MePlatonin was discovered in plants in the late nineties, but its role, signaling, and crosstalk with other phytohormones remain unknown. Research on melatonin in plants has risen dramatically in recent years and the role of this putative plant hormone under biotic and abiotic stressnts are exposed to a variety of environmental stresses (biotic and abiotic) during the course of development. Stressful conditions has been reported. In the present review, we discuss the main functions of melatonin in the growth and development of plants, its role under abiotic stresses, such as water stress (waterlogging and drought), extreme temperature (low and high), salinity, heavy metal, and light-induced stress. Similarly, we also discuss the role of melatonin under biotic stresses (antiviral, antibacterialalter the basic metabolism of the affected plants. Plants must cope with environmental constraints to effectively complete their life cycle. Plant produce and regulate various biomolecules to adapt to adverse environmental conditions. Melatonin (N-acetyl-5-methoxytryptamine) is a ubiquitous molecule that is present in plants, animals, and antifungal effects). Moreover, the present review meticulously discusses the crosstalk of melatoninmicroorganisms. It has been revealed as an indolic chemical compound with structural similarities with other phytohormonevital compounds such as auxins, gibberellic acids, cytokinins, ethylene, and salicyltryptophan, serotonin, and indole-3-acetic acid under normal and stressful conditions and reports melatonin receptors and signaling in plants. All these aspects of melatonin suggest that phytomelatonin is a key player in crop improvement and(IAA). In plants, melatonin is a putative hormone involved in the regulation of plant growth and productivity, even under biotic and abiotic stress regulationconditions.
- melatonin
- phytohormone
- biotic stress
- abiotic stress
- plant growth
1. Introduction
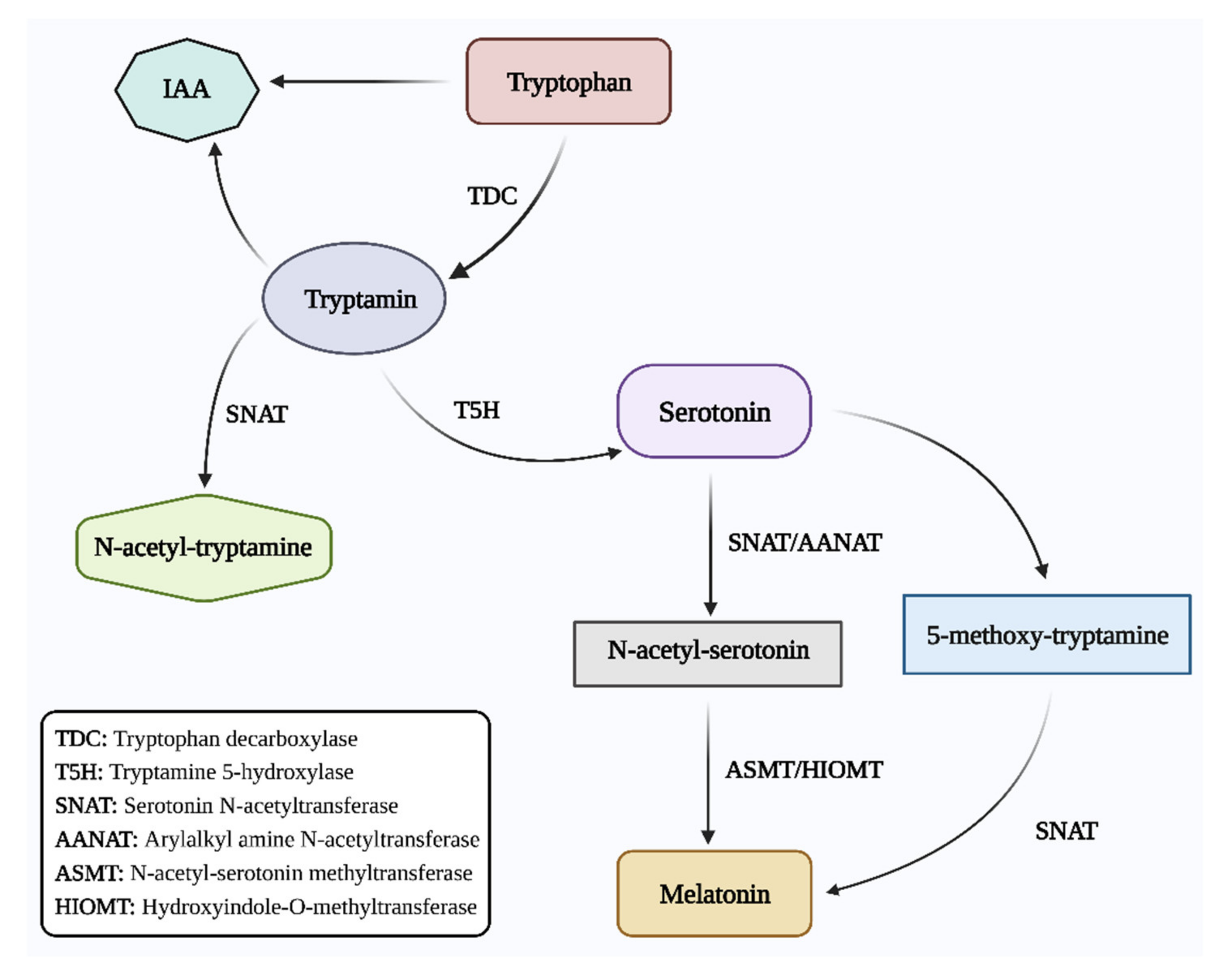
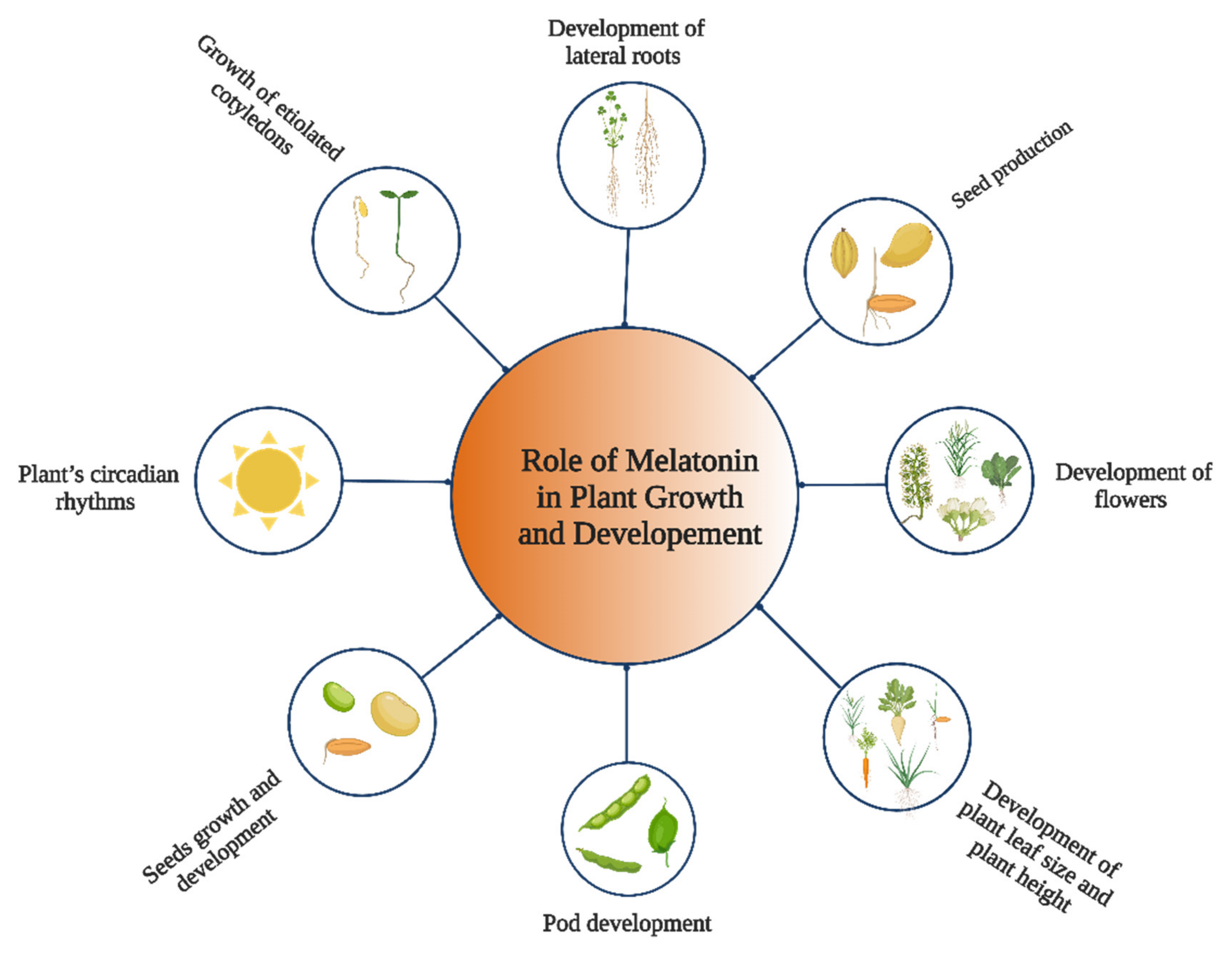
2. Role of Melatonin in Plant Growth and Development
3. Role of Melatonin under Abiotic Stress
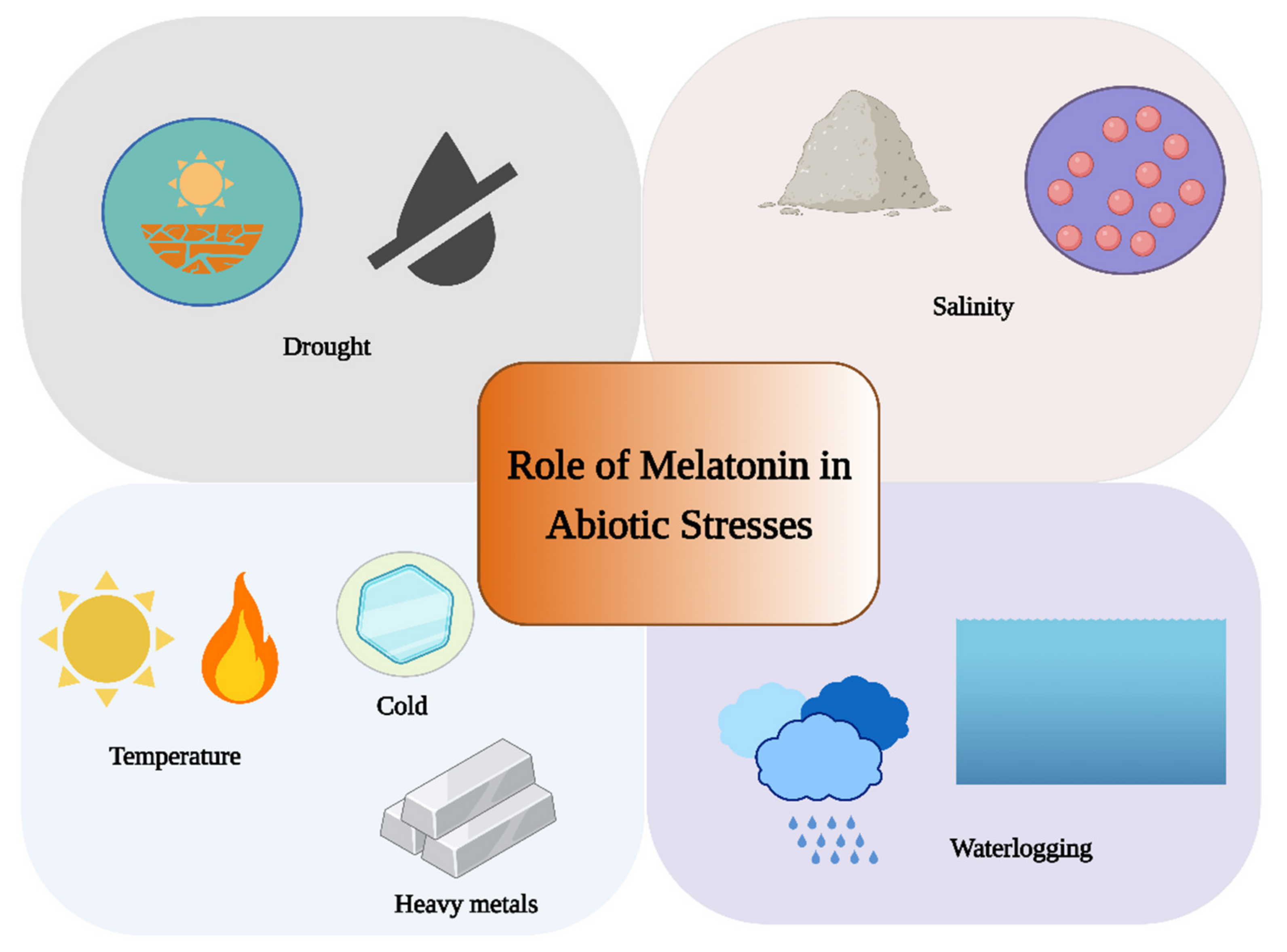
3.1. Role of Melatonin under Water Stress
3.2. Role of Melatonin under Extreme Temperature
3.3. Role of Melatonin under Salt Stress
3.4. Role of Melatonin under Heavy Metal Stress
3.5. Role of Melatonin under Light-Induced Stress
4. Role of Melatonin in Biotic Stress
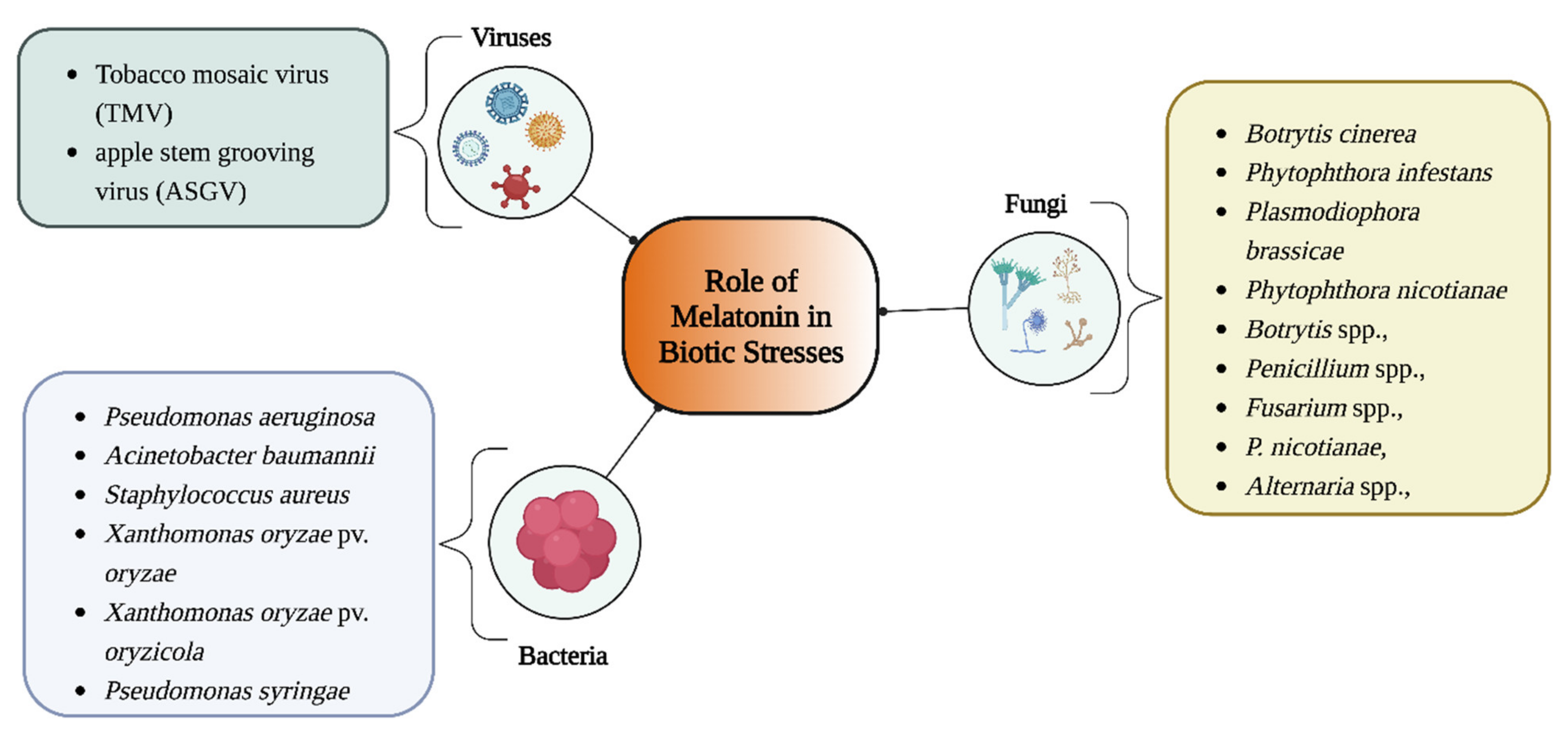
4.1. Antiviral Effects of Melatonin
4.2. Antibacterial Effects of Melatonin
4.3. Antifungal Effects of Melatonin
Melatonin was shown to have therapeutic advantages in animal models of Candida sepsis and conventional antimycotic therapy, where it could reduce interleukin-6 concentrations and shorten the amount of time needed for recovery from Candida sepsis in rats [86][73]. Melatonin promoted tomato fruit resistance to Botrytis cinerea by controlling the production of H2O2 and the jasmonic acid signaling pathway [87][74]. In watermelon and other cucurbits, a rise in melatonin accumulation in plants increases resistance to foliar diseases, such as powdery mildew and soil-borne oomycetes, through alterations in the transcript accumulation of the genes linked to PTI and ETI [81][72]. The prevalence of Plasmodiophora brassicae infection of A. thaliana and the number of pathogen sporangia decreased following melatonin treatment. This decrease was ascribed to the high expression of the JA-responsive PR3 and PR4 genes [12][51]. Melatonin and ethylicin, an oomycete antifungal, work synergistically to prevent the growth of Phytophthora nicotianae in vitro and in vivo by disrupting the fungus’ amino acid metabolic homeostasis [88][75]. Melatonin is exogenously applied to replant soil to promote apple seedling growth, boost potassium levels, and induce photosynthesis, all of which alleviate replant disease [89][76]. Other fungi, such as Botrytis spp., Penicillium spp., Fusarium spp., P. nicotianae, and Alternaria spp., also showed similar results [12][51]. Additionally, several studies have examined the function of endophytic rhizobacteria in enhancing plants’ capacity to synthesize melatonin [90][77]. A number of different theories have explained melatonin’s preventive function against plant fungal infections. For example, some scientists have suggested that melatonin’s defense mechanism involves its capacity to maintain H2O2 cellular concentrations and the production and control of antioxidant enzyme activities [85][78]. Transcriptomic data have recently shown that exogenous melatonin administration activates PTI- and ETI-related genes in watermelon and A. thaliana [81][72]. Additionally, melatonin is essential for controlling the levels of ROS and reactive nitrogen species (RNS) in plants, which are signals for numerous cellular and physiological responses to biotic and abiotic stresses. These responses can be triggered directly by ROS/RNS scavengers or indirectly by genes that control the redox network [91][79].References
- Fan, J.; Xie, Y.; Zhang, Z.; Chen, L. Melatonin: A multifunctional factor in plants. Int. J. Mol. Sci. 2018, 19, 1528.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin: A new plant hormone and/or a plant master regulator? Trends Plant Sci. 2019, 24, 38–48.
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175.
- Posmyk, M.M.; Janas, K.M. Melatonin in plants. Acta Physiol. Plant. 2009, 31, 1.
- Choi, G.H.; Lee, H.Y.; Back, K. Chloroplast overexpression of rice caffeic acid O-methyltransferase increases melatonin production in chloroplasts via the 5-methoxytryptamine pathway in transgenic rice plants. J. Pineal Res. 2017, 63, e12412.
- Lee, K.; Lee, H.Y.; Back, K. Rice histone deacetylase 10 and Arabidopsis histone deacetylase 14 genes encode N-acetylserotonin deacetylase, which catalyzes conversion of N-acetylserotonin into serotonin, a reverse reaction for melatonin biosynthesis in plants. J. Pineal Res. 2018, 64, e12460.
- Kolář, J.; Macháčková, I.; Eder, J.; Prinsen, E.; Van Dongen, W.; Van Onckelen, H.; Illnerová, H. Melatonin: Occurrence and daily rhythm in Chenopodium rubrum. Phytochemistry 1997, 44, 1407–1413.
- Kolář, J.; Johnson, C.H.; Macháčková, I. Exogenously applied melatonin (N-acetyl-5-methoxytryptamine) affects flowering of the short-day plant Chenopodium rubrum. Physiol. Plant. 2003, 118, 605–612.
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707.
- Arnao, M.B.; Hernández-Ruiz, J. Protective effect of melatonin against chlorophyll degradation during the senescence of barley leaves. J. Pineal Res. 2009, 46, 58–63.
- Wen, D.; Gong, B.; Sun, S.; Liu, S.; Wang, X.; Wei, M.; Yang, F.; Li, Y.; Shi, Q. Promoting roles of melatonin in adventitious root development of Solanum lycopersicum L. by regulating auxin and nitric oxide signaling. Front. Plant Sci. 2016, 7, 718.
- Bajwa, V.S.; Shukla, M.R.; Sherif, S.M.; Murch, S.J.; Saxena, P.K. Role of melatonin in alleviating cold stress in Arabidopsis thaliana. J. Pineal Res. 2014, 56, 238–245.
- Saqib, S.; Zaman, W.; Ayaz, A.; Habib, S.; Bahadur, S.; Hussain, S.; Muhammad, S.; Ullah, F. Postharvest disease inhibition in fruit by synthesis and characterization of chitosan iron oxide nanoparticles. Biocatal. Agric. Biotechnol. 2020, 28, 101729.
- Ayaz, A.; Saqib, S.; Huang, H.; Zaman, W.; Lü, S.; Zhao, H. Genome-wide comparative analysis of long-chain acyl-CoA synthetases (LACSs) gene family: A focus on identification, evolution and expression profiling related to lipid synthesis. Plant Physiol. Biochem. 2021, 161, 1–11.
- Wang, Y.; Reiter, R.J.; Chan, Z. Phytomelatonin: A universal abiotic stress regulator. J. Exp. Bot. 2018, 69, 963–974.
- Sun, C.; Liu, L.; Wang, L.; Li, B.; Jin, C.; Lin, X. Melatonin: A master regulator of plant development and stress responses. J. Integr. Plant Biol. 2021, 63, 126–145.
- Al Azzawi, T.N.I.; Khan, M.; Hussain, A.; Shahid, M.; Imran, Q.M.; Mun, B.-G.; Lee, S.-U.; Yun, B.-W. Evaluation of Iraqi rice cultivars for their tolerance to drought stress. Agronomy 2020, 10, 1782.
- Rolly, N.K.; Imran, Q.M.; Shahid, M.; Imran, M.; Khan, M.; Lee, S.-U.; Hussain, A.; Lee, I.-J.; Yun, B.-W. Drought-induced AtbZIP62 transcription factor regulates drought stress response in Arabidopsis. Plant Physiol. Biochem. 2020, 156, 384–395.
- Shi, H.; Jiang, C.; Ye, T.; Tan, D.-X.; Reiter, R.J.; Zhang, H.; Liu, R.; Chan, Z. Comparative physiological, metabolomic, and transcriptomic analyses reveal mechanisms of improved abiotic stress resistance in bermudagrass by exogenous melatonin. J. Exp. Bot. 2015, 66, 681–694.
- Zuo, B.; Zheng, X.; He, P.; Wang, L.; Lei, Q.; Feng, C.; Zhou, J.; Li, Q.; Han, Z.; Kong, J. Overexpression of MzASMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis thaliana plants. J. Pineal Res. 2014, 57, 408–417.
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302.
- Meng, J.F.; Xu, T.F.; Wang, Z.Z.; Fang, Y.L.; Xi, Z.M.; Zhang, Z.W. The ameliorative effects of exogenous melatonin on grape cuttings under water-deficient stress: Antioxidant metabolites, leaf anatomy, and chloroplast morphology. J. Pineal Res. 2014, 57, 200–212.
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.; Yu, M.; Shabala, S. Hypoxia-induced increase in GABA content is essential for restoration of membrane potential and preventing ROS-induced disturbance to ion homeostasis. Plant Commun. 2021, 2, 100188.
- Moustafa-Farag, M.; Mahmoud, A.; Arnao, M.B.; Sheteiwy, M.S.; Dafea, M.; Soltan, M.; Elkelish, A.; Hasanuzzaman, M.; Ai, S. Melatonin-induced water stress tolerance in plants: Recent advances. Antioxidants 2020, 9, 809.
- Gu, X.; Xue, L.; Lu, L.; Xiao, J.; Song, G.; Xie, M.; Zhang, H. Melatonin enhances the waterlogging tolerance of Prunus persica by modulating antioxidant metabolism and anaerobic respiration. J. Plant Growth Regul. 2021, 40, 2178–2190.
- Kang, K.; Lee, K.; Park, S.; Kim, Y.S.; Back, K. Enhanced production of melatonin by ectopic overexpression of human serotonin N-acetyltransferase plays a role in cold resistance in transgenic rice seedlings. J. Pineal Res. 2010, 49, 176–182.
- Lee, H.-J.; Back, K. 2-Hydroxymelatonin confers tolerance against combined cold and drought stress in tobacco, tomato, and cucumber as a potent anti-stress compound in the evolution of land plants. Melatonin Res. 2019, 2, 35–46.
- Posmyk, M.M.; Bałabusta, M.; Wieczorek, M.; Sliwinska, E.; Janas, K.M. Melatonin applied to cucumber (Cucumis sativus L.) seeds improves germination during chilling stress. J. Pineal Res. 2009, 46, 214–223.
- Turk, H.; Erdal, S.; Genisel, M.; Atici, O.; Demir, Y.; Yanmis, D. The regulatory effect of melatonin on physiological, biochemical and molecular parameters in cold-stressed wheat seedlings. Plant Growth Regul. 2014, 74, 139–152.
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wu, D.; Jing, D.; Liang, G. Melatonin treatment maintains quality and delays lignification in loquat fruit during cold storage. Sci. Hortic. 2021, 284, 110126.
- Zhao, J.; Lu, Z.; Wang, L.; Jin, B. Plant responses to heat stress: Physiology, transcription, noncoding RNAs, and epigenetics. Int. J. Mol. Sci. 2020, 22, 117.
- Wang, X.; Zhang, H.; Xie, Q.; Liu, Y.; Lv, H.; Bai, R.; Ma, R.; Li, X.; Zhang, X.; Guo, Y.-D. SlSNAT interacts with HSP40, a molecular chaperone, to regulate melatonin biosynthesis and promote thermotolerance in tomato. Plant Cell Physiol. 2020, 61, 909–921.
- Tal, O.; Haim, A.; Harel, O.; Gerchman, Y. Melatonin as an antioxidant and its semi-lunar rhythm in green macroalga Ulva sp. J. Exp. Bot. 2011, 62, 1903–1910.
- Hernández, I.G.; Gomez, F.J.V.; Cerutti, S.; Arana, M.V.; Silva, M.F. Melatonin in Arabidopsis thaliana acts as plant growth regulator at low concentrations and preserves seed viability at high concentrations. Plant Physiol. Biochem. 2015, 94, 191–196.
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593.
- Imran, M.; Shazad, R.; Bilal, S.; Imran, Q.M.; Khan, M.; Kang, S.-M.; Khan, A.L.; Yun, B.-W.; Lee, I.-J. Exogenous Melatonin mediates the regulation of endogenous nitric oxide in Glycine max L. to reduce effects of drought stress. Environ. Exp. Bot. 2021, 188, 104511.
- Su, J.; Yang, X.; Shao, Y.; Chen, Z.; Shen, W. Molecular hydrogen–induced salinity tolerance requires melatonin signalling in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 476–490.
- Nawaz, K.; Chaudhary, R.; Sarwar, A.; Ahmad, B.; Gul, A.; Hano, C.; Abbasi, B.H.; Anjum, S. Melatonin as master regulator in plant growth, development and stress alleviator for sustainable agricultural production: Current status and future perspectives. Sustainability 2020, 13, 294.
- Zhang, N.; Zhang, H.J.; Zhao, B.; Sun, Q.Q.; Cao, Y.Y.; Li, R.; Wu, X.X.; Weeda, S.; Li, L.; Ren, S. The RNA-seq approach to discriminate gene expression profiles in response to melatonin on cucumber lateral root formation. J. Pineal Res. 2014, 56, 39–50.
- Zhang, H.J.; Zhang, N.A.; Yang, R.C.; Wang, L.; Sun, Q.Q.; Li, D.B.; Cao, Y.Y.; Weeda, S.; Zhao, B.; Ren, S. Melatonin promotes seed germination under high salinity by regulating antioxidant systems, ABA and GA 4 interaction in cucumber (Cucumis sativus L.). J. Pineal Res. 2014, 57, 269–279.
- Khan, M.; Rolly, N.K.; Al Azzawi, T.N.I.; Imran, M.; Mun, B.-G.; Lee, I.-J.; Yun, B.-W.J.A. Lead (Pb)-Induced Oxidative Stress Alters the Morphological and Physio-Biochemical Properties of Rice (Oryza sativa L.). Agronomy 2021, 11, 409.
- Khan, M.; Al Azzawi, T.N.I.; Imran, M.; Hussain, A.; Mun, B.-G.; Pande, A.; Yun, B.-W. Effects of lead (Pb)-induced oxidative stress on morphological and physio-biochemical properties of rice. Biocell 2021, 45, 1413.
- Kamal, A.; Haroon, U.; Manghwar, H.; Alamer, K.H.; Alsudays, I.M.; Althobaiti, A.T.; Iqbal, A.; Akbar, M.; Anar, M.; Nazish, M. Biological Applications of Ball-Milled Synthesized Biochar-Zinc Oxide Nanocomposite Using Zea mays L. Molecules 2022, 27, 5333.
- Hasan, M.K.; Ahammed, G.J.; Yin, L.; Shi, K.; Xia, X.; Zhou, Y.; Yu, J.; Zhou, J. Melatonin mitigates cadmium phytotoxicity through modulation of phytochelatins biosynthesis, vacuolar sequestration, and antioxidant potential in Solanum lycopersicum L. Front. Plant Sci. 2015, 6, 601.
- Posmyk, M.M.; Kuran, H.; Marciniak, K.; Janas, K.M. Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J. Pineal Res. 2008, 45, 24–31.
- Nawaz, M.A.; Jiao, Y.; Chen, C.; Shireen, F.; Zheng, Z.; Imtiaz, M.; Bie, Z.; Huang, Y. Melatonin pretreatment improves vanadium stress tolerance of watermelon seedlings by reducing vanadium concentration in the leaves and regulating melatonin biosynthesis and antioxidant-related gene expression. J. Plant Physiol. 2018, 220, 115–127.
- Nazir, M.; Asad Ullah, M.; Mumtaz, S.; Siddiquah, A.; Shah, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var purpurascens). Molecules 2020, 25, 1072.
- Wei, Z.; Li, C.; Gao, T.; Zhang, Z.; Liang, B.; Lv, Z.; Zou, Y.; Ma, F. Melatonin increases the performance of Malus hupehensis after UV-B exposure. Plant Physiol. Biochem. 2019, 139, 630–641.
- Yin, Y.; Tian, X.; Yang, J.; Yang, Z.; Tao, J.; Fang, W. Melatonin mediates isoflavone accumulation in germinated soybeans (Glycine max L.) under ultraviolet-B stress. Plant Physiol. Biochem. 2022, 175, 23–32.
- Yao, J.W.; Ma, Z.; Ma, Y.Q.; Zhu, Y.; Lei, M.Q.; Hao, C.Y.; Chen, L.Y.; Xu, Z.Q.; Huang, X. Role of melatonin in UV-B signaling pathway and UV-B stress resistance in Arabidopsis thaliana. Plant Cell Environ. 2021, 44, 114–129.
- Moustafa-Farag, M.; Almoneafy, A.; Mahmoud, A.; Elkelish, A.; Arnao, M.B.; Li, L.; Ai, S. Melatonin and its protective role against biotic stress impacts on plants. Biomolecules 2019, 10, 54.
- Moon, Y.-S.; Ali, S. A fruitful decade of bacterial ACC deaminase biotechnology: A pragmatic approach towards abiotic stress relief in plants. Theor. Exp. Plant Physiol. 2022, 34, 109–129.
- Gul, S.L.; Moon, Y.-S.; Hamayun, M.; Khan, S.A.; Iqbal, A.; Khan, M.A.; Hussain, A.; Shafique, M.; Kim, Y.-H.; Ali, S. Porostereum spadiceum-AGH786 Regulates the Growth and Metabolites Production in Triticum aestivum L. Under Salt Stress. Curr. Microbiol. 2022, 79, 159.
- Khan, M.; Nazar, T.; Pande, A.; Mun, B.-G.; Lee, D.; Hussain, A.; Lee, B.-H.; Yun, B.-W.J.F. The Role of Nitric Oxide-Induced ATILL6 in Growth and Disease Resistance in Arabidopsis thaliana. Front. Plant Sci. 2021, 12, 1314.
- Shahid, M.; Imran, Q.M.; Hussain, A.; Khan, M.; Lee, S.U.; Mun, B.G.; Yun, B.-W. Comprehensive analyses of nitric oxide-induced plant stem cell-related genes in Arabidopsis thaliana. Genes 2019, 10, 190.
- Khan, A.H.; Min, L.; Ma, Y.; Wu, Y.; Ding, Y.; Li, Y.; Xie, S.; Ullah, A.; Shaban, M.; Manghwar, H. High day and night temperatures distinctively disrupt fatty acid and jasmonic acid metabolism, inducing male sterility in cotton. J. Exp. Bot. 2020, 71, 6128–6141.
- Ali, Q.; Zheng, H.; Rao, M.J.; Ali, M.; Hussain, A.; Saleem, M.H.; Nehela, Y.; Sohail, M.A.; Ahmed, A.M.; Kubar, K.A. Advances, limitations, and prospects of biosensing technology for detecting phytopathogenic bacteria. Chemosphere 2022, 296, 133773.
- Santamaria, M.E.; Martínez, M.; Cambra, I.; Grbic, V.; Diaz, I. Understanding plant defence responses against herbivore attacks: An essential first step towards the development of sustainable resistance against pests. Transgenic Res. 2013, 22, 697–708.
- Hussain, A.; Ding, X.; Alariqi, M.; Manghwar, H.; Hui, F.; Li, Y.; Cheng, J.; Wu, C.; Cao, J.; Jin, S. Herbicide resistance: Another hot agronomic trait for plant genome editing. Plants 2021, 10, 621.
- Nabavi, S.M.; Nabavi, S.F.; Sureda, A.; Xiao, J.; Dehpour, A.R.; Shirooie, S.; Silva, A.S.; Baldi, A.; Khan, H.; Daglia, M. Anti-inflammatory effects of Melatonin: A mechanistic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S4–S16.
- Vielma, J.R.; Bonilla, E.; Chacín-Bonilla, L.; Mora, M.; Medina-Leendertz, S.; Bravo, Y. Effects of melatonin on oxidative stress, and resistance to bacterial, parasitic, and viral infections: A review. Acta Trop. 2014, 137, 31–38.
- Huang, S.-H.; Liao, C.-L.; Chen, S.-J.; Shi, L.-G.; Lin, L.; Chen, Y.-W.; Cheng, C.-P.; Sytwu, H.-K.; Shang, S.-T.; Lin, G.-J. Melatonin possesses an anti-influenza potential through its immune modulatory effect. J. Funct. Foods 2019, 58, 189–198.
- Boga, J.A.; Caballero, B.; Potes, Y.; Perez-Martinez, Z.; Reiter, R.J.; Vega-Naredo, I.; Coto-Montes, A. Therapeutic potential of melatonin related to its role as an autophagy regulator: A review. J. Pineal Res. 2019, 66, e12534.
- Zhou, Y.; Manghwar, H.; Hu, W.; Liu, F. Degradation Mechanism of Autophagy-Related Proteins and Research Progress. Int. J. Mol. Sci. 2022, 23, 7301.
- Cheng, S.; Wang, Q.; Manghwar, H.; Liu, F. Autophagy-Mediated Regulation of Different Meristems in Plants. Int. J. Mol. Sci. 2022, 23, 6236.
- Zhao, L.; Chen, L.; Gu, P.; Zhan, X.; Zhang, Y.; Hou, C.; Wu, Z.; Wu, Y.F.; Wang, Q.C. Exogenous application of melatonin improves plant resistance to virus infection. Plant Pathol. 2019, 68, 1287–1295.
- Chen, L.; Wang, M.R.; Li, J.W.; Feng, C.H.; Cui, Z.H.; Zhao, L.; Wang, Q.C. Exogenous application of melatonin improves eradication of apple stem grooving virus from the infected in vitro shoots by shoot tip culture. Plant Pathol. 2019, 68, 997–1006.
- Tekbas, O.F.; Ogur, R.; Korkmaz, A.; Kilic, A.; Reiter, R.J. Melatonin as an antibiotic: New insights into the actions of this ubiquitous molecule. J. Pineal Res. 2008, 44, 222–226.
- Chen, X.; Sun, C.; Laborda, P.; He, Y.; Zhao, Y.; Li, C.; Liu, F. Melatonin treatments reduce the pathogenicity and inhibit the growth of Xanthomonas oryzae pv. oryzicola. Plant Pathol. 2019, 68, 288–296.
- Shi, H.; Chen, Y.; Tan, D.X.; Reiter, R.J.; Chan, Z.; He, C. Melatonin induces nitric oxide and the potential mechanisms relate to innate immunity against bacterial pathogen infection in Arabidopsis. J. Pineal Res. 2015, 59, 102–108.
- Lee, H.Y.; Back, K. Melatonin is required for H2O2− and NO− mediated defense signaling through MAPKKK 3 and OXI 1 in Arabidopsis thaliana. J. Pineal Res. 2017, 62, e12379.
- Mandal, M.K.; Suren, H.; Ward, B.; Boroujerdi, A.; Kousik, C. Differential roles of melatonin in plant-host resistance and pathogen suppression in cucurbits. J. Pineal Res. 2018, 65, e12505.
- Yavuz, T.; Kaya, D.; Behçet, M.; Ozturk, E.; Yavuz, Ö. Effects of melatonin on Candida sepsis in an experimental rat model. Adv. Ther. 2007, 24, 91–100.
- Liu, C.; Chen, L.; Zhao, R.; Li, R.; Zhang, S.; Yu, W.; Sheng, J.; Shen, L. Melatonin induces disease resistance to Botrytis cinerea in tomato fruit by activating jasmonic acid signaling pathway. J. Agric. Food Chem. 2019, 67, 6116–6124.
- Zhang, S.; Liu, S.; Zhang, J.; Reiter, R.J.; Wang, Y.; Qiu, D.; Luo, X.; Khalid, A.R.; Wang, H.; Feng, L. Synergistic anti-oomycete effect of melatonin with a biofungicide against oomycetic black shank disease. J. Pineal Res. 2018, 65, e12492.
- Li, C.; Zhao, Q.; Gao, T.; Wang, H.; Zhang, Z.; Liang, B.; Wei, Z.; Liu, C.; Ma, F. The mitigation effects of exogenous melatonin on replant disease in apple. J. Pineal Res. 2018, 65, e12523.
- Ma, Y.; Jiao, J.; Fan, X.; Sun, H.; Zhang, Y.; Jiang, J.; Liu, C. Endophytic bacterium Pseudomonas fluorescens RG11 may transform tryptophan to melatonin and promote endogenous melatonin levels in the roots of four grape cultivars. Front. Plant Sci. 2017, 7, 2068.
- Aghdam, M.S.; Fard, J.R. Melatonin treatment attenuates postharvest decay and maintains nutritional quality of strawberry fruits (Fragaria × anannasa cv. Selva) by enhancing GABA shunt activity. Food Chem. 2017, 221, 1650–1657.
- Arnao, M.B.; Hernández-Ruiz, J. Melatonin as a chemical substance or as phytomelatonin rich-extracts for use as plant protector and/or biostimulant in accordance with EC legislation. Agronomy 2019, 9, 570.
