You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is a comparison between Version 1 by Fuad Gandhi Torizal and Version 2 by Sirius Huang.
Human pluripotent stem cells (hPSCs) have become a powerful tool to generate the various kinds of cell types comprising the human body. Organoid technology has emerged as a platform to generate a physiologically relevant tissue-like structure from PSCs. Compared to an actual human organ, this structure more closely represents a three-dimensional microenvironment than the conventional monolayer culture system for transplantation, disease modeling, and drug development.
- hPSCs derived-organoids
- culture strategy
- disease modeling
- drug screening
- regenerative therapy
1. Introduction
Animal models are a routinely used platform for understanding many biological processes related to human disease. Although they can partially represent human physiological and pathological conditions, these models cannot accurately provide similar features for human tissue physiology and disease mechanisms due to the interspecies genomic divergence which impacts various complex cellular activities [1][2][1,2]. In actual translational applications, this discrepancy can reduce accuracy, which potentially contributes to a low success rate in their utilization (for example, clinical trials of newly developed drugs) [3]. On the other hand, commonly used two-dimensional human cell cultures often do not completely represent actual human tissue function and architecture [4].
Organoids are three-dimensional miniature organ-like structures that recapitulate physiologically relevant tissue function through self-organization in vitro. The generation of a human pluripotent stem cells (hPSCs) derived organoid can facilitate the improvement of tissue culture by addressing current limitations in disease modeling or drug screening platforms, as well as providing new insights into their application in regenerative therapy [3].
The development of human-induced pluripotent stem cells (hiPSCs) allows for greater experimental accessibility with fewer ethical constraints than the usage of human embryonic stem cells (hESCs), which require a sacrificial human embryo. Moreover, PSCs can be generated either from a healthy or diseased individual. This can be useful for personalized medicine applications, such as disease modeling [5], drug screening [6], or even regenerative therapy [7].
Currently, hPSCs-derived organoid production mostly involves a complex multistep protocol during their differentiation and maturation process, which makes the production of organoids technically challenging. Various methods have been developed to produce organoids from PSCs. However, some important factors related to the organoid culture system need to be considered to achieve the optimum culture conditions for each specific translational purpose.
2. The Main Features of hPSCs Derived-Organoids
Organoids have become a promising research tool for elucidating the mechanism of various diseases and for testing the effect of substance exposure, which provides a precise preclinical setting for research [8]. Recently, the concept of organoid transplantation has emerged as a potential method for obtaining insight into alternative partial tissue replacement for regeneration [9]. Nevertheless, possible improvements to the current culture methodologies still need to be explored to achieve better overall reliability for translational applications.
At present, numerous studies of hPSCs-derived organoids have succeeded in representing a broad spectrum of specific organ types for different applications. A 3D organoid can enable crosstalk between different cells inside its structure, something which cannot be achieved by a conventional monolayer culture. Therefore, these 3D “miniaturized organs” possess decent potential to become physiologically relevant models and better predictive tools than monolayer cultures, which reflect the cellular interactions between different cells within the organ during their development, healthy or diseased conditions.
Generally, an organoid needs to meet some criteria which confirm the authenticity of the original organ [10]. First, the organoid should be a three-dimensional (3D) structure consisting of multiple cell types that retain the identity of the specific organ. Secondly, this 3D structure should be formed by self-organization according to the similar intrinsic organization principles of the organ. Thirdly, the organoid should recapitulate the key features that represent the functional capability of the actual organ (both in terms of structure and physiological activity) [11][12][11,12].
3. Culture Strategy to Generate hPSCs Derived-Organoids
Currently, several approaches have been developed to generate a miniaturized tissue-like organoid comprising the complex functional cellular components derived from hPSCs. These methods mostly involve a differentiation process via a stepwise treatment using specific combined factors to direct the hPSC cell fates.
This step can be improved by creating or adjusting the in vitro culture condition to stimulate subsequent organ-specific lineage differentiation using different available culture platforms. Depending on the particular application and expected organoid type, several factors need to be considered when choosing a culture strategy to obtain the best hPSCs derived-organoid production outcomes (Table 1).
Table 1.
A brief comparison of the common culture platform for organoid production.
| Consideration | Animal Model | hPSCs Differentiation or Coculture in Monolayer |
hPSCs Derived-Organoid | ||
|---|---|---|---|---|---|
| Matrigel Embedded /Dome |
Static Suspension Culture | Dynamic Suspension Culture | |||
| Culture maintenance | Not required, but difficult animal handling | Easy | Relatively easy |
Relatively Easy |
Relatively easy |
| Represent actual human organ’s physiology | Partial, limited by interspecies variability | Poor | good | Good | Good |
| Tissue complexity | Very good | Poor | good | Good | Good |
| ECM and cell-cell interaction | Very good, native tissue condition | Poor | good | Good | Good |
| Ability to represent organogenesis/developmental biology | No | No | Yes | Yes | Yes |
| Represent actual human organs physiology | Partial, limited by interspecies variability | Poor | good | Good | Good |
| Scalability | Not available | Low | Low | High | High |
| Relative cost production | high | Relatively high | Relatively low | Low | Low |
| Application for personalized medicine and non-xenogeneic transplantation | Not possible | possible | Not possible | Possible | Possible |
3.1. Direct Organoid Differentiation from hPSCs EBs
Several methodologies were previously developed for generating hPSCs-derived organoids to represent miniaturized specific tissues or organs. The simplest strategy is to directly induce differentiation toward organoid formation from the three-dimensional structure of hPSCs (which are referred to as embryoid bodies (EBs)). This structure can be generated using the dynamic suspension culture, hanging drop techniques, or by using Matrigel (Figure 1). Subsequently, one can employ a stepwise treatment using a cocktail of biomolecule inducers, which mostly consists of lineage-specific signaling factors (e.g., small molecule and/or growth factors) to mimic the cellular signaling during organogenesis in embryo development. This biomolecule formulation differs between the organoid types, depending ultimately on which pathways that needed to direct the hPSCs lineage. For example, hPSCs differentiation into the hepatic lineage requires exposure to a high concentration of Activin A during the endodermal differentiation followed by BMP4 to further direct the endoderm cells into hepatic competent cells. Here, final liver organoid maturation can be induced by HGF and OSM in the differentiation medium [13][44].
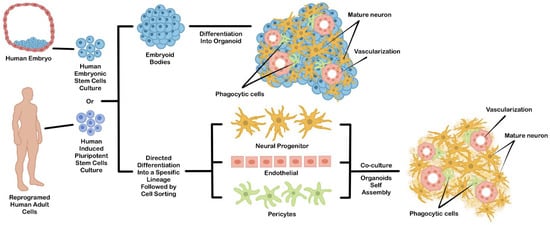
Figure 1. An example of general strategies used to generate hPSCs-derived brain organoids. This organoid can be generated directly from differentiated EBs (upper chart) or by coculture from independently differentiated hPSCs-derived cell types (lower chart).
Direct EBs-based differentiation permits intrinsic self-organization during directed differentiation, which generates three-dimensional structures consisting of multiple cell types comprising a specific organ [14][52]. Due to their spontaneous morphogenesis during differentiation, this approach can be utilized for developing a model for the developmental study of a certain organ during embryogenesis. However, the emergence of undesirable cell types that did not complement the expected organ is more difficult to control during differentiation. This problem may reduce reliability in other translational applications, such as transplantation or drug testing.
3.2. Coculture of Multiple Differentiated Cells
The problem of cell heterogeneity coming from undesirable differentiated cell types often makes the reproducibility of the resulting organoid quite low. To solve this problem, hPSCs can be differentiated separately into specific cell types, sorted, and then mixed to form an organoid. This technique can be a solution for addressing the uncontrollable heterogeneous cell population problems. The hPSCs-derived cellular components can be achieved by performing an in vitro co-culture of multiple PSCs-derived cells or PSCs-derived progenitor cells (Figure 1). For example, the specific progenitor cell type can be individually differentiated from PSCs using a monolayer culture. Then, each cell component is purified and co-cultured together to induce their self-organization into a 3D structure. Since the organoid was assembled from an independent and specific hPSCs-derived cell type, the occurrence of other cells which are not normally part of the expected organs can be minimized. Additionally, the cellular diversity inside the organoids can be controlled by mixing each cell type based on the actual cellular composition ratio of the original organ [15][53]. This approach can be further improved by some engineering techniques, such as 3D cell bioprinting or scaffold based-template to partially direct the arrangement of each cellular component inside the organoid structure [16][54].
4. General Culture Platform to Generate the hPSCs Derived-Organoids
4.1. Matrigel Embedded Technique
As similar as in vivo organ development, extracellular matrix (ECM) remodeling plays an essential role in organoid morphogenesis. This component is very important (mainly for the branching formation that dictates the functional architecture of certain organs, such as the salivary glands, lungs, or kidneys) [17][55]. Matrigel is an extracellular matrix extracted from Engelbreth-Holm-Swarm mouse sarcomas which are routinely used in various cell culture applications [18][56]. During organoid differentiation, the Matrigel dome serves as structural support and provides a three-dimensional niche for the organoid assembly [18][19][20][21][56,57,58,59] (Figure 2a).
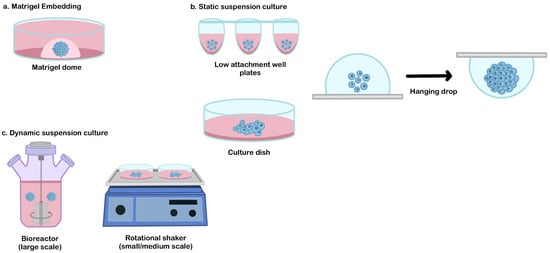
Figure 2. The common culture platform for the hPSCs derived-organoid. (a) Matrigel embedding technique, (b) static suspension culture, and (c) dynamic suspension culture.
Despite its benefits in organoid culture, Matrigel also has some disadvantages. The variations in biochemical composition and mechanical variability of this sarcoma-derived-ECM complex may result in single- and batch-to-batch variability during the hPSCs-derived organoids culture [22][60]. Although this approach can be used for developmental study or disease modeling, this method may not be compatible with therapeutic applications. For example, xenogeneic materials need to be eliminated to achieve safe transplantable organoids. These animal-derived components such as Matrigel may potentially induce antigenicity and transmit pathogens such as viruses into the human recipient [23][61].
Recently, several efforts have been made to find synthetic alternatives for Matrigel [22][24][25][60,62,63]. These alternative materials may provide a chemically defined and xeno-free niche that can be independently tuned by adjusting various variables (such as their composition) and by using polymerization methods to produce expected mechanical and biological properties [22][24][60,62]. Moreover, by using a bioengineering approach, these synthetic matrices can be further utilized to provide a specific boundary guiding tissue morphogenesis [26][64].
Another challenge of the Matrigel embedded method is related to its application in organoid-based transplantation of large organs. Due to its technical difficulties in scaling up, this technique may be limited to small-scale drug screening and disease modeling.
4.2. Static Suspension Culture
A static suspension culture can be used as an alternative method to produce broad quantities of organoid production, from a small scale (e.g., patterned well plates or hanging drop method), medium-scale (e.g., low attachment culture dish [27][28][65,66]), or larger scale (e.g., oxygen-permeable static tissue culture bag [29][67]) (Figure 2b). This approach can be selected when the usage of ECM embedded is not required and when the interference of hydrodynamic conditions needs to be avoided to generate a specific lineage that is sensitive to shear stress (such as in hepatic differentiation) [30][68].
Traditionally, the formation of a 3D structure relies on spontaneous aggregation in the static suspension culture. This simple method has been adapted to generate hPSCs-derived organoids in medium or large-scale organoid productions (for example, using a low attachment culture dish [27][28][65,66] or culture bag [29][67]). The main drawback of this technique is the failure to control EBs aggregation. Due to the epithelial characteristic of hPSCs, spontaneous aggregation often occurs and results in a random size. These conditions may impact the variability of their 3D-differentiated organoid structure. Therefore, several alternative methods have been developed to improve aggregation control, such as the hanging drop method or patterned culture vessel (Figure 2b). The hanging drop method is a technique used to induce cellular assembly by utilizing gravitational forces. In this method, a cell suspension droplet is hung on the reversed surface, and the EBs are formed from the surface tension properties of the culture medium. This method is relatively simple during cell inoculation, but the medium replacement is very challenging due to the limited droplet volume that can be hung on the culture vessel surface [31][69]. Patterned well plates (e.g., microwell plates, U or V-bottom-well plates) can be used as a tool to force and control cell aggregation [32][33][14,70]. By adjusting the cell number, precise control of the EBs size can be enabled, which significantly reduces the variability of the resulting organoids. This culture system has potential for use in drug screening or disease modeling but is difficult to be applied for the therapeutic purposes of large organs that require a large number of organoids (due to the difficulties involved in their scalability).
4.3. Dynamic Suspension Culture
Dynamic suspension culture is a culture technique that is commonly used to produce a medium to a large number of organoids for various applications (such as transplantation of large organs) [23][61]. A dynamic culture environment can be achieved by using rotating or stirring mechanisms to promote the formation of a spherical 3D formation and control its initial size. The simple platform that is routinely used for organoid generation is by using the rotational shaker or stirred bioreactor (Figure 2c). These types of suspension cultures enable better control of the size and uniformity of organoids population in the culture system. The size control of initial EBs influences the dynamic profile of their pluripotency and lineage specification during differentiation [34][71]. The spheroid size and uniformity can be controlled by adjusting the inoculation density and manipulating the medium dynamics (such as rotation/stirring speed, fluidic flow, or medium volume) [23][35][61,72].
Hydrodynamic condition is an important biomechanical factor involved in dynamic suspension culture that may affect the differentiation process of hPSCs. Despite its importance in controlling agglomeration and medium mixing, the amount of hydrodynamic shear force needs to be carefully considered. A previous comparison study showed that diverse hydrodynamic conditions coming from the shear force in various culture vessels which were set up (such as rotational cultures, ring-shaped culture vessels, or spinner flasks) may implicate the differentiation tendencies of hPSCs. The results indicated that higher shear stress may improve the hPSC EBs differentiation tendency toward an ectoderm and mesoderm lineage rather than an endoderm lineage [34][71]. A study conducted by Wolfe, et al. revealed that the shear force was potentially inducing early germ specification into ectodermal and mesodermal lineage on stress magnitude, ranging from 1.5 to 15 dynes cm−2 [36][73]. This result also corresponds well with another study conducted by Vosough et al. which reports several spontaneously differentiated-mesodermal populations from hepatic differentiation (which is presumably exposed by excessive shear stress in the stirred tank bioreactor) [30][68].
