Valve replacement is the mainstay of treatment for end-stage valvular heart disease, but varying degrees of defects exist in clinically applied valve implants. A mechanical heart valve requires long-term anti-coagulation, but the formation of blood clots is still inevitable. A biological heart valve eventually decays following calcification due to glutaraldehyde cross-linking toxicity and a lack of regenerative capacity. The goal of tissue-engineered heart valves is to replace normal heart valves and overcome the shortcomings of heart valve replacement commonly used in clinical practice. Surface biofunctionalization has been widely used in various fields of research to achieve functionalization and optimize mechanical properties.
- surface biofunctionalization
- tissue engineering
- valve implants
1. Introduction
2. Biofunctionalization of the Tissue Engineered Heart Valves
2.1. Non-Glutaraldehyde Cross-Linking
Glutaraldehyde (GLUT) is an aliphatic dialdehyde that can be attached to an amine functional group on collagen by the Schiff alkali reaction [12][29]; it has been shown to be reversible with easy hydrolysis [12][13][29,30]. GLUT or the presence of some groups such as aldehyde groups are highly cytotoxic and do not facilitate cell adhesion and growth on the valve, further leading to valve calcification [14][15][31,32]. In addition, GLUT can cause a strong immune response in the body, which leads to valve degeneration through the activation of macrophages, phagocytes, and T lymphocytes, and the aggregation of platelets [16][17][18][33,34,35]. Due to the defects in GLUT cross-linking, the implanted BHV is susceptible to structural damage and loss of function, resulting in a reduction in its durability and the need for reoperation in approximately only 15 years [19][20][36,37]. An ideal biomaterial cross-linker should be cytotoxic and less expensive. It can improve the mechanical properties of the material and inhibit calcification. Many fixed ECM-derived scaffolds of cross-linking agents are available, which can be divided into (1) chemical cross-linking agents and (2) natural cross-linking agents. Chemical cross-linkers include carbodiimide [1-ethyl-3-(3-dimethylaminopropyl)-carbondiimide (EDC)], epoxy compounds, six methylene diisocyanates, glycerin, and alginate [21][22][23][24][38,39,40,41]. Natural cross-linkers mainly include genipin, nordihydroguaiaretic acid, tannic acid, and proanthocyanidins [25][42]. Besides the aforementioned cross-linking agents, researchers have developed many uncommon cross-linking agents, and their effect is also worth looking forward to. Guo et al. [26][27][43,44] and Xu et al. [28][45] explored the comprehensive properties of the prepared BHV by radical polymerization and cross-linking, respectively. The results showed that the free radical polymerization cross-linking treatment had similar ECM stability and biaxial mechanical properties to the GLUT treatment. Furthermore, the samples showed better cytocompatibility, endothelialization potential, and anti-calcification potential and lower immune response in vivo after the free radical polymerization cross-linking treatment (Figure 12). Curcumin, quercetin, and other polyphenols have calcification inhibition potential similar to that of proanthocyanidins in cross-linked collagen and elastin scaffolds [29][30][46,47]. Curcumin [31][48] and quercetin [32][49] were developed as cross-linking reagents for valve materials. The cross-linked samples were superior to GLUT in terms of mechanical properties, blood compatibility, and cell compatibility.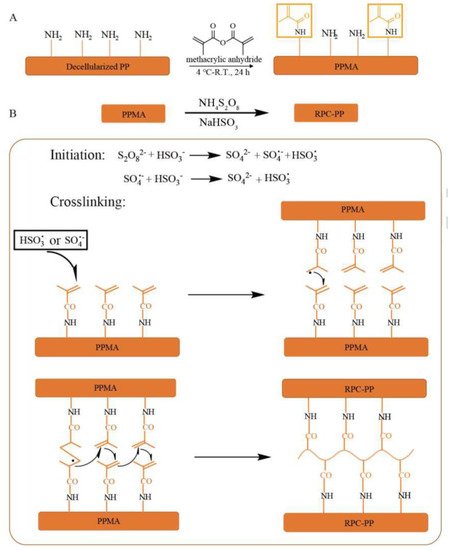
| Cross-Linking Agent | Advantages Compared with GLUT Treatment | Cross-Linking Mechanisms |
Reference |
|---|---|---|---|
| Methacrylic anhydride | Improve the biocompatibility and anti-calcification performance | Radicals react with vinyl on collagen | [26][27][28][43,44,45] |
| Procyanidins | Anti-calcification | Hydrogen bond | [29][46[30],47] |
| Curcumin | Anti-calcification | Hydrogen bond | [31][48] |
| Quercetin | Increasing significantly the ultimate tensile strength and anti-calcification |
Hydrogen bond | [32][49] |
| 1-Ethyl-3-(3-dimethylaminopropyl)carbodiimide |
| Composition of Hydrogels | Advantages of Hydrogels | Mechanism | Reference | ||||
|---|---|---|---|---|---|---|---|
| VEGF-Loaded hyaluronic acid hydrogel |
Improve adhesion and growth potential, with less platelet adhesion and less calcification | Cross-linking the hydroxyl group of hyaluronic acid with the epoxide of BDDE |
[62][79] | ||||
| TGF-β1-loaded polyethylene glycol nanoparticles | Advantageous biocompatibility | Combining with PEG nanoparticles by carbodiimide | [78][95] | VEGF-loaded elastin hydrogel | Improve endothelialization potential | Cross-linking reaction between the amine groups of soluble elastin and hexamethylene diisocyanate | |
| VEGF-loaded polycaprolactone nanoparticles | Acceleration of endothelialization | Michael addition reaction | [63][80] | ||||
| [ | 79 | ] | [96] | Sulfobetaine methacrylate and methacrylated hyaluronic acid | Improve endothelialization and anti-calcification properties |
Radical polymerization | [65 |
| Improving the biocompatibility | Amide and ester bond formation | of side groups |
[41][58] | ||||
| (3-glycidyloxypropyl) trimethoxysilane | Improving the cytocompatibility, endothelialization, hemocompatibility, and anti-calcification properties |
Epoxy reacts with amino groups; inorganic polymerization | [42][59] | ||||
| Dialdehyde pectin | Enhancing anti-calcification and anti- coagulation |
Schiff base reaction | [43][60] | ||||
| Alginate (oxidized alginate) | Improving the cytocompatibility, hemocompatibility, and anti-calcification properties |
Amino reaction with carboxyl groups | [44][61] | ||||
| Rose Bengal | Less cytotoxicity and better endothelialization potential |
Photoinduced cross-linking | [45][62] | ||||
| Triglycidyl amine | Anti-calcification | Reactive epoxy reacts with amine groups | [46][[49][6347][48],64,65,66] |
2.2. Modification Strategies after Decellularization
The decellularized porcine aortic valve is a promising alternative to the ideal tissue-engineered valve scaffold [50][51][52][53][67,68,69,70]. Pretreatment of the porcine aortic valve with fixatives and detergents can greatly stabilize xenografts, improve their persistence, and reduce immunogenic updates. However, biofunctionalized decellularized porcine valves, such as Synerggraft, have failed due to decreased mechanical properties and poor cell aggregation [54][71]. Therefore, surface biofunction has attracted widespread attention as an important means to improve the performance of decellularized valves.2.2.1. Hydrogel Coating
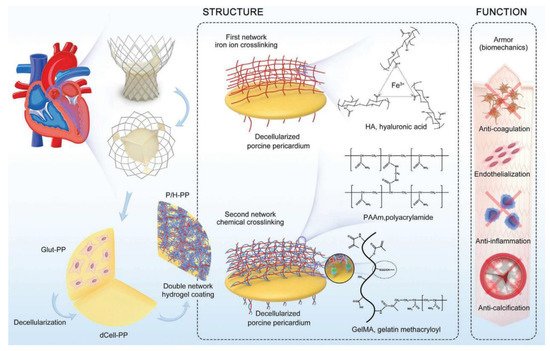
Physical coatings can give the substrate an ideal morphology and corresponding biological function, which is simple, fast, and efficient compared with chemical modification. In recent years, hydrogels have been widely used in engineered tissue due to their good biocompatibility and adjustable mechanical properties, but their application has been limited due to poor mechanical durability. Therefore, hydrogel coatings based on decellularized scaffolds have been developed by many researchers. These coatings provide good biocompatibility and function as carriers for decellularized scaffolds, while decellularized scaffolds provide sufficient mechanical properties. A variety of hydrogels, including natural (e.g., collagen [35][52], fibrin [55][56][72,73], and hyaluronic acid [56][57][73,74]), synthetic (e.g., polyethylene glycol [58][59][75,76] and polyvinyl alcohol [60][77]), and composite hydrogels (e.g., type I collagen with chondroitin sulfate [61][78]), have been developed, which provide good biocompatibility and bioactivity for engineered tissue applications. Synthetic hydrogels are composed of synthetic polymers, which have the advantages of adjustable mechanical properties and structure and easy control of chemical composition compared with natural hydrogels. Taking enhanced endothelialization as an example, the researchers seemed to prefer to load vascular endothelial growth factor (VEGF) into hydrogels as a core factor and coat decellularized scaffolds using natural hydrogels such as hyaluronic acid [62][79], elastin [63][80], and alginate [64][81]. The types and functions of synthetic hydrogels are much more complex. They not only focus on solving the side effects of heart valve implants but also make more detailed adjustments to the mechanical properties and fatigue resistance of the hybrid scaffold. Luo et al. used methacrylic anhydride to modify the decellularized heart valves and applied a mixed hydrogel made of sulfa methacrylate and hyaluronic acid methacrylate to the surface to improve the anti-calcification performance of BHVs. This strategy could achieve both endothelialization and anti-calcification, thus helping improve the main drawbacks of the existing commercial BHV products [65][82]. In addition, Jahnavi et al.| RBC-based rapamycin and atorvastatin calcium-loaded poly(lactic- | |||||||
| co-glycolic) acid nanoparticles | ] | [ | 82] | ||||
| Anticoagulation, anti-inflammation, anti-calcification, and endothelialization properties | Amidation reaction | [ | 80][97] | Hyaluronic acid and hydrophilic polyacrylamide |
Improve endothelialization, biocompatibility, and anticalcifification properties | Ionic and chemical Cross-linking |
[67] |
| OPG-loaded polycaprolactone nanoparticles | [ | 84 | ] | ||||
| Anti-calcification | Michael addition reaction | [ | 84][101] | SDF-1a-loaded MMP degradable hydrogel | Promote cell growth and mediate the tissue remodeling |
Michael-type addition reaction | [68][85] |
| Rivaroxaban-loaded nanogels | Acceleration of endothelialization and antithrombogenicity | Self-assembly | Polyhedral oligomeric silsesquioxane–polyethylene glycol hybrid hydrogel | Have anti-calcification potential | Formation of hydrogel network connecting POSS and MMP peptide using four-arm PEG-MAL | [69][86] | |
| Chondroitin sulfate hydrogel |
Improve endothelialization and shield against deterioration | Polymerization under UV lamps | [70][87] |
2.2.2. Cross-Linked with Nanocomposite
The advent of nanotechnology has made anti-tumor interventions possible. It also provides new ideas for TEHV. The nanoparticles have good biocompatibility. Furthermore, their surfaces have a variety of active groups, and researchers can modify them according to their purpose [71][88]. Common nanoparticles include lipids [72][73][89,90], polymers [74][91], inorganics [75][76][92,93], metals [77][94], and so forth. Nanoparticles are used in tissue-engineered heart valves mainly for gene transmission or the transmission of biologically active factors. This can effectively solve the limitation of cell seeding before TEHV implantation. A polyethylene glycol nanoparticle containing transforming growth factor-β1 was developed through a carbon diimide-modified fibrous valve scaffold that improved the ECM microenvironment of the tissue-engineered heart valves [78][95]. Based on this concept, researchers encapsulated VEGF in polycaprolactone (PCL) nanoparticles (Figure 34). Then, using the Michael addition reaction, PCL nanoparticles were introduced to the decellularized aortic valve, and a hybrid valve was prepared. The results showed that the mixed valve could effectively accelerate endothelialization [79][96]. A large number of nanoparticles used in tissue-engineered heart valves in recent years are biocompatible polymers, such as PEGs, PCL, and so forth. Recently, Hu et al. [80][97] were inspired by the natural biological systems and reported a new approach for cross-linking amino and carboxyl groups. The heart valves with erythrocyte membrane bionic drug-carrier nanoparticles were modified using amino and carboxyl groups remaining after GLUT cross-linking. This modified heart valve not only preserved the structural integrity, stability, and mechanical properties of GLUT-treated BHV but also significantly improved anticoagulation, anti-inflammatory, anti-calcification, and endothelialization properties.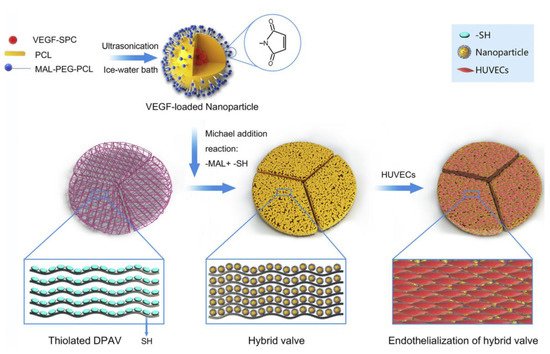
| Composition of Nanocomposite | Advantages of Nanocomposite | Mechanism | Reference |
|---|---|---|---|
| [ | |||
| 85 | |||
| ] | |||
| [ | 102 | ] | |
| Polyhedral oligomeric silsesquioxane- nanocomposite |
Acceleration of endothelialization potential | Reaction of the silanol groups of cyclohexanechlorohydrine-functionalized POSS with isocyanate |
[86][87]104[88][103,,105] |
