Allergic rhinitis (AR) is a common medical condition affecting up to 40% of the general population. A type 2 immunity determines eosinophilic inflammation that, in turn, elicits typical nasal symptoms. Type 2 immunity is eminently characterized by polarization of innate and adaptative B and T cells, increased production of type 2 cytokines, including interleukin-4 (IL-4), IL-5, and IL-13, and impaired function of allergen-specific T regulatory cells (Tregs). This immunologic derangement promotes allergic inflammation, characterized by an abundant eosinophilic infiltrate and the presence of mast cells. The mast cells are activated by allergen exposure and release pro-inflammatory mediators, including histamine. These mediators interact with specific receptors and, consequently, are responsible for the appearance of typical AR symptoms: nasal itching, sneezing, rhinorrhea, and nasal congestion.
1. The Rationale for Probiotic Use in Allergic Rhinitis Management
There is a well-established awareness that the prevalence of allergic diseases has been impressively raised in the last decades. Consequently, several attempts have been made to explain this important phenomenon. In this context, the hygiene hypothesis has garnered much acclaim and has been mainly studied
[1][18]. The observation has sustained the so-called allergy epidemics in which poor hygienic conditions protect from allergies while “clean” settings arrange for allergies. Namely, adequate microbiota biodiversity guarantees a correct maturation of the immune system in infants
[2][19]. Contrarily, the impaired composition of microbiota facilitates poor immunity maturation and favors allergy
[3][20]. Moreover, newborns display a type 2 immunity as this immune disposition protects the fetus from the potential maternal rejection as recognized non-self.
Allergic subjects present dysbiosis and impaired biodiversity in the gut and target organs
[4][21]. Dysbiosis harms AR as it promotes allergic inflammation. However, there is evidence that some environmental factors may contribute to eubiosis
[5][22]. In particular, exposure to microbial fragments (e.g., farming settings) promotes balanced microbiota preventing allergy
[6][23].
Moreover, a gut-airways axis permits the crosstalk between the digestive tract and respiratory mucosa. In other words, enteric microbiota significantly affects respiratory microbiota and vice versa
[7][24]. Subjects with AR present a dysbiosis associated with inflammatory pathways and symptom severity
[8][25]. Therefore, the combination of all this evidence suggests the possibility of manipulating the immune system to return a physiological response in AR. In this regard, probiotics have been proposed as an ideal candidate to counteract dysbiosis in allergic rhinitis
[9][26]. Probiotics are “live microorganisms which confer a beneficial effect on the host”, as proposed by the World Health Organization
[10][27].
Probiotic supplementation may exert manifold benefits due to its complex mechanism of action
[11][28]. Probiotics represent the current strategy to heal dysbiosis revitalize biodiversity, and balance microbiota. However, the mechanisms of action are complicated and still not completely defined. The detailed description of the probiotics mechanisms of action goes beyond the purpose of the present review. Consequently, a summary of the concept will be presented. Many recent reviews are available
[12][13][14][15][16][17][18][19][20][21][22][23][24][29,30,31,32,33,34,35,36,37,38,39,40,41].
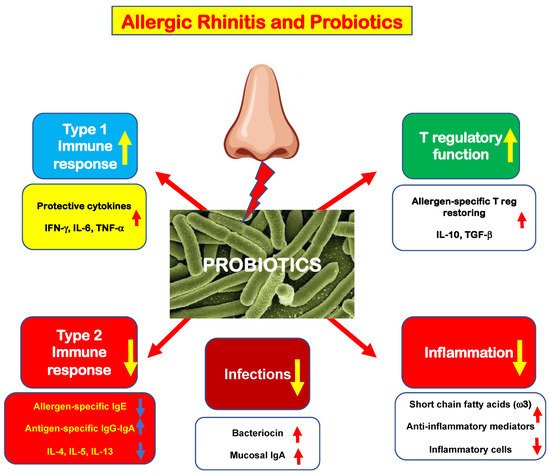
Briefly, probiotics act on innate immunity through the toll-like receptor, switching off the antigen-presenting machinery. The activation of innate immunity displays several positive effects, as schematically represented in Figure 1. However, some effects on immune results have to be presumed as the present evidence derives from small studies.
Figure 1. Schematic representation of the possible probiotic mechanisms of action in allergic rhinitis.
First, probiotics may down-regulate type 2 immunity by reducing allergen-specific IgE and “pro-allergic” cytokine production; contextually, probiotics stimulate allergen-specific IgG and IgA production. Consistently, probiotics may expand type 1 immunity, increasing the production of “protective” cytokines (IFN-γ, IL-6, and TNF-α). As a result, probiotics make allergic subjects less prone to infections and also promote the synthesis of anti-infective agents, such as bacteriocin and secretory IgA. In addition, probiotics may dampen the inflammatory cascade by stimulating the production of anti-inflammatory mediators, including short-chain fatty acids (mainly ω3), that reduce cellular infiltrate. Finally, probiotics may restore the function of allergen-specific Tregs and consequently increase the production of regulatory cytokines (IL-10 and TGF-β).
Therefore, there is evidence that probiotics could confer some beneficial effects to allergic subjects rebalancing their immunity and reinforcing mucosal defense against pathogens. However, more methodologically robust studies are needed to confirm these possible effects of probiotics.
2. New Evidence in the Literature
Four more studies on the use of probiotics in the treatment of AR have been published in the past year, which were not covered by the meta-analyses mentioned above. Therefore, they will be presented here to provide updated knowledge about this issue.
The first study
ai
smed to investigate the probiotic prophylactic treatment in children with AR
[25][48].
The tested compound was a probiotic mixture including
Bifidobacterium animalis subsp.
Lactis BB12 and
Enterococcus faecium L3. The outcome measures were nasal symptom scores and the need for rescue medication. The study included 250 children and adolescents. Subjects were stratified into two groups: actively treated (150) or control (100). The probiotic mixture was administered three months before the pollen season. Patients treated with probiotics experienced less severe symptoms than controls. Consistently, probiotic-treated children used less symptomatic drugs than controls. Consequently, the investigators concluded that the mixture containing BB12 and L3 exerted significant preventive effects on the AR course.
The second study explored the effects of a commercial probiotic preparation containing four strains:
Lactobacillus acidophilus LA02,
Lactobacillus delbrueckii LDB01,
Lactobacillus rhamnosus LR04, and
Streptococcus thermophilus FP04
[26][49].
Twenty-eight AR patients took the probiotic mixture for 60 days; they were evaluated at baseline, after the treatment, and during a two-month follow-up. The probiotic product significantly reduced rhinitis total symptom scores and visual analog scale. Consistently, probiotics reduced the peripheral eosinophils, the levels of type 2 cytokines IL-4 and IL-5, and increased biodiversity in stool microbiota composition. Interestingly, the microbiota changes correlated with clinical and immunological parameters. Consequently, the study concluded that this probiotic preparation could be envisaged as a helpful add-on strategy in AR patients. The outcomes provided by this study were particularly impressive, mainly concerning the cytological and immunological results. It has to be noted that such positive results were not observed by most other studies exploring these aspects. However, it should be emphasized that this study should be considered a preliminary study conducted on a small sample of patients. Therefore, these results, which are undoubtedly attractive, need confirmation by studies conducted with a more robust and appropriate methodology.
Another study evaluated an intranasally administered probiotic assemblage containing
Lactobacillus rhamnosus SP1,
Lactobacillus paracasei 101/37, and
Lactococcus lactis L1A, compared with placebo
[27][50].
Participants were subdivided into two groups: actively treated (12) and placebo-treated (12). Treatment lasted three weeks. Outcome measures were quality of life scores, total nasal symptom score, peak nasal inspiration flow, fractional exhaled nitric oxide, and cytokines assay. Cultures were also performed to detect the colonization of strains. Unfortunately, the only significant result concerned a slight decrease in IL-17. Therefore, the authors concluded that the topical administration of a probiotic mixture was ineffective in AR patients. Probably, the duration and the topical route were inadequate to observe benefits.
Finally, an Australian study investigated the effects of a probiotic drink (“NC-Seasonal-Biotic”) containing four strains:
Lactobacillus reuteri GL 104,
Lactobacillus plantarum LPL28,
Lactobacillus rhamnosus MP108, and
Bifidobacterium lactis BI04, and a fructooligosaccharide as prebiotic, compared with placebo
[28][51]. Forty patients concluded the 10–12-week intervention period. The active group showed a significant improvement in clinical parameters and consistent quality of life scores. In addition, the probiotic drink restored the Th1/Th2 ratio.
These recent studies, therefore, added new proof evidencing that probiotics could help manage patients suffering from AR. However, two main lessons have to be acknowledged. First, the probiotic supplementation duration should be adequate to allow colonization and completion of the immunological mechanism of action. Second, the effectiveness depends on the specificity of single strains.
Moreover, the outcomes reported in AR were substantially consistent with what has been reported in the asthma model
[29][30][31][52,53,54].