Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is a comparison between Version 2 by Rita Xu and Version 1 by Kyoungtae Kim.
Quantum dots are nanocrystals with bright and tunable fluorescence. Due to their unique property, quantum dots are sought after for their potential in several applications in biomedical sciences as well as industrial use.
- quantum dots
- mammalian
- fungal
- plants
- trafficking
- toxicity
1. Introduction
Due to their potential in applied science, quantum dots and their toxicity have been intensively studied and reviewed in the past few decades [1,2,3,4,5,6,7][1][2][3][4][5][6][7]. Quantum dots (QDs) are nanosized (2–10 nm) semiconductor crystals with distinguished chemical and physical properties, enabling them to emit a wide range of bright, photobleaching-resistant light [8]. QDs’ fluorescence is size-tunable, which allows for simple adjustment of QDs’ size and composition to achieve desired color [5,9,10,11,12,13,14,15,16,17,18,19,20][5][9][10][11][12][13][14][15][16][17][18][19][20]. It was found that increased diameter of QDs caused a redshift in fluorescence [17]. Additionally, quantum dots with greater height in dimension display a longer photoluminescence lifetime and increased emission wavelength [15]. Thus, larger quantum dots emit more stable fluorescence in higher emission ranges. In addition to QDs’ size, the shape of QDs also significantly influence QDs’ stability and optical property [19,20,21,22,23][19][20][21][22][23]. Some of the most used quantum dot shapes include spherical QDs, cylindrical QDs, pyramidal QDs, conical QDs, tetrahedral QDs, and lens-shaped QDs [19,20,21,24][19][20][21][24]. It was found that tetrahedral QDs have sharper edge absorption and are more confined than spherical QDs. On the other hand, spherical QDs at 3.1 nm showed to be most efficient in photon absorption and required less excitation energy compared to other types of QD shapes [19,21][19][21].
Quantum dots are typically sorted based on their core type, shape, structure, size, and ligands. Common quantum dot cores include cadmium, indium, and carbon encapsulated by chalcogenides such as selenides, tellurides, and sulfide [25,26,27,28,29][25][26][27][28][29]. However, core-only quantum dots have been shown to be unstable due to the deterioration of materials [30,31][30][31]. For core-type QDs such as cadmium selenide quantum dots (CdSe QDs), an oxidizing environment causes oxidation of the selenide (Se) layer on the QDs’ surface, thus weakening the overall structure of QDs and leading to leakage of cadmium ions. When adding an additional shell layer such as zinc sulfide (ZnS), the oxidation of Se is reduced, thus decreasing the amount of Cd ion leakage overall [30]. Compared to core-only QDs, quantum dots with a core–shell structure are considered superior when it comes to structural stability and photoluminescent quantum yield (PLQY) [31,32,33][31][32][33]. It is known that long-term exposure to environmental factors such as blue light and UV light quenches QDs’ fluorescence. The addition of a protective shell has shown to be effective in increasing QD resistance against photobleaching [34,35,36,37,38][34][35][36][37][38]. In addition to increased QDs’ stability, the presence of an exterior shell has been found to reduce the toxicity of quantum dots, which broadens their application range [39]. Therefore, a new type of quantum dot called core–shell quantum dots has been developed and is currently widely used. The shell of some quantum dots is decorated with ligands that provide further stability [40,41,42][40][41][42]. The addition of ligands allows QDs to have specific interactions with various environmental factors, which is useful in certain applications such as biosensing and particle detection [43,44,45,46][43][44][45][46]. Appropriate ligand choice could also aid the dispersion of QDs in an aqueous solution, thus minimizing the aggregation of QDs and resulting in accurate emission of size-dependent QDs [47].
Due to their unique characteristics, quantum dots have become a promising candidate for a range of important applications. Quantum dots are sought after for their potential in biomedical science, particularly for biosensing, drug delivery, cell tracking, disease detection, and potential antimicrobial/antibiotic remedies [48,49,50,51,52,53][48][49][50][51][52][53]. Furthermore, quantum dots are currently heavily utilized in several commercialized products, such as electronic devices, solar cells, LEDs, cosmetics, plastics, and other products essential to daily life [54,55,56,57][54][55][56][57]. As quantum dots gained attention in recent years, a few studies have shown the potential toxicity of quantum dots to mammalian cells, fungal cells, plants, and other organisms [25,58,59,60,61,62,63][25][58][59][60][61][62][63].
2. Core-Type Quantum Dots
As of today, a wide variety of quantum dots have been developed, each with slightly different properties and potential applications [13,32,58,67,68,69,70,71,72,73,74][13][32][58][64][65][66][67][68][69][70][71]. Some of the most common quantum dots that have captured current interest in the scientific community are cadmium QDs, indium QDs, graphene QDs, and carbon dots [26,56,63,72,75,76,77,78,79,80,81,82][26][56][63][69][72][73][74][75][76][77][78][79]. Each of these types of quantum dots possess a unique set of advantages and disadvantages, which make them suitable for slightly different applications (Figure 1). Among these, cadmium-based quantum dots are the most intensively researched.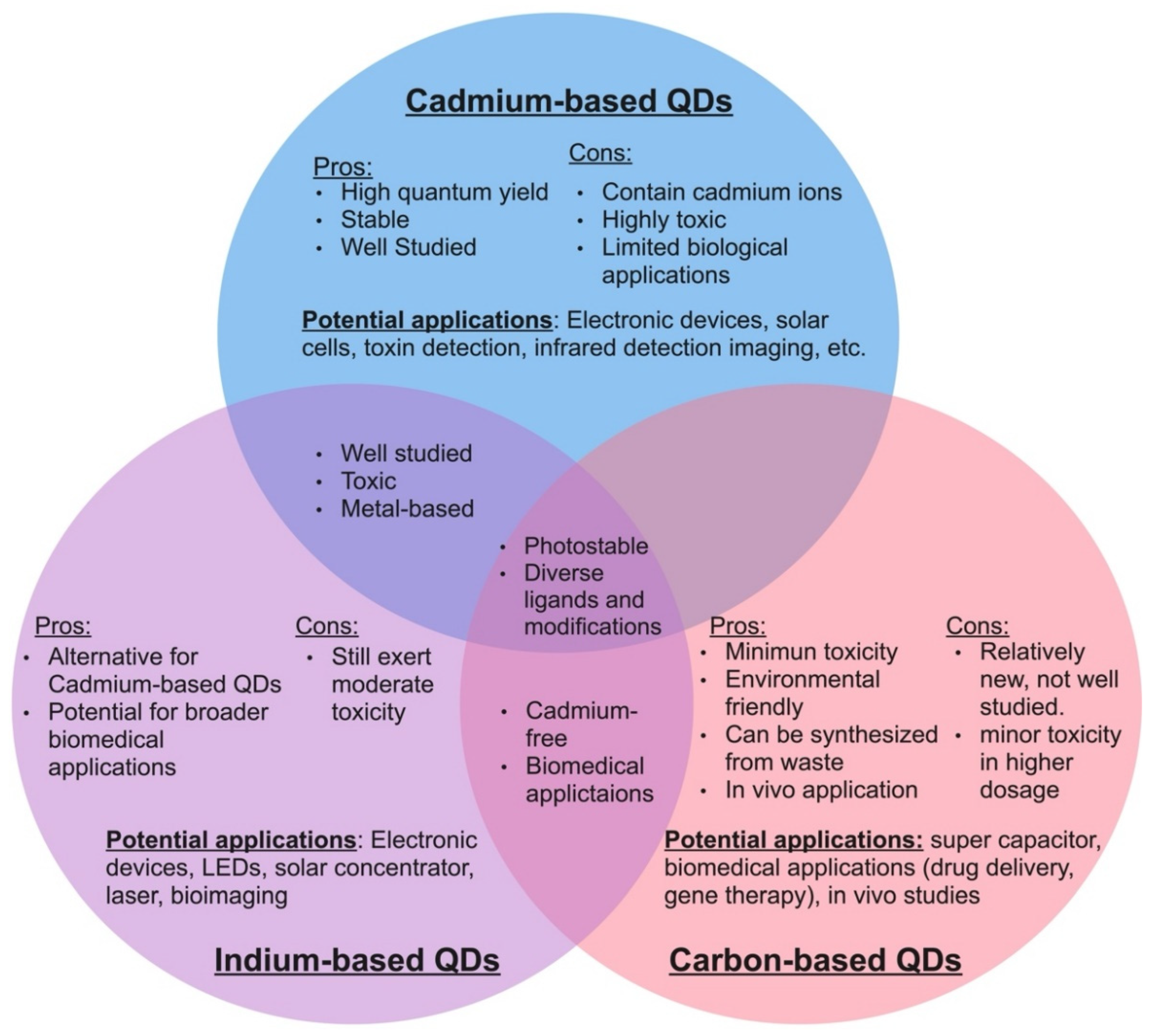
Figure 1. Characteristics of the three major quantum dots core types.
3. Quantum Dots in Fungal Cells
3.1. Interaction and Intracellular Trafficking
As decomposers and nutrient recyclers [159,160[110][111][112][113],161,162], fungi are an important component of ourthe ecosystem. Therefore, before applying QDs in mass industrial-produced products, it is important to assess how QDs from discarded products will interact and affect fungal systems. For fungal cells, the rigid cell wall is a crucial factor when it comes to QD interactions. In a recent study, Qdots 625 ITKTM (QDs) were engineered on the surface of the budding yeast Saccharomyces cerevisiae by covalently binding yeast surface protein with the -SH group of modified QDs to investigate the interaction between QDs and the yeast surface. Results showed that Qdots 625 ITKTM (QDs) on the cell wall of the mother cell remained on its progeny for up to two generations. Although QDs remained attached to the cell surface for some time, there was no difference in yeast surface morphology when examined with an electron microscope. This study also found that engineered QD attachment on yeast surface did not affect the growth and viability of yeast [163][114]. In contrast, a different study found that small, free CdTe QDs, around 4.1–5.8 nm quantum dots, readily internalized into fungal cells and induced cytotoxicity by breaking cell wall components as well as causing apoptotic blebbing in the cytoplasm [52]. Consistently, recent data found that in the ascomycete fungus Fusarium oxysporum, CdSe/ZnS QDs easily internalized and uniformly distributed throughout hyphae after 3 h of incubation. It was also observed that QDs formed large defined clusters inside fungal cells after 16 h of incubation. Afterward, the team removed QDs from the media by washing cells and placing them in a QD-free media. Four hours after QDs were removed, a small amount of quantum dots remained inside the cells, while large aggregates were found on the cell surface and media. This led the team to hypothesize that internalized QDs are eventually secreted out, likely by exocytosis, but the exact mechanism of QD release remained unclear. Furthermore, the results in this study showed that only a high concentration, 500 nM of CdSe/ZnS QDs, compromised the growth and germination of Fusarium oxysporum [52]. Overall, in these findings, wresearchers could conjecture that internalized QDs had more effect on yeast viability compared to surface-attached QDs. Consistent with data seen in mammalian systems, QDs are also exported out of the cell by exocytosis, yet the exact exit pathway in yeast remains a mystery. To the best of ourthe knowledge, the current data only indicate the uptake of QDs by fungal cells, however, the endocytic route and QDs’ exact intracellular trafficking are unknown. There is also a lack of studies demonstrating the trafficking of other types of QDs, such as InP-based and carbon-based quantum dots in yeast. This presents a knowledge gap in the field that requires immediate attention and research.3.2. Toxicity Effect of QDs on Fungal Cells
Similar to the mammalian system, the protective zinc shell was also found to be effective in limiting QD toxicity in fungi. As demonstrated in a study, yeast cells treated with core-type CdSe QDs were found to have a significant reduction in mitochondrial membrane potential and acquired severe cell wall damage, while treatment of core–shell structure CdSe/ZnS QDs showed less impact on yeast cells overall [164][115]. The lower toxicity effect in the presence of a ZnS shell suggested that core leakage seems to be a major mechanism in QD toxicity. Interestingly, further studies show that the release of cadmium ions seems to be only one of the factors contributing to QD toxicity. In Saccharomyces cerevisiae, a systemic knockout mutant screening identified 114 KO mutant strains to develop tolerance to the presence of CdS QDs. These mutant strains were then tested with cadmium ions in the form of CdSO4. The results showed that there were only 11 CdS-QD-tolerant mutant strains showing resistance to cadmium ions, supporting that the cellular response to cadmium ions and cadmium-based quantum dots is different [165][116]. Thus, the toxicity of CdS QDs was not solely due to the release of cadmium ions but rather resulted from the interaction with QDs. Another group of researchers demonstrated the impacts of CdS QDs against Saccharomyces cerevisiae. CdS QDs were found to induce the deletion of genes associated with stress response, metabolic processes, mitochondrial organization, DNA repair, and cellular transportation. Exposure to CdS QDs also led to an increased level of reactive oxygen species, reduced oxygen consumption, lowered both reduced and oxidized glutathione levels, and altered the mitochondrial membrane potential and mitochondrial morphology [166][117]. Although core–shell quantum dots are considered to cause less cellular damage, treatment of CdSe/ZnS QDs still altered the expression of many genes in S. Cerevisiae. Using RNA sequencing, it was found that most upregulated genes are associated with cellular component regulation, rRNA metabolic processes, macromolecule methylation, maturation of large and small subunits of ribosomes, and DNA replication in the cell cycle. Downregulated genes include genes involved in oxidation–reduction processes, small-molecule metabolic processes, proteolysis, transmembrane import and transportation, chemical responses, and electron transport chains [167][118]. For the mammalian cellular system, the majority of available data suggested that InP-based QDs are less toxic than Cd-based QDs. However, this was not observed in the fungal system. In 2021, Horstmann et al. were the first to compare the transcriptome of S. cerevisiae exposed to cadmium-based QDs (CdSe/ZnS QDs) to indium-based QDs (InP/ZnS QDs). They found that InP/ZnS inhibited yeast growth when treated at a high concentration of 100 µg/mL, while CdSe/ZnS prolonged the lag phase of yeast cells, out-compromising the final optical density at the same dosage. Furthermore, the team measured the ROS level of QD-treated cells to examine whether the inhibitory effect of InP/ZnS QDs was due to increased ROS level. They found that the ROS level in InP/ZnS-QD-treated cells decreased, while a significant elevation in ROS level was observed for CdSe/ZnS-treated cells. Furthermore, the team’s RNA-seq analysis revealed that InP/ZnS QD cells showed to have an increased expression in genes associated with antioxidant defense and peroxisome structure, while the expression of genes associated with metabolic activities was significantly decreased. On the other hand, CdSe/ZnS QDs altered genes associated with protein metabolic processes, cell wall organization, and cellular homeostasis. Interestingly, both QDs changed the level of genes associated with transmembrane transport and translation [76][73]. Unlike the increasing trend in the research effort on indium-based QDs in the mammalian system, available data regarding their interaction with the fungal system are limited. As the initial comparison of the two quantum dots points out the difference in the cellular response to each QD type, it is crucial to investigate the possible effect indium-based QDs have on fungal populations as wresearchers examine the possibility of using them as an alternative to cadmium-based QDs. A few recent studies have found carbon dots to be a promising candidate for antimicrobial and antifungal drugs [168,169,170,171,172,173][119][120][121][122][123][124]. It was found that while carbon dots were non-toxic to the human cells HCT-116 and TM4 at a low concentration of 3.5 mg/mL, they had dose-dependent antimicrobial activity on fungal cells [173][124]. Similarly, nitrogen-doped graphene quantum dots (NDGQDs) significantly inhibited the growth of several fungal strains, including P. citrinum, C. albicans, and Ammophilus fumigatus, while exerted minimal impact on mouse fibroblasts cells [168][119]. Additionally, combining high photothermal light and carbon-based quantum dots was found to enhance QDs’ antifungal activities [169][120]. All this evidence suggests that fungal cells are more sensitive to carbon-based quantum dots compared to mammalian cells, and thus could potentially become effective antimicrobial agents. It is worth noting that the above studies only focused on the viability of fungal cells, yet the mechanism by which carbon dots reduce fungal viability remains to be understood. Future research regarding the specific impact of carbon-based QDs on fungal transcriptomic, proteomic, and metabolic changes is greatly needed. While the impacts of different types of QDs are under investigation, it is important to also consider examining the interaction between QDs and fungal cells, as well as the mechanisms that induced these cellular responses. To the best of ourthe knowledge, currently, there are very few studies focusing on the specific QD–fungal cell interactions, the QD intracellular trafficking pathway in fungal cells, QD interactions with specific organelles, and how these interactions contribute to QD toxicity in fungal cells (Table 1). This knowledge would allow uresearchers to further understand the mechanisms of QDs’ toxicity and develop strategies to limit their toxicity for safer QD applications, therefore this could be the target of future research.Table 1. Relevant articles cited in the fungal system section.
| Article Title | Author | Reference | |
|---|---|---|---|
| Fungal Importance Extends beyond Litter Decomposition in Experimental Early-Successional Streams | Frossard et al. | [159] | [110] |
| Socialism in Soil? The Importance of Mycorrhizal Fungal Networks for Facilitation in Natural Ecosystems | Van der Heijden et al. | [160] | [111] |
| The Missing Metric: An Evaluation of Fungal Importance in Wetland Assessments | Onufrak et al. | [161] | [112] |
| Hildebrand, F. Metagenomic Assessment of the Global Diversity and Distribution of Bacteria and Fungi. | Bahram et al. | [162] | [113] |
| Determining the Fate of Fluorescent Quantum Dots on Surface of Engineered Budding S. Cerevisiae Cell Molecular Landscape | Chouhan et al. | [163] | [114] |
| The Interactions between CdSe Quantum Dots and Yeast Saccharomyces Cerevisiae: Adhesion of Quantum Dots to the Cell Surface and the Protection Effect of ZnS Shell | Mei et al. | [164] | [115] |
| Yeast Populations Evolve to Resist CdSe Quantum Dot Toxicity | Strtak et al. | [165] | [116] |
| Nucleo-Mitochondrial Interaction of Yeast in Response to Cadmium Sulfide Quantum Dot Exposure | Pasquali et al. | [166] | [117] |
| Transcriptome Profile Alteration with Cadmium Selenide/Zinc Sulfide Quantum Dots in Saccharomyces Cerevisiae | Horstmann et al. | [167] | [118] |
| Preparation and Characterization of B, S, and N-Doped Glucose Carbon Dots: Antibacterial, Antifungal, and Antioxidant Activity | Ezati et al. | [168] | [119] |
| Green Synthesis of Multifunctional Carbon Dots for Anti-Cancer and Anti-Fungal Applications | Zhao et al. | [169] | [120] |
| Amine-Coated Carbon Dots (NH2-FCDs) as Novel Antimicrobial Agent for Gram-Negative Bacteria | Devkota et al. | [170] | [121] |
| Carbon Dots as an Emergent Class of Antimicrobial Agents | Ghirardello et al. | [171] | [122] |
| Antimicrobial Activity and Characterization of Pomegranate Peel-Based Carbon Dots | Qureshi et al. | [172] | [123] |
| One-Pot Microbial Approach to Synthesize Carbon Dots from Baker’s Yeast-Derived Compounds for the Preparation of Antimicrobial Membrane | Ghorbani et al. | [173] | [124] |
| Toxicity of CdTe Quantum Dots on Yeast Saccharomyces Cerevisiae | Han et al. | [52] | |
| Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots | Oh et al. | [83] | [80] |
References
- Joglekar, P.; Mandalkar, D.; Nikam, M.; Pande, N.; Dubal, A. Review Article on Quantum Dots: Synthesis, Properties and Application. Int. J. Res. Advent. Technol. 2019, 7, 510–515.
- Wang, L.; Li, W.; Yin, L.; Liu, Y.; Guo, H.; Lai, J.; Han, Y.; Li, G.; Li, M.; Zhang, J.; et al. Full-Color Fluorescent Carbon Quantum Dots. Sci. Adv. 2020, 6, eabb6772.
- He, L.; Yang, L.; Liu, B.; Zhang, J.; Zhang, C.; Liu, S.; Chen, S.; Zapien, J.A.; Alamry, K.A.; Asiri, A.M.; et al. One-Pot Synthesis of Color-Tunable Copper Doped Zinc Sulfide Quantum Dots for Solid-State Lighting Devices. J. Alloys Compd. 2019, 787, 537–542.
- Cai, K.-B.; Huang, H.-Y.; Hsieh, M.-L.; Chen, P.-W.; Chiang, S.-E.; Chang, S.H.; Shen, J.-L.; Liu, W.-R.; Yuan, C.-T. Two-Dimensional Self-Assembly of Boric Acid-Functionalized Graphene Quantum Dots: Tunable and Superior Optical Properties for Efficient Eco-Friendly Luminescent Solar Concentrators. ACS Nano 2022, 16, 3994–4003.
- Gu, Y.P.; Cui, R.; Zhang, Z.L.; Xie, Z.X.; Pang, D.W. Ultrasmall Near-Infrared Ag 2Se Quantum Dots with Tunable Fluorescence for in Vivo Imaging. J. Am. Chem. Soc. 2012, 134, 79–82.
- Nakane, Y.; Tsukasaki, Y.; Sakata, T.; Yasuda, H.; Jin, T. Aqueous Synthesis of Glutathione-Coated PbS Quantum Dots with Tunable Emission for Non-Invasive Fluorescence Imaging in the Second near-Infrared Biological Window (1000–1400 Nm). Chem. Commun. 2013, 49, 7584–7586.
- Bhandari, S.; Hao, B.; Waters, K.; Lee, C.H.; Idrobo, J.C.; Zhang, D.; Pandey, R.; Yap, Y.K. Two-Dimensional Gold Quantum Dots with Tunable Bandgaps. ACS Nano 2019, 13, 4347–4353.
- Campbell-Ricketts, T.E.J.; Kleemans, N.A.J.M.; Nötzel, R.; Silov, A.Y.; Koenraad, P.M. The Role of Dot Height in Determining Exciton Lifetimes in Shallow InAs/GaAs Quantum Dots. Appl. Phys. Lett. 2010, 96, 033102.
- Sadi, I.; Sellami, K.; Yahyaoui, M.; Testelin, C.; Boujdaria, K. Electron and Hole Energy Levels in InAs/GaAs Quantum Dots: Size and Magnetic Field Effects. J. Appl. Phys. 2011, 109, 033703.
- Heitz, R.; Stier, O.; Mukhametzhanov, I.; Madhukar, A.; Bimberg, D. Quantum Size Effect in Self-Organized InAs/GaAs Quantum Dots. Phys. Rev. B 2000, 62, 11017–11028.
- Schmidt, K.H.; Medeiros-Ribeiro, G.; Garcia, J.; Petroff, P.M. Size Quantization Effects in InAs Self-Assembled Quantum Dots. Appl. Phys. Lett. 1998, 70, 1727–1729.
- Imran, A.; Jiang, J.; Eric, D.; Yousaf, M. Size and Shape Dependent Optical Properties of InAs Quantum Dots. In Proceedings of the 2017 International Conference on Optical Instruments and Technology Micro/Nano Photonics: Materials and Devices, Beijing, China, 28–30 October 2017; Volume 10622.
- Wen, L.; Qiu, L.; Wu, Y.; Hu, X.; Zhang, X. Aptamer-Modified Semiconductor Quantum Dots for Biosensing Applications. Sensors 2017, 17, 1736.
- Chua, S.J.; Ngo, S.; Yoon, Y.C.; Fan, S.F.; Chua, W.J. Effects of Size and Shape on Electronic States of Quantum Dots. Phys. Rev. B 2006, 74, 245331–245332.
- Zhang, H.; Guyot-Sionnest, P. Shape-Controlled HgTe Colloidal Quantum Dots and Reduced Spin-Orbit Splitting in the Tetrahedral Shape. J. Phys. Chem. Lett. 2020, 11, 6860–6866.
- Liang, L.; Xie, W. Influence of the Shape of Quantum Dots on Their Optical Absorptions. Physica. B Condens. Matter 2015, 462, 15–17.
- Yang, X.F.; Chen, X.S.; Lu, W.; Fu, Y. Effects of Shape and Strain Distribution of Quantum Dots on Optical Transition in the Quantum Dot Infrared Photodetectors. Nanoscale Res. Lett. 2008, 3, 534.
- Mo, D.; Hu, L.; Zeng, G.; Chen, G.; Wan, J.; Yu, Z.; Huang, Z.; He, K.; Zhang, C.; Cheng, M. Cadmium-Containing Quantum Dots: Properties, Applications, and Toxicity. Appl. Microbiol. Biotechnol. 2017, 101, 2713–2733.
- Kays, J.C.; Saeboe, A.M.; Toufanian, R.; Kurant, D.E.; Dennis, A.M. Shell-Free Copper Indium Sulfide Quantum Dots Induce Toxicity in Vitro and in Vivo. Nano Lett. 2020, 20, 1980–1991.
- Nair, A.; Haponiuk, J.T.; Thomas, S.; Gopi, S. Natural Carbon-Based Quantum Dots and Their Applications in Drug Delivery: A Review. Biomed. Pharmacother. 2020, 132, 110834.
- Kloepfer, J.A.; Bradforth, S.E.; Nadeau, J.L. Photophysical Properties of Biologically Compatible CdSe Quantum Dot Structures. J. Phys. Chem. B 2005, 109, 9996–10003.
- Zielony, E.; Płaczek-Popko, E.; Kamyczek, P.; Henrykowski, A.; Karczewski, G. Raman Spectroscopy of CdTe/ZnTe Quantum Dot Structures. Opt. Appl. 2013, 43, 181–185.
- Derfus, A.M.; Chan, W.C.W.; Bhatia, S.N. Probing the Cytotoxicity of Semiconductor Quantum Dots. Nano Lett. 2004, 4, 11–18.
- Selopal, G.S.; Zhao, H.; Wang, Z.M.; Rosei, F. Core/Shell Quantum Dots Solar Cells. Adv. Funct. Mater. 2020, 30, 1908762.
- Li, H.; Jiang, X.; Wang, A.; Chu, X.; Du, Z. Simple Synthesis of CuInS2/ZnS Core/Shell Quantum Dots for White Light-Emitting Diodes. Front. Chem. 2020, 8, 669.
- Vasudevan, D.; Gaddam, R.R.; Trinchi, A.; Cole, I. Core-Shell Quantum Dots: Properties and Applications. J. Alloys Compd. 2015, 636, 395–404.
- Tan, Y.; Jin, S.; Hamers, R.J. Photostability of Cdse Quantum Dots Functionalized with Aromatic Dithiocarbamate Ligands. ACS Appl. Mater. Interfaces 2013, 5, 12975–12983.
- Yu, S.; Zhang, X.; Li, L.; Xu, J.; Song, Y.; Liu, X.; Wu, S.; Zhang, J. High Photostability and Luminescent Efficiency of Quantum Dots: Ultrathin Epitaxial Al Self-Passivation Layer with a Homogeneous Ligand. Mater. Res. Express 2019, 6, 0850f7.
- Jo, J.H.; Kim, J.H.; Lee, S.H.; Jang, H.S.; Jang, D.S.; Lee, J.C.; Park, K.U.; Choi, Y.; Ha, C.; Yang, H. Photostability Enhancement of InP/ZnS Quantum Dots Enabled by In2O3 Overcoating. J. Alloys Compd. 2015, 647, 6–13.
- Christensen, E.A.; Kulatunga, P.; Lagerholm, B.C. A Single Molecule Investigation of the Photostability of Quantum Dots. PLoS ONE 2012, 7, e44355.
- Bailes, J. Photostability of Semiconductor Quantum Dots in Response to UV Exposure. Methods Mol. Biol. 2020, 2118, 343–349.
- Wang, Z.; Tang, M. The Cytotoxicity of Core-Shell or Non-Shell Structure Quantum Dots and Reflection on Environmental Friendly: A Review. Environ. Res. 2021, 194, 110593.
- Green, M. The Nature of Quantum Dot Capping Ligands. J. Mater. Chem. 2010, 20, 5797–5809.
- Nam, E.; Lee, C.; Kim, S.J.; Chung, H.K.; Chae, H. Stability and Dispersion Improvement of Quantum-Dot Films by Hydrosilylation between Quantum-Dot Ligands and a Siloxane Matrix. Opt. Express 2019, 27, 20037–20046.
- Gao, Y.; Aerts, M.; Sandeep, C.S.S.; Talgorn, E.; Savenije, T.J.; Kinge, S.; Siebbeles, L.D.A.; Houtepen, A.J. Photoconductivity of PbSe Quantum-Dot Solids: Dependence on Ligand Anchor Group and Length. ACS Nano 2012, 6, 9606–9614.
- Yuan, C.; Zhang, K.; Zhang, Z.; Wang, S. Highly Selective and Sensitive Detection of Mercuric Ion Based on a Visual Fluorescence Method. Anal. Chem. 2012, 84, 9792–9801.
- Sung, T.W.; Lo, Y.L. Highly Sensitive and Selective Sensor Based on Silica-Coated CdSe/ZnS Nanoparticles for Cu2+ Ion Detection. Sens. Actuators B Chem. 2012, 165, 119–125.
- Ding, Y.; Shen, S.Z.; Sun, H.; Sun, K.; Liu, F. Synthesis of L-Glutathione-Capped-ZnSe Quantum Dots for the Sensitive and Selective Determination of Copper Ion in Aqueous Solutions. Sens. Actuators B Chem. 2014, 203, 35–43.
- Zhao, Q.; Rong, X.; Ma, H.; Tao, G. Dithizone Functionalized CdSe/CdS Quantum Dots as Turn-on Fluorescent Probe for Ultrasensitive Detection of Lead Ion. J. Hazard. Mater. 2013, 250, 45–52.
- Boles, M.A.; Ling, D.; Hyeon, T.; Talapin, D.V. Erratum: The Surface Science of Nanocrystals. Nat. Mater. 2016, 15, 364.
- Hong, S.; Lee, C. The Current Status and Future Outlook of Quantum Dot-Based Biosensors for Plant Virus Detection. Plant Pathol. J. 2018, 34, 85–92.
- Matea, C.T.; Mocan, T.; Tabaran, F.; Pop, T.; Mosteanu, O.; Puia, C.; Iancu, C.; Mocan, L. Quantum Dots in Imaging, Drug Delivery and Sensor Applications. Int. J. Nanomed. 2017, 12, 5421–5431.
- Zhao, C.; Song, X.; Liu, Y.; Fu, Y.; Ye, L.; Wang, N.; Wang, F.; Li, L.; Mohammadniaei, M.; Zhang, M.; et al. Synthesis of Graphene Quantum Dots and Their Applications in Drug Delivery. J. Nanobiotechnol. 2020, 18, 1–32.
- Joo, K.I.; Lei, Y.; Lee, C.L.; Lo, J.; Xie, J.; Hamm-Alvarez, S.F.; Wang, P. Site-Specific Labeling of Enveloped Viruses with Quantum Dots for Single Virus Tracking. ACS Nano 2008, 2, 1553–1562.
- van’t Padje, A.; Oyarte Galvez, L.; Klein, M.; Hink, M.A.; Postma, M.; Shimizu, T.; Kiers, E.T. Temporal Tracking of Quantum-Dot Apatite across in Vitro Mycorrhizal Networks Shows How Host Demand Can Influence Fungal Nutrient Transfer Strategies. ISME J. 2021, 15, 435–449.
- Rajendiran, K.; Zhao, Z.; Pei, D.S.; Fu, A. Antimicrobial Activity and Mechanism of Functionalized Quantum Dots. Polymers 2019, 11, 1670.
- Liu, M.; Yazdani, N.; Yarema, M.; Jansen, M.; Wood, V.; Sargent, E.H. Colloidal Quantum Dot Electronics. Nat. Electron. 2021, 4, 548–558.
- Song, J.-W. Grow Light for Plant Factory Using Quantum Dot LED. J. Int. Counc. Electr. Eng. 2016, 6, 13–16.
- Eren, G.O.; Sadeghi, S.; Bahmani Jalali, H.; Ritter, M.; Han, M.; Baylam, I.; Melikov, R.; Onal, A.; Oz, F.; Sahin, M.; et al. Cadmium-Free and Efficient Type-II InP/ZnO/ZnS Quantum Dots and Their Application for LEDs. ACS Appl. Mater. Interfaces 2021, 13, 32022–32030.
- Li, J.; Han, Y.; Li, X.; Xiong, L.; Wei, L.; Cheng, X. Analysis of Methylparaben in Cosmetics Based on a Chemiluminescence H2O2−NaIO4−CNQDs System. Luminescence 2021, 36, 79–84.
- Valizadeh, A.; Mikaeili, H.; Samiei, M.; Farkhani, S.M.; Zarghami, N.; Kouhi, M.; Akbarzadeh, A.; Davaran, S. Quantum Dots: Synthesis, Bioapplications, and Toxicity. Nanoscale Res. Lett. 2012, 7, 1–14.
- Han, X.; Lai, L.; Tian, F.; Jiang, F.L.; Xiao, Q.; Li, Y.; Yu, Q.; Li, D.; Wang, J.; Zhang, Q.; et al. Toxicity of CdTe Quantum Dots on Yeast Saccharomyces Cerevisiae. Small 2012, 8, 2680–2689.
- Liang, Y.; Zhang, T.; Tang, M. Toxicity of Quantum Dots on Target Organs and Immune System. J. Appl. Toxicol. 2022, 42, 17–40.
- Duan, J.; Yu, Y.; Li, Y.; Yu, Y.; Li, Y.; Huang, P.; Zhou, X.; Peng, S.; Sun, Z. Developmental Toxicity of CdTe QDs in Zebrafish Embryos and Larvae. J. Nanoparticle Res. 2013, 15, 1–11.
- Nguyen, K.C.; Rippstein, P.; Tayabali, A.F.; Willmore, W.G. Mitochondrial Toxicity of Cadmium Telluride Quantum Dot Nanoparticles in Mammalian Hepatocytes. Toxicol. Sci. 2015, 146, 31–42.
- Banerjee, R.; Goswami, P.; Chakrabarti, M.; Chakraborty, D.; Mukherjee, A.; Mukherjee, A. Cadmium Selenide (CdSe) Quantum Dots Cause Genotoxicity and Oxidative Stress in Allium Cepa Plants. Mutat. Res. Toxicol. Environ. Mutagen. 2021, 865, 503338.
- Zahra, Z.; Habib, Z.; Hyun, S.; Sajid, M. Nanowaste: Another Future Waste, Its Sources, Release Mechanism, and Removal Strategies in the Environment. Sustainability 2022, 14, 2041.
- Reshma, V.G.; Mohanan, P.V. Quantum Dots: Applications and Safety Consequences. J. Lumin. 2019, 205, 287–298.
- Tsoi, K.M.; Dai, Q.; Alman, B.A.; Chan, W.C.W. Are Quantum Dots Toxic? Exploring the Discrepancy between Cell Culture and Animal Studies. Acc. Chem. Res. 2013, 46, 662–671.
- Bottrill, M.; Green, M. Some Aspects of Quantum Dot Toxicity. Chem. Commun. 2011, 47, 7039–7050.
- Hauck, T.S.; Anderson, R.E.; Fischer, H.C.; Newbigging, S.; Chan, W.C.W. In Vivo Quantum-Dot Toxicity Assessment. Small 2010, 6, 138–144.
- Hardman, R. A Toxicologic Review of Quantum Dots: Toxicity Depends on Physicochemical and Environmental Factors. Env. Health Perspect. 2006, 114, 165–172.
- Lu, J.; Tang, M.; Zhang, T. Review of Toxicological Effect of Quantum Dots on the Liver. J. Appl. Toxicol. 2019, 39, 72–86.
- Jain, S.; Bharti, S.; Bhullar, G.K.; Tripathi, S.K. I-III-VI Core/Shell QDs: Synthesis, Characterizations and Applications. J. Lumin. 2020, 219, 116912.
- Morozova, S.; Alikina, M.; Vinogradov, A.; Pagliaro, M. Silicon Quantum Dots: Synthesis, Encapsulation, and Application in Light-Emitting Diodes. Front. Chem. 2020, 8, 191.
- Borovaya, M.N.; Burlaka, O.M.; Naumenko, A.P.; Blume, Y.B.; Yemets, A.I. Extracellular Synthesis of Luminescent CdS Quantum Dots Using Plant Cell Culture. Nanoscale Res. Lett. 2016, 11, 100.
- Janus, Ł.; Piatkowski, M.; Radwan-Pragłowska, J.; Bogdał, D.; Matysek, D. Chitosan-Based Carbon Quantum Dots for Biomedical Applications: Synthesis and Characterization. Nanomaterials 2019, 9, 274.
- Iravani, S.; Varma, R.S. Green Synthesis, Biomedical and Biotechnological Applications of Carbon and Graphene Quantum Dots. A Review. Environ. Chem. Lett. 2020, 18, 703–727.
- Mirhosseini Moghaddam, M.; Baghbanzadeh, M.; Sadeghpour, A.; Glatter, O.; Kappe, C.O. Continuous-Flow Synthesis of CdSe Quantum Dots: A Size-Tunable and Scalable Approach. Chem. A Eur. J. 2013, 19, 11629–11636.
- Kato, I.T.; Santos, C.C.; Benetti, E.; Tenório, D.P.L.A.; Cabral Filho, P.E.; Sabino, C.P.; Fontes, A.; Santos, B.S.; Prates, R.A.; Ribeiro, M.S. CdTe/CdS-MPA Quantum Dots as Fluorescent Probes to Label Yeast Cells: Synthesis, Characterization and Conjugation with Concanavalin A. In Proceedings of the Colloidal Nanocrystals for Biomedical Applications VII, San Francisco, CA, USA, 21–22 January 2012; Volume 8232.
- Rzigalinski, B.A.; Strobl, J.S. Cadmium-Containing Nanoparticles: Perspectives on Pharmacology and Toxicology of Quantum Dots. Toxicol. Appl. Pharm. 2009, 238, 280–288.
- Gallo, V.; Srivastava, V.; Bulone, V.; Zappettini, A.; Villani, M.; Marmiroli, N.; Marmiroli, M. Proteomic Analysis Identifies Markers of Exposure to Cadmium Sulphide Quantum Dots (Cds Qds). Nanomaterials 2020, 10, 1214.
- Horstmann, C.; Kim, K. Comparing Transcriptome Profiles of Saccharomyces Cerevisiae Cells Exposed to Cadmium Selenide/Zinc Sulfide and Indium Phosphide/Zinc Sulfide. Genes 2021, 12, 428.
- Reznik, I.; Zlatov, A.; Baranov, M.; Zakoldaev, R.; Veniaminov, A.; Moshkalev, S.; Orlova, A. Photophysical Properties of Multilayer Graphene–Quantum Dots Hybrid Structures. Nanomaterials 2020, 10, 714.
- Tian, P.; Tang, L.; Teng, K.S.; Lau, S.P. Graphene Quantum Dots from Chemistry to Applications. Mater. Today Chem. 2018, 10, 221–258.
- Mansuriya, B.D.; Altintas, Z. Applications of Graphene Quantum Dots in Biomedical Sensors. Sensors 2020, 20, 1072.
- Alaghmandfard, A.; Sedighi, O.; Tabatabaei Rezaei, N.; Abedini, A.A.; Malek Khachatourian, A.; Toprak, M.S.; Seifalian, A. Recent Advances in the Modification of Carbon-Based Quantum Dots for Biomedical Applications. Mater. Sci. Eng. C 2021, 120.
- Gong, Y.; Dong, Z. Transfer, Transportation, and Accumulation of Cerium-Doped Carbon Quantum Dots: Promoting Growth and Development in Wheat. Ecotoxicol. Environ. Saf. 2021, 226, 112852.
- Chen, N.; He, Y.; Su, Y.; Li, X.; Huang, Q.; Wang, H.; Zhang, X.; Tai, R.; Fan, C. The Cytotoxicity of Cadmium-Based Quantum Dots. Biomaterials 2012, 33, 1238–1244.
- Oh, E.; Liu, R.; Nel, A.; Gemill, K.B.; Bilal, M.; Cohen, Y.; Medintz, I.L. Meta-Analysis of Cellular Toxicity for Cadmium-Containing Quantum Dots. Nat. Nanotechnol. 2016, 11, 479–486.
- Hu, L.; Zhong, H.; He, Z. Toxicity Evaluation of Cadmium-Containing Quantum Dots: A Review of Optimizing Physicochemical Properties to Diminish Toxicity. Colloids Surf. B Biointerfaces 2021, 200, 111609.
- Zhang, B.; Wang, Y.; Hu, R.; Roy, I.; Yong, K.T. Cadmium-Free Quantum Dots for Biophotonic Imaging and Sensing. In Handbook of Photonics for Biomedical Engineering; Springer: Berlin/Heidelberg, Germany, 2017.
- Su, Y.; Hu, M.; Fan, C.; He, Y.; Li, Q.; Li, W.; Wang, L.H.; Shen, P.; Huang, Q. The Cytotoxicity of CdTe Quantum Dots and the Relative Contributions from Released Cadmium Ions and Nanoparticle Properties. Biomaterials 2010, 31, 4829–4834.
- Tarantini, A.; Wegner, K.D.; Dussert, F.; Sarret, G.; Beal, D.; Mattera, L.; Lincheneau, C.; Proux, O.; Truffier-Boutry, D.; Moriscot, C.; et al. Physicochemical Alterations and Toxicity of InP Alloyed Quantum Dots Aged in Environmental Conditions: A Safer by Design Evaluation. NanoImpact 2019, 14, 100168.
- Veronesi, G.; Moros, M.; Castillo-Michel, H.; Mattera, L.; Onorato, G.; Wegner, K.D.; Ling, W.L.; Reiss, P.; Tortiglione, C. In Vivo Biotransformations of Indium Phosphide Quantum Dots Revealed by X-Ray Microspectroscopy. ACS Appl. Mater. Interfaces 2019, 11, 35630–35640.
- Brunetti, V.; Chibli, H.; Fiammengo, R.; Galeone, A.; Malvindi, M.A.; Vecchio, G.; Cingolani, R.; Nadeau, J.L.; Pompa, P.P. InP/ZnS as a Safer Alternative to CdSe/ZnS Core/Shell Quantum Dots: In Vitro and in Vivo Toxicity Assessment. Nanoscale 2013, 5, 307–317.
- Allocca, M.; Mattera, L.; Bauduin, A.; Miedziak, B.; Moros, M.; de Trizio, L.; Tino, A.; Reiss, P.; Ambrosone, A.; Tortiglione, C. An Integrated Multilevel Analysis Profiling Biosafety and Toxicity Induced by Indium- and Cadmium-Based Quantum Dots in Vivo. Environ. Sci. Technol. 2019, 53, 3938–3947.
- Ramasamy, P.; Kim, N.; Kang, Y.S.; Ramirez, O.; Lee, J.S. Tunable, Bright, and Narrow-Band Luminescence from Colloidal Indium Phosphide Quantum Dots. Chem. Mater. 2017, 29, 6893–6899.
- Wu, Y.; Li, C.; van der Mei, H.C.; Busscher, H.J.; Ren, Y. Carbon Quantum Dots Derived from Different Carbon Sources for Antibacterial Applications. Antibiotics 2021, 10, 623.
- Yoo, D.; Park, Y.; Cheon, B.; Park, M.H. Carbon Dots as an Effective Fluorescent Sensing Platform for Metal Ion Detection. Nanoscale Res. Lett. 2019, 14, 1–13.
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.P.; Yang, L. Carbon Dots as Potent Antimicrobial Agents. Theranostics 2020, 10, 671–686.
- Guo, R.; Li, L.; Wang, B.; Xiang, Y.; Zou, G.; Zhu, Y.; Hou, H.; Ji, X. Functionalized Carbon Dots for Advanced Batteries. Energy Storage Mater. 2021, 37, 8–39.
- Belza, J.; Opletalová, A.; Poláková, K. Carbon Dots for Virus Detection and Therapy. Microchim. Acta 2021, 188, 1–23.
- Kang, C.; Huang, Y.; Yang, H.; Yan, X.F.; Chen, Z.P. A Review of Carbon Dots Produced from Biomass Wastes. Nanomaterials 2020, 10, 2316.
- Park, S.J.; Yang, H.K. Ultra-Fast Synthesis of Carbon Dots Using the Wasted Coffee Residues for Environmental Remediation. Curr. Appl. Phys. 2022, 36, 9–15.
- Janus, Ł.; Piątkowski, M.; Radwan-Pragłowska, J.; Sierakowska, A. Carbon Quantum Dots (CQDs) Prepared from Waste Biomass as a New Class of Biomaterials with Luminescent Properties. Inz. Miner. 2020, 2020.
- Bagheri, Z.; Ehtesabi, H.; Hallaji, Z.; Latifi, H.; Behroodi, E. Investigation the Cytotoxicity and Photo-Induced Toxicity of Carbon Dot on Yeast Cell. Ecotoxicol. Environ. Saf. 2018, 161, 245–250.
- Zhang, Q.; Shi, R.; Li, Q.; Maimaiti, T.; Lan, S.; Ouyang, P.; Ouyang, B.; Bai, Y.; Yu, B.; Yang, S.T. Low Toxicity of Fluorescent Carbon Quantum Dots to White Rot Fungus Phanerochaete Chrysosporium. J. Environ. Chem. Eng. 2021, 9, 104633.
- Haque, E.; Kim, J.; Malgras, V.; Raghava Reddy, K.; Ward, A.C.; You, J.; Bando, Y.; Hossain, M.S.A.; Yamauchi, Y. Recent Advances in Graphene Quantum Dots: Synthesis, Properties, and Applications. Small Methods 2018, 2, 1800050.
- Tabish, T.A.; Scotton, C.J.; Ferguson, J.D.C.; Lin, L.; der Veen, A.V.; Lowry, S.; Ali, M.; Jabeen, F.; Winyard, P.G.; Zhang, S. Biocompatibility and Toxicity of Graphene Quantum Dots for Potential Application in Photodynamic Therapy. Nanomedicine 2018, 13, 1923–1937.
- Henna, T.K.; Pramod, K. Graphene Quantum Dots Redefine Nanobiomedicine. Mater. Sci. Eng. C 2020, 110, 110651.
- Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Carbon Dots and Graphene Quantum Dots in Electrochemical Biosensing. Nanomaterials 2019, 9, 634.
- Jiang, D.; Ni, D.; Liu, F.; Zhang, L.; Liu, L.; Pu, X. A Fluorescent Imaging Assay of Cast in Renal Disease Based on Graphene Quantum Dots and Fe3O4 Nanoparticles. Clin. Chim. Acta 2016, 454, 94–101.
- Mansuriya, B.D.; Altintas, Z. Graphene Quantum Dot-Based Electrochemical Immunosensors for Biomedical Applications. Materials 2020, 13, 96.
- Kang, I.; Yoo, J.M.; Kim, D.; Kim, J.; Cho, M.K.; Lee, S.E.; Kim, D.J.; Lee, B.C.; Lee, J.Y.; Kim, J.J.; et al. Graphene Quantum Dots Alleviate Impaired Functions in Niemann-Pick Disease Type C in Vivo. Nano Lett. 2021, 21, 2339–2346.
- Tak, K.; Sharma, R.; Dave, V.; Jain, S.; Sharma, S. Clitoria Ternatea Mediated Synthesis of Graphene Quantum Dots for the Treatment of Alzheimer’s Disease. ACS Chem. Neurosci. 2020, 11, 3741–3748.
- Kim, D.; Yoo, J.M.; Hwang, H.; Lee, J.; Lee, S.H.; Yun, S.P.; Park, M.J.; Lee, M.J.; Choi, S.; Kwon, S.H.; et al. Graphene Quantum Dots Prevent α-Synucleinopathy in Parkinson’s Disease. Nat. Nanotechnol. 2018, 13, 812–818.
- Liu, Q.; Sun, J.; Gao, K.; Chen, N.; Sun, X.; Ti, D.; Bai, C.; Cui, R.; Qu, L. Graphene Quantum Dots for Energy Storage and Conversion: From Fabrication to Applications. Mater. Chem. Front. 2020, 4, 421–436.
- Arikpo, J.U.; Onuu, M.U. Graphene Growth and Characterization: Advances, Present Challenges and Prospects. J. Mater. Sci. Res. 2019, 8, 37–68.
- Frossard, A.; Gerull, L.; Mutz, M.; Gessner, M.O. Fungal Importance Extends beyond Litter Decomposition in Experimental Early-Successional Streams. Environ. Microbiol. 2012, 14, 2971–2983.
- van der Heijden, M.G.A.; Horton, T.R. Socialism in Soil? The Importance of Mycorrhizal Fungal Networks for Facilitation in Natural Ecosystems. J. Ecol. 2009, 97, 1139–1150.
- Onufrak, A.; Rúa, M.A.; Hossler, K. The Missing Metric: An Evaluation of Fungal Importance in Wetland Assessments. Wetlands 2020, 40, 825–838.
- Bahram, M.; Netherway, T.; Frioux, C.; Ferretti, P.; Coelho, L.P.; Geisen, S.; Bork, P.; Hildebrand, F. Metagenomic Assessment of the Global Diversity and Distribution of Bacteria and Fungi. Environ. Microbiol. 2021, 23, 316–326.
- Chouhan, R.S.; Qureshi, A.; Niazi, J.H. Determining the Fate of Fluorescent Quantum Dots on Surface of Engineered Budding S. Cerevisiae Cell Molecular Landscape. Biosens. Bioelectron. 2015, 69, 26–33.
- Mei, J.; Yang, L.Y.; Lai, L.; Xu, Z.Q.; Wang, C.; Zhao, J.; Jin, J.C.; Jiang, F.L.; Liu, Y. The Interactions between CdSe Quantum Dots and Yeast Saccharomyces Cerevisiae: Adhesion of Quantum Dots to the Cell Surface and the Protection Effect of ZnS Shell. Chemosphere 2014, 112, 92–99.
- Strtak, A.; Sathiamoorthy, S.; Tang, P.S.; Tsoi, K.M.; Song, F.; Anderson, J.B.; Chan, W.C.W.; Shin, J.A. Yeast Populations Evolve to Resist CdSe Quantum Dot Toxicity. Bioconjug. Chem. 2017, 28, 1205–1213.
- Pasquali, F.; Agrimonti, C.; Pagano, L.; Zappettini, A.; Villani, M.; Marmiroli, M.; White, J.C.; Marmiroli, N. Nucleo-Mitochondrial Interaction of Yeast in Response to Cadmium Sulfide Quantum Dot Exposure. J. Hazard Mater. 2017, 324, 744–752.
- Horstmann, C.; Kim, D.S.; Campbell, C.; Kim, K. Transcriptome Profile Alteration with Cadmium Selenide/Zinc Sulfide Quantum Dots in Saccharomyces Cerevisiae. Biomolecules 2019, 9, 653.
- Ezati, P.; Rhim, J.W.; Molaei, R.; Priyadarshi, R.; Roy, S.; Min, S.; Kim, Y.H.; Lee, S.G.; Han, S. Preparation and Characterization of B, S, and N-Doped Glucose Carbon Dots: Antibacterial, Antifungal, and Antioxidant Activity. Sustain. Mater. Technol. 2022, 32, e00397.
- Zhao, S.; Huang, L.; Xie, Y.; Wang, B.; Wang, F.; Lan, M. Green Synthesis of Multifunctional Carbon Dots for Anti-Cancer and Anti-Fungal Applications. Chin. J. Chem. Eng. 2021, 37, 97–104.
- Devkota, A.; Pandey, A.; Yadegari, Z.; Dumenyo, K.; Taheri, A. Amine-Coated Carbon Dots (NH2-FCDs) as Novel Antimicrobial Agent for Gram-Negative Bacteria. Front. Nanotechnol. 2021, 3, 78.
- Ghirardello, M.; Ramos-Soriano, J.; Galan, M.C. Carbon Dots as an Emergent Class of Antimicrobial Agents. Nanomaterials 2021, 11, 1877.
- Qureshi, W.A.; Vivekanandan, B.; Jayaprasath, J.A.; Ali, D.; Alarifi, S.; Deshmukh, K. Antimicrobial Activity and Characterization of Pomegranate Peel-Based Carbon Dots. J. Nanomater. 2021, 2021, 1–6.
- Ghorbani, M.; Tajik, H.; Moradi, M.; Molaei, R.; Alizadeh, A. One-Pot Microbial Approach to Synthesize Carbon Dots from Baker’s Yeast-Derived Compounds for the Preparation of Antimicrobial Membrane. J. Environ. Chem. Eng. 2022, 10, 107525.
More
