Gan, J.; Wang, J.; Wang, T.; Liu, Y.; Liu, W.; Zhou, Y.; Gao, X.; et al. Porous Gelatin Microspheres for Controlled Drug Delivery with High Hemostatic Efficacy. Colloids Surf. B Biointerfaces 2021, 207, 112013.
- Fan, Z.; Cheng, P.; Yin, G.; Wang, Z.; Han, J. In Situ Forming Oxidized Salecan/Gelatin Injectable Hydrogels for Vancomycin Delivery and 3D Cell Culture. J. Biomater. Sci. Polym. Ed. 2020, 31, 762–780.
- Nouri-Felekori, M.; Khakbiz, M.; Nezafati, N.; Mohammadi, J.; Eslaminejad, M.B. Comparative Analysis and Properties Evaluation of Gelatin Microspheres Crosslinked with Glutaraldehyde and 3-Glycidoxypropyltrimethoxysilane as Drug Delivery Systems for the Antibiotic Vancomycin. Int. J. Pharm. 2019, 557, 208–220.
- Chen, X.; Fan, M.; Tan, H.; Ren, B.; Yuan, G.; Jia, Y.; Li, J.; Xiong, D.; Xing, X.; Niu, X.; et al. Magnetic and Self-Healing Chitosan-Alginate Hydrogel Encapsulated Gelatin Microspheres via Covalent Cross-Linking for Drug Delivery. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 101, 619–629.
- Jahanban-Esfahlan, R.; Derakhshankhah, H.; Haghshenas, B.; Massoumi, B.; Abbasian, M.; Jaymand, M. A Bio-Inspired Magnetic Natural Hydrogel Containing Gelatin and Alginate as a Drug Delivery System for Cancer Chemotherapy. Int. J. Biol. Macromol. 2020, 156, 438–445.
- Vaghasiya, K.; Ray, E.; Singh, R.; Jadhav, K.; Sharma, A.; Khan, R.; Katare, O.P.; Verma, R.K. Efficient, Enzyme Responsive and Tumor Receptor Targeting Gelatin Nanoparticles Decorated with Concanavalin-A for Site-Specific and Controlled Drug Delivery for Cancer Therapy. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 123, 112027.
- Zhou, K.; Zhu, Y.; Chen, X.; Li, L.; Xu, W. Redox- and MMP-2-Sensitive Drug Delivery Nanoparticles Based on Gelatin and Albumin for Tumor Targeted Delivery of Paclitaxel. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111006.
- Albadran, H.A.; Monteagudo-Mera, A.; Khutoryanskiy, V.V.; Charalampopoulos, D. Development of Chitosan-Coated Agar-Gelatin Particles for Probiotic Delivery and Targeted Release in the Gastrointestinal Tract. Appl. Microbiol. Biotechnol. 2020, 104, 5749–5757.
- Demir, G.C.; Erdemli, Ö.; Keskin, D.; Tezcaner, A. Xanthan-Gelatin and Xanthan-Gelatin-Keratin Wound Dressings for Local Delivery of Vitamin C. Int. J. Pharm. 2022, 614, 121436.
- Amit, C.; Muralikumar, S.; Janaki, S.; Lakshmipathy, M.; Therese, K.L.; Umashankar, V.; Padmanabhan, P.; Narayanan, J. Designing and Enhancing the Antifungal Activity of Corneal Specific Cell Penetrating Peptide Using Gelatin Hydrogel Delivery System. Int. J. Nanomed. 2019, 14, 605–622.
- Galdopórpora, J.M.; Morcillo, M.F.; Ibar, A.; Perez, C.J.; Tuttolomondo, M.V.; Desimone, M.F. Development of Silver Nanoparticles/Gelatin Thermoresponsive Nanocomposites: Characterization and Antimicrobial Activity. Curr. Pharm. Des. 2019, 25, 4121–4129.
- Luo, R.; Huang, Y.; Yuan, X.; Yuan, Z.; Zhang, L.; Han, J.; Zhao, Y.; Cai, Q. Controlled Co-Delivery System of Magnesium and Lanthanum Ions for Vascularized Bone Regeneration. Biomed. Mater. 2021, 16, 065024.
- Fan, C.; Zhan, S.-H.; Dong, Z.-X.; Yang, W.; Deng, W.-S.; Liu, X.; Wang, D.-A.; Sun, P. Cross-Linked Gelatin Microsphere-Based Scaffolds as a Delivery Vehicle of MC3T3-E1 Cells: In Vitro and in Vivo Evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 108, 110399.
- Tang, Y.; Tong, X.; Conrad, B.; Yang, F. Injectable and in Situ Crosslinkable Gelatin Microribbon Hydrogels for Stem Cell Delivery and Bone Regeneration in Vivo. Theranostics 2020, 10, 6035–6047.
- Yuan, Z.; Yuan, X.; Zhao, Y.; Cai, Q.; Wang, Y.; Luo, R.; Yu, S.; Wang, Y.; Han, J.; Ge, L.; et al. Injectable GelMA Cryogel Microspheres for Modularized Cell Delivery and Potential Vascularized Bone Regeneration. Small 2021, 17, e2006596.
- Bai, Y.; Moeinzadeh, S.; Kim, S.; Park, Y.; Lui, E.; Tan, H.; Zhao, W.; Zhou, X.; Yang, Y.P. Development of PLGA-PEG-COOH and Gelatin-Based Microparticles Dual Delivery System and E-Beam Sterilization Effects for Controlled Release of BMP-2 and IGF-1. Part. Part. Syst. Charact. 2020, 37, 2000180.
- Yu, J.R.; Janssen, M.; Liang, B.J.; Huang, H.-C.; Fisher, J.P. A Liposome/Gelatin Methacrylate Nanocomposite Hydrogel System for Delivery of Stromal Cell-Derived Factor-1α and Stimulation of Cell Migration. Acta Biomater. 2020, 108, 67–76.
- Attarwala, H.Z.; Suri, K.; Amiji, M.M. Pharmacokinetics and Biodistribution Analysis of Small Interference RNA for Silencing Tissue Transglutaminase-2 in Celiac Disease After Oral Administration in Mice Using Gelatin-Based Multicompartmental Delivery Systems. Bioelectricity 2020, 2, 167–174.
- Takanche, J.S.; Kim, J.-E.; Kim, J.-S.; Yi, H.-K. Guided Bone Regeneration with a Gelatin Layer and Adenoviral Delivery of C-Myb Enhances Bone Healing in Rat Tibia. Regen. Med. 2020, 15, 1877–1890.
- Man, K.; Barroso, I.A.; Brunet, M.Y.; Peacock, B.; Federici, A.S.; Hoey, D.A.; Cox, S.C. Controlled Release of Epigenetically-Enhanced Extracellular Vesicles from a GelMA/Nanoclay Composite Hydrogel to Promote Bone Repair. Int. J. Mol. Sci. 2022, 23, 832.
- Sharma, P.K.; Singh, Y. Glyoxylic Hydrazone Linkage-Based PEG Hydrogels for Covalent Entrapment and Controlled Delivery of Doxorubicin. Biomacromolecules 2019, 20, 2174–2184.
- Chai, D.; Hao, B.; Hu, R.; Zhang, F.; Yan, J.; Sun, Y.; Huang, X.; Zhang, Q.; Jiang, H. Delivery of Oridonin and Methotrexate via PEGylated Graphene Oxide. ACS Appl. Mater. Interfaces 2019, 11, 22915–22924.
- Emami, J.; Maghzi, P.; Hasanzadeh, F.; Sadeghi, H.; Mirian, M.; Rostami, M. PLGA-PEG-RA-Based Polymeric Micelles for Tumor Targeted Delivery of Irinotecan. Pharm. Dev. Technol. 2018, 23, 41–54.
- Gong, Y.-H.; Shu, M.; Xie, J.-H.; Zhang, C.; Cao, Z.; Jiang, Z.-Z.; Liu, J. Enzymatic Synthesis of PEG-Poly(Amine-Co-Thioether Esters) as Highly Efficient PH and ROS Dual-Responsive Nanocarriers for Anticancer Drug Delivery. J. Mater. Chem. B 2019, 7, 651–664.
- Manjili, H.K.; Malvandi, H.; Mousavi, M.S.; Attari, E.; Danafar, H. In Vitro and in Vivo Delivery of Artemisinin Loaded PCL-PEG-PCL Micelles and Its Pharmacokinetic Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 926–936.
- Nasab, S.H.; Amani, A.; Ebrahimi, H.A.; Hamidi, A.A. Design and Preparation of a New Multi-Targeted Drug Delivery System Using Multifunctional Nanoparticles for Co-Delivery of SiRNA and Paclitaxel. J. Pharm. Anal. 2021, 11, 163–173.
- Nguyen, P.T.H.; Le, B.T.; Ninh, H.D.; La, D.D. Ultrasonic-Assisted Synthesis of Fe–BTC–PEG Metal–Organic Complex: An Effective and Safety Nanocarrier for Anticancer Drug Delivery. ACS Omega 2021, 6, 33419–33427.
- Ni, R.; Duan, D.; Li, B.; Li, Z.; Li, L.; Ming, Y.; Wang, X.; Chen, J. Dual-Modified PCL-PEG Nanoparticles for Improved Targeting and Therapeutic Efficacy of Docetaxel against Colorectal Cancer. Pharm. Dev. Technol. 2021, 26, 910–921.
- Wu, J.; Wang, X.; Zhu, B.; He, Q.; Ren, F.; Tong, F.; Jiang, W.; He, X. PH-Sensitive Magnetic Drug Delivery System via Layer-by-Layer Self-Assembly of CS/PEG and Its Controlled Release of DOX. J. Biomater. Sci. Polym. Ed. 2020, 31, 1057–1070.
- Yang, J.-G.; Zhang, J.; Chen, X.-J.; Zhou, G. Stable Loading and Delivery of Icaritin Using PEG-PCL Micelles for Effective Treatment of Oral Squamous Cell Carcinoma. Curr. Drug. Deliv. 2021, 18, 975–983.
- Alami-Milani, M.; Zakeri-Milani, P.; Valizadeh, H.; Salehi, R.; Jelvehgari, M. Preparation and Evaluation of PCL-PEG-PCL Micelles as Potential Nanocarriers for Ocular Delivery of Dexamethasone. Iran. J. Basic Med. Sci. 2018, 21, 153–164.
- Sharma, P.K.; Halder, M.; Srivastava, U.; Singh, Y. Antibacterial PEG-Chitosan Hydrogels for Controlled Antibiotic/Protein Delivery. ACS Appl. Bio Mater. 2019, 2, 5313–5322.
- Singh, S.; Alrobaian, M.M.; Molugulu, N.; Agrawal, N.; Numan, A.; Kesharwani, P. Pyramid-Shaped PEG-PCL-PEG Polymeric-Based Model Systems for Site-Specific Drug Delivery of Vancomycin with Enhance Antibacterial Efficacy. ACS Omega 2020, 5, 11935–11945.
- Yamashita, S.; Katsumi, H.; Sakane, T.; Yamamoto, A. Bone-Targeting Dendrimer for the Delivery of Methotrexate and Treatment of Bone Metastasis. J. Drug Target. 2018, 26, 818–828.
- Zhang, Y.; Lane, M.E.; Moore, D.J. An Investigation of the Influence of PEG 400 and PEG-6-Caprylic/Capric Glycerides on Dermal Delivery of Niacinamide. Polymers 2020, 12, 2907.
- Zhang, M.; Xu, C.; Liu, D.; Han, M.K.; Wang, L.; Merlin, D. Oral Delivery of Nanoparticles Loaded With Ginger Active Compound, 6-Shogaol, Attenuates Ulcerative Colitis and Promotes Wound Healing in a Murine Model of Ulcerative Colitis. J. Crohn’s Colitis 2018, 12, 217–229.
- Tan, X.; Yin, N.; Liu, Z.; Sun, R.; Gou, J.; Yin, T.; Zhang, Y.; He, H.; Tang, X. Hydrophilic and Electroneutral Nanoparticles to Overcome Mucus Trapping and Enhance Oral Delivery of Insulin. Mol. Pharm. 2020, 17, 3177–3191.
- Ghuman, H.; Matta, R.; Tompkins, A.; Nitzsche, F.; Badylak, S.F.; Gonzalez, A.L.; Modo, M. ECM Hydrogel Improves the Delivery of PEG Microsphere-Encapsulated Neural Stem Cells and Endothelial Cells into Tissue Cavities Caused by Stroke. Brain Res. Bull. 2021, 168, 120–137.
- Amani, A.; Kabiri, T.; Shafiee, S.; Hamidi, A. Preparation and Characterization of PLA-PEG-PLA/PEI/DNA Nanoparticles for Improvement of Transfection Efficiency and Controlled Release of DNA in Gene Delivery Systems. Iran. J. Pharm. Res. 2019, 18, 125–141.
- Poudel, S.; Napit, P.R.; Briski, K.P.; Mattheolabakis, G. Oral Delivery of Nucleic Acids with Passive and Active Targeting to the Intestinal Tissue Using Polymer-Based Nanocarriers. Pharmaceutics 2021, 13, 1075.
- Yu, M.; Wang, K.; Zhang, H.; Liu, Q.; Wang, J.; Cao, L.; Li, W.; Wang, K.; Hong, Z. DOTAP-Incorporated PEG-PLGA Nanoparticles for Efficient In Vitro and In Vivo Gene Delivery. J. Biomed. Nanotechnol. 2018, 14, 281–293.
- Kwak, G.; Cheng, J.; Kim, H.; Song, S.; Lee, S.J.; Yang, Y.; Jeong, J.H.; Lee, J.E.; Messersmith, P.B.; Kim, S.H. Sustained Exosome-Guided Macrophage Polarization Using Hydrolytically Degradable PEG Hydrogels for Cutaneous Wound Healing: Identification of Key Proteins and MiRNAs, and Sustained Release Formulation. Small 2022, 18, 2200060.
- Jiang, T.; Liu, S.; Wu, Z.; Li, Q.; Ren, S.; Chen, J.; Xu, X.; Wang, C.; Lu, C.; Yang, X.; et al. Smart Hydrogel Promotes Diabetic Wound Healing by Optimizing Cellular Functions and Relieving Oxidative Stress. Mater. Today Bio 2022, 16, 100365.
- Mohamady Hussein, M.A.; Guler, E.; Rayaman, E.; Cam, M.E.; Sahin, A.; Grinholc, M.; Sezgin Mansuroglu, D.; Sahin, Y.M.; Gunduz, O.; Muhammed, M.; et al. Dual-Drug Delivery of Ag-Chitosan Nanoparticles and Phenytoin via Core-Shell PVA/PCL Electrospun Nanofibers. Carbohydr. Polym. 2021, 270, 118373.
- Zavan, B.; Gardin, C.; Guarino, V.; Rocca, T.; Cruz Maya, I.; Zanotti, F.; Ferroni, L.; Brunello, G.; Chachques, J.-C.; Ambrosio, L.; et al. Electrospun PCL-Based Vascular Grafts: In Vitro Tests. Nanomaterials 2021, 11, 751.
- Brandt, J.V.; Piazza, R.D.; Dos Santos, C.C.; Vega-Chacón, J.; Amantéa, B.E.; Pinto, G.C.; Magnani, M.; Piva, H.L.; Tedesco, A.C.; Primo, F.L.; et al. Synthesis and Colloidal Characterization of Folic Acid-Modified PEG-b-PCL Micelles for Methotrexate Delivery. Colloids Surf. B Biointerfaces 2019, 177, 228–234.
- De Lima, J.M.; Castellano, L.R.C.; Bonan, P.R.F.; de Medeiros, E.S.; Hier, M.; Bijian, K.; Alaoui-Jamali, M.A.; da Cruz Perez, D.E.; da Silva, S.D. Chitosan/PCL Nanoparticles Can Improve Anti-Neoplastic Activity of 5-Fluorouracil in Head and Neck Cancer through Autophagy Activation. Int. J. Biochem. Cell Biol. 2021, 134, 105964.
- Hassankhani Rad, A.; Asiaee, F.; Jafari, S.; Shayanfar, A.; Lavasanifar, A.; Molavi, O. Poly(Ethylene Glycol)-Poly(ε-Caprolactone)-Based Micelles for Solubilization and Tumor-Targeted Delivery of Silibinin. Bioimpacts 2020, 10, 87–95.
- Jalilzadeh, N.; Samadi, N.; Salehi, R.; Dehghan, G.; Iranshahi, M.; Dadpour, M.R.; Hamishehkar, H. Novel Nano-Vehicle for Delivery and Efficiency of Anticancer Auraptene against Colon Cancer Cells. Sci. Rep. 2020, 10, 1606.
- Rezvani, M.; Mohammadnejad, J.; Narmani, A.; Bidaki, K. Synthesis and in Vitro Study of Modified Chitosan-Polycaprolactam Nanocomplex as Delivery System. Int. J. Biol. Macromol. 2018, 113, 1287–1293.
- Yu, T.; Wu, C.; Zhu, C.; He, Y.; Yang, D.; Cheng, Y.; Gao, X. Oral Administration of Liposome-Apatinib and Locally Delivery of Docetaxel/MPEG-PCL by Fibrin Glue Synergistically Improve Therapeutic Effect in Colorectal Cancer. J. Biomed. Nanotechnol. 2018, 14, 2077–2091.
- Mirzaeei, S.; Mansurian, M.; Asare-Addo, K.; Nokhodchi, A. Metronidazole- and Amoxicillin-Loaded PLGA and PCL Nanofibers as Potential Drug Delivery Systems for the Treatment of Periodontitis: In Vitro and In Vivo Evaluations. Biomedicines 2021, 9, 975.
- Ramazani, A.; Keramati, M.; Malvandi, H.; Danafar, H.; Kheiri Manjili, H. Preparation and in Vivo Evaluation of Anti-Plasmodial Properties of Artemisinin-Loaded PCL-PEG-PCL Nanoparticles. Pharm. Dev. Technol. 2018, 23, 911–920.
- Al-Lawati, H.; Vakili, M.R.; Lavasanifar, A.; Ahmed, S.; Jamali, F. Delivery and Biodistribution of Traceable Polymeric Micellar Diclofenac in the Rat. J. Pharm. Sci. 2019, 108, 2698–2707.
- Samy, K.E.; Cao, Y.; Kim, J.; Konichi da Silva, N.R.; Phone, A.; Bloomer, M.M.; Bhisitkul, R.B.; Desai, T.A. Co-Delivery of Timolol and Brimonidine with a Polymer Thin-Film Intraocular Device. J. Ocul. Pharmacol. Ther. 2019, 35, 124–131.
- Kurd, M.; Sadegh Malvajerd, S.; Rezaee, S.; Hamidi, M.; Derakhshandeh, K. Oral Delivery of Indinavir Using MPEG-PCL Nanoparticles: Preparation, Optimization, Cellular Uptake, Transport and Pharmacokinetic Evaluation. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2123–2133.
- Danafar, H.; Jaberizadeh, H.; Andalib, S. In Vitro and in Vivo Delivery of Gliclazide Loaded MPEG-PCL Micelles and Its Kinetic Release and Solubility Study. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1625–1636.
- Dziadek, M.; Dziadek, K.; Checinska, K.; Zagrajczuk, B.; Golda-Cepa, M.; Brzychczy-Wloch, M.; Menaszek, E.; Kopec, A.; Cholewa-Kowalska, K. PCL and PCL/Bioactive Glass Biomaterials as Carriers for Biologically Active Polyphenolic Compounds: Comprehensive Physicochemical and Biological Evaluation. Bioact. Mater. 2020, 6, 1811–1826.
- Li, L.; Gatto, G.J.; Brand, R.M.; Krovi, S.A.; Cottrell, M.L.; Norton, C.; van der Straten, A.; Johnson, L.M. Long-Acting Biodegradable Implant for Sustained Delivery of Antiretrovirals (ARVs) and Hormones. J. Control. Release 2021, 340, 188–199.
- Khodaei, M.; Rostamizadeh, K.; Taromchi, A.H.; Monirinasab, H.; Fathi, M. DDAB Cationic Lipid-MPEG, PCL Copolymer Hybrid Nano-Carrier Synthesis and Application for Delivery of SiRNA Targeting IGF-1R into Breast Cancer Cells. Clin. Transl. Oncol. 2021, 23, 1167–1178.
- Zhang, H.-T.; Yu, M.; Niu, Y.-J.; Liu, W.-Z.; Pang, W.-H.; Ding, J.; Wang, J.-C. Polyarginine-Mediated SiRNA Delivery: A Mechanistic Study of Intracellular Trafficking of PCL-R15/SiRNA Nanoplexes. Mol. Pharm. 2020, 17, 1685–1696.
- Kong, D.; Shi, Y.; Gao, Y.; Fu, M.; Kong, S.; Lin, G. Preparation of BMP-2 Loaded MPEG-PCL Microspheres and Evaluation of Their Bone Repair Properties. Biomed. Pharmacother. 2020, 130, 110516.
- Chen, M.; Feng, Z.; Guo, W.; Yang, D.; Gao, S.; Li, Y.; Shen, S.; Yuan, Z.; Huang, B.; Zhang, Y.; et al. PCL-MECM-Based Hydrogel Hybrid Scaffolds and Meniscal Fibrochondrocytes Promote Whole Meniscus Regeneration in a Rabbit Meniscectomy Model. ACS Appl. Mater. Interfaces 2019, 11, 41626–41639.
- Wei, Y.; Wu, Y.; Zhao, R.; Zhang, K.; Midgley, A.C.; Kong, D.; Li, Z.; Zhao, Q. MSC-Derived SEVs Enhance Patency and Inhibit Calcification of Synthetic Vascular Grafts by Immunomodulation in a Rat Model of Hyperlipidemia. Biomaterials 2019, 204, 13–24.
- Qian, Y.; Zhou, X.; Zhang, F.; Diekwisch, T.G.H.; Luan, X.; Yang, J. Triple PLGA/PCL Scaffold Modification Including Silver-Impregnation, Collagen-Coating, and Electrospinning Significantly Improve Biocompatibility, Antimicrobial, and Osteogenic Properties for Oro-Facial Tissue Regeneration. ACS Appl. Mater. Interfaces 2019, 11, 37381–37396.
- Stromberg, Z.R.; Lisa Phipps, M.; Magurudeniya, H.D.; Pedersen, C.A.; Rajale, T.; Sheehan, C.J.; Courtney, S.J.; Bradfute, S.B.; Hraber, P.; Rush, M.N.; et al. Formulation of Stabilizer-Free, Nontoxic PLGA and Elastin-PLGA Nanoparticle Delivery Systems. Int. J. Pharm. 2021, 597, 120340.
- Anversa Dimer, F.; de Souza Carvalho-Wodarz, C.; Goes, A.; Cirnski, K.; Herrmann, J.; Schmitt, V.; Pätzold, L.; Abed, N.; De Rossi, C.; Bischoff, M.; et al. PLGA Nanocapsules Improve the Delivery of Clarithromycin to Kill Intracellular Staphylococcus Aureus and Mycobacterium Abscessus. Nanomedicine 2020, 24, 102125.
- Gebreel, R.M.; Edris, N.A.; Elmofty, H.M.; Tadros, M.I.; El-Nabarawi, M.A.; Hassan, D.H. Development and Characterization of PLGA Nanoparticle-Laden Hydrogels for Sustained Ocular Delivery of Norfloxacin in the Treatment of Pseudomonas Keratitis: An Experimental Study. Drug Des. Dev. Ther. 2021, 15, 399–418.
- Govoni, M.; Lamparelli, E.P.; Ciardulli, M.C.; Santoro, A.; Oliviero, A.; Palazzo, I.; Reverchon, E.; Vivarelli, L.; Maso, A.; Storni, E.; et al. Demineralized Bone Matrix Paste Formulated with Biomimetic PLGA Microcarriers for the Vancomycin Hydrochloride Controlled Delivery: Release Profile, Citotoxicity and Efficacy against S. Aureus. Int. J. Pharm. 2020, 582, 119322.
- Jadidi, A.; Salahinejad, E.; Sharifi, E.; Tayebi, L. Drug-Delivery Ca-Mg Silicate Scaffolds Encapsulated in PLGA. Int. J. Pharm. 2020, 589, 119855.
- Sun, M.; Zhu, C.; Long, J.; Lu, C.; Pan, X.; Wu, C. PLGA Microsphere-Based Composite Hydrogel for Dual Delivery of Ciprofloxacin and Ginsenoside Rh2 to Treat Staphylococcus Aureus-Induced Skin Infections. Drug Deliv. 2020, 27, 632–641.
- Allavena, P.; Palmioli, A.; Avigni, R.; Sironi, M.; La Ferla, B.; Maeda, A. PLGA Based Nanoparticles for the Monocyte-Mediated Anti-Tumor Drug Delivery System. J. Biomed. Nanotechnol. 2020, 16, 212–223.
- Chung, K.; Ullah, I.; Kim, N.; Lim, J.; Shin, J.; Lee, S.C.; Jeon, S.; Kim, S.H.; Kumar, P.; Lee, S.-K. Intranasal Delivery of Cancer-Targeting Doxorubicin-Loaded PLGA Nanoparticles Arrests Glioblastoma Growth. J. Drug Target. 2020, 28, 617–626.
- Nguyen, H.X.; Banga, A.K. Delivery of Methotrexate and Characterization of Skin Treated by Fabricated PLGA Microneedles and Fractional Ablative Laser. Pharm. Res. 2018, 35, 68.
- Trujillo-Nolasco, R.M.; Morales-Avila, E.; Ocampo-García, B.E.; Ferro-Flores, G.; Gibbens-Bandala, B.V.; Escudero-Castellanos, A.; Isaac-Olive, K. Preparation and in Vitro Evaluation of Radiolabeled HA-PLGA Nanoparticles as Novel MTX Delivery System for Local Treatment of Rheumatoid Arthritis. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109766.
- Yao, W.; Yao, J.; Qian, F.; Que, Z.; Yu, P.; Luo, T.; Zheng, D.; Zhang, Z.; Tian, J. Paclitaxel-Loaded and Folic Acid-Modified PLGA Nanomedicine with Glutathione Response for the Treatment of Lung Cancer. Acta Biochim. Biophys. Sin. 2021, 53, 1027–1036.
- Yin, Y.; Wang, J.; Yang, M.; Du, R.; Pontrelli, G.; McGinty, S.; Wang, G.; Yin, T.; Wang, Y. Penetration of the Blood-Brain Barrier and the Anti-Tumour Effect of a Novel PLGA-LysoGM1/DOX Micelle Drug Delivery System. Nanoscale 2020, 12, 2946–2960.
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-Coated PLGA Nanoparticles for the Nasal Delivery of Ropinirole Hydrochloride: In Vitro and Ex Vivo Evaluation of Efficacy and Safety. Int. J. Pharm. 2020, 589, 119776.
- Nigam, K.; Kaur, A.; Tyagi, A.; Nematullah, M.; Khan, F.; Gabrani, R.; Dang, S. Nose-to-Brain Delivery of Lamotrigine-Loaded PLGA Nanoparticles. Drug Deliv. Transl. Res. 2019, 9, 879–890.
- Sun, Y.; Long, D. Preparation, Characterization and in Vitro/in Vivo Evaluation of Lovastatin-Loaded PLGA Microspheres by Local Administration for Femoral Head Necrosis. Drug Des. Dev. Ther. 2021, 15, 601–610.
- Saffari, T.M.; Chan, K.; Saffari, S.; Zuo, K.J.; McGovern, R.M.; Reid, J.M.; Borschel, G.H.; Shin, A.Y. Combined Local Delivery of Tacrolimus and Stem Cells in Hydrogel for Enhancing Peripheral Nerve Regeneration. Biotechnol. Bioeng. 2021, 118, 2804–2814.
- Pan, S.; Qi, Z.; Li, Q.; Ma, Y.; Fu, C.; Zheng, S.; Kong, W.; Liu, Q.; Yang, X. Graphene Oxide-PLGA Hybrid Nanofibres for the Local Delivery of IGF-1 and BDNF in Spinal Cord Repair. Artif. Cells Nanomed. Biotechnol. 2019, 47, 651–664.
- Attias Cohen, S.; Kingma, P.S.; Whitsett, J.A.; Goldbart, R.; Traitel, T.; Kost, J. SP-D Loaded PLGA Nanoparticles as Drug Delivery System for Prevention and Treatment of Premature Infant’s Lung Diseases. Int. J. Pharm. 2020, 585, 119387.
- Mahmoud, M.Y.; Sapare, S.; Curry, K.C.; Demuth, D.R.; Steinbach-Rankins, J.M. Rapid Release Polymeric Fibers for Inhibition of Porphyromonas Gingivalis Adherence to Streptococcus Gordonii. Front. Chem. 2019, 7, 926.
- Song, Y.; Shi, Y.; Zhang, L.; Hu, H.; Zhang, C.; Yin, M.; Chu, L.; Yan, X.; Zhao, M.; Zhang, X.; et al. Synthesis of CSK-DEX-PLGA Nanoparticles for the Oral Delivery of Exenatide to Improve Its Mucus Penetration and Intestinal Absorption. Mol. Pharm. 2019, 16, 518–532.
- Mohammadpour, F.; Hadizadeh, F.; Tafaghodi, M.; Sadri, K.; Mohammadpour, A.H.; Kalani, M.R.; Gholami, L.; Mahmoudi, A.; Chamani, J. Preparation, in Vitro and in Vivo Evaluation of PLGA/Chitosan Based Nano-Complex as a Novel Insulin Delivery Formulation. Int. J. Pharm. 2019, 572, 118710.
- Wang, W.; Yu, C.; Zhang, F.; Li, Y.; Zhang, B.; Huang, J.; Zhang, Z.; Jin, L. Improved Oral Delivery of Insulin by PLGA Nanoparticles Coated with 5β-Cholanic Acid Conjugated Glycol Chitosan. Biomed. Mater. 2021, 16, 064103.
- Jo, A.; Ringel-Scaia, V.M.; McDaniel, D.K.; Thomas, C.A.; Zhang, R.; Riffle, J.S.; Allen, I.C.; Davis, R.M. Fabrication and Characterization of PLGA Nanoparticles Encapsulating Large CRISPR-Cas9 Plasmid. J. Nanobiotechnol. 2020, 18, 16.
- Zhu, J.; Qin, F.; Ji, Z.; Fei, W.; Tan, Z.; Hu, Y.; Zheng, C. Mannose-Modified PLGA Nanoparticles for Sustained and Targeted Delivery in Hepatitis B Virus Immunoprophylaxis. AAPS PharmSciTech 2019, 21, 13.
- Li, W.; Liu, Y.; Zhang, P.; Tang, Y.; Zhou, M.; Jiang, W.; Zhang, X.; Wu, G.; Zhou, Y. Tissue-Engineered Bone Immobilized with Human Adipose Stem Cells-Derived Exosomes Promotes Bone Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 5240–5254.
- Ko, K.-W.; Park, S.-Y.; Lee, E.H.; Yoo, Y.-I.; Kim, D.-S.; Kim, J.Y.; Kwon, T.G.; Han, D.K. Integrated Bioactive Scaffold with Polydeoxyribonucleotide and Stem-Cell-Derived Extracellular Vesicles for Kidney Regeneration. ACS Nano 2021, 15, 7575–7585.
- Ramesh, B.; Cherian, K.M.; Fakoya, A.O.J. Fabrication and Electrospinning of 3D Biodegradable Poly-l-Lactic Acid (PLLA) Nanofibers for Clinical Application. In Stem Cell Nanotechnology: Methods and Protocols; Turksen, K., Ed.; Methods in Molecular Biology; Springer: New York, NY, USA, 2020; pp. 119–128.
- Ghaeini-Hesaroeiye, S.; Boddohi, S.; Vasheghani-Farahani, E. Dual Responsive Chondroitin Sulfate Based Nanogel for Antimicrobial Peptide Delivery. Int. J. Biol. Macromol. 2020, 143, 297–304.
- He, J.; Hu, X.; Cao, J.; Zhang, Y.; Xiao, J.; Peng, L.; Chen, D.; Xiong, C.; Zhang, L. Chitosan-Coated Hydroxyapatite and Drug-Loaded Polytrimethylene Carbonate/Polylactic Acid Scaffold for Enhancing Bone Regeneration. Carbohydr. Polym. 2021, 253, 117198.
- Kiani, M.H.; Ali, S.; Qadry, A.; Arshad, R.; Aslam, A.; Shahnaz, G. Polyethylene Imine Conjugated Supramolecular Stereocomplexed Nanomicelles for Intracellular Delivery of Rifampicin against Mycobacterium Bovis. Colloids Surf. B Biointerfaces 2021, 206, 111976.
- Lim, D.-J.; McCormick, J.; Skinner, D.; Zhang, S.; Elder, J.B.; McLemore, J.G.; Allen, M.; West, J.M.; Grayson, J.W.; Rowe, S.M.; et al. Controlled Delivery of Ciprofloxacin and Ivacaftor via Sinus Stent in a Preclinical Model of Pseudomonas Sinusitis. Int. Forum Allergy Rhinol. 2020, 10, 481–488.
- Cardoso, M.M.; Peca, I.N.; Lopes, T.; Gardner, R.; Bicho, A. Double-Walled Poly-(D,L-Lactide-Co-Glycolide) (PLGA) and Poly(L-Lactide) (PLLA) Nanoparticles for the Sustained Release of Doxorubicin. Polymers 2021, 13, 3230.
- Tan, W.; Gao, C.; Feng, P.; Liu, Q.; Liu, C.; Wang, Z.; Deng, Y.; Shuai, C. Dual-Functional Scaffolds of Poly(L-Lactic Acid)/Nanohydroxyapatite Encapsulated with Metformin: Simultaneous Enhancement of Bone Repair and Bone Tumor Inhibition. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111592.
- Tu, H.; Dai, F.; Cheng, G.; Yuan, M.; Zhou, X.; Wang, Y.; Zhang, R.; Zheng, Y.; Cheng, Y.; Deng, H. Incorporation of Layered Rectorite into Biocompatible Core-Sheath Nanofibrous Mats for Sustained Drug Delivery. ACS Biomater. Sci. Eng. 2021, 7, 4509–4520.
- Wang, Y.; Zhu, P.; Li, G.; Zhu, S.; Liu, K.; Liu, Y.; He, J.; Lei, J. Amphiphilic Carboxylated Cellulose-g-Poly(l-Lactide) Copolymer Nanoparticles for Oleanolic Acid Delivery. Carbohydr. Polym. 2019, 214, 100–109.
- Wang, Y.; Cui, S.; Wu, B.; Zhang, Q.; Jiang, W. PLA-Based Core-Shell Structure Stereocomplexed Nanoparticles with Enhanced Loading and Release Profile of Paclitaxel. Front. Biosci. (Landmark Ed.) 2021, 26, 517–532.
- Birhanu, G.; Tanha, S.; Akbari Javar, H.; Seyedjafari, E.; Zandi-Karimi, A.; Kiani Dehkordi, B. Dexamethasone Loaded Multi-Layer Poly-l-Lactic Acid/Pluronic P123 Composite Electrospun Nanofiber Scaffolds for Bone Tissue Engineering and Drug Delivery. Pharm. Dev. Technol. 2019, 24, 338–347.
- Lugasi, L.; Grinberg, I.; Rudnick-Glick, S.; Okun, E.; Einat, H.; Margel, S. Designed Proteinoid Polymers and Nanoparticles Encapsulating Risperidone for Enhanced Antipsychotic Activity. J. Nanobiotechnol. 2020, 18, 149.
- Nanaki, S.G.; Spyrou, K.; Bekiari, C.; Veneti, P.; Baroud, T.N.; Karouta, N.; Grivas, I.; Papadopoulos, G.C.; Gournis, D.; Bikiaris, D.N. Hierarchical Porous Carbon-PLLA and PLGA Hybrid Nanoparticles for Intranasal Delivery of Galantamine for Alzheimer’s Disease Therapy. Pharmaceutics 2020, 12, 227.
- Liu, W.; Lee, B.-S.; Mieler, W.F.; Kang-Mieler, J.J. Biodegradable Microsphere-Hydrogel Ocular Drug Delivery System for Controlled and Extended Release of Bioactive Aflibercept In Vitro. Curr. Eye Res. 2019, 44, 264–274.
- Rudeen, K.M.; Liu, W.; Mieler, W.F.; Kang-Mieler, J.J. Simultaneous Release of Aflibercept and Dexamethasone from an Ocular Drug Delivery System. Curr. Eye Res. 2022, 47, 1034–1042.
- Chung, H.-J.; Kim, J.-T.; Kim, H.-J.; Kyung, H.-W.; Katila, P.; Lee, J.-H.; Yang, T.-H.; Yang, Y.-I.; Lee, S.-J. Epicardial Delivery of VEGF and Cardiac Stem Cells Guided by 3-Dimensional PLLA Mat Enhancing Cardiac Regeneration and Angiogenesis in Acute Myocardial Infarction. J. Control. Release 2015, 205, 218–230.
- Kong, Y.; Zhao, Y.; Li, D.; Shen, H.; Yan, M. Dual Delivery of Encapsulated BM-MSCs and BMP-2 Improves Osteogenic Differentiation and New Bone Formation. J. Biomed. Mater. Res. A 2019, 107, 2282–2295.
- Heidariyan, Z.; Ghanian, M.H.; Ashjari, M.; Farzaneh, Z.; Najarasl, M.; Rezaei Larijani, M.; Piryaei, A.; Vosough, M.; Baharvand, H. Efficient and Cost-Effective Generation of Hepatocyte-like Cells through Microparticle-Mediated Delivery of Growth Factors in a 3D Culture of Human Pluripotent Stem Cells. Biomaterials 2018, 159, 174–188.
- Kang, I.-G.; Kim, J.; Park, S.; Kim, H.-E.; Han, C.-M. PLLA Membrane with Embedded Hydroxyapatite Patterns for Improved Bioactivity and Efficient Delivery of Growth Factor. Macromol. Biosci. 2020, 20, e2000136.
- Wu, L.; Gu, Y.; Liu, L.; Tang, J.; Mao, J.; Xi, K.; Jiang, Z.; Zhou, Y.; Xu, Y.; Deng, L.; et al. Hierarchical Micro/Nanofibrous Membranes of Sustained Releasing VEGF for Periosteal Regeneration. Biomaterials 2020, 227, 119555.
- Xia, B.; Lv, Y. Dual-Delivery of VEGF and NGF by Emulsion Electrospun Nanofibrous Scaffold for Peripheral Nerve Regeneration. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 82, 253–264.
- Muniyandi, P.; Palaninathan, V.; Mizuki, T.; Mohamed, M.S.; Hanajiri, T.; Maekawa, T. Scaffold Mediated Delivery of Dual MiRNAs to Transdifferentiate Cardiac Fibroblasts. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112323.
- Javazmi, L.; Young, A.; Ash, G.J.; Low, T. Kinetics of Slow Release of Nitrogen Fertiliser from Multi-Layered Nanofibrous Structures. Sci. Rep. 2021, 11, 4871.
- Swanson, W.B.; Zhang, Z.; Xiu, K.; Gong, T.; Eberle, M.; Wang, Z.; Ma, P.X. Scaffolds with Controlled Release of Pro-Mineralization Exosomes to Promote Craniofacial Bone Healing without Cell Transplantation. Acta Biomater. 2020, 118, 215–232.
More
