Spine metastases are a common life-threatening complication of advanced-stage malignancies and often result in poor prognosis. Symptomatic spine metastases develop in the course of about 10% of malignant neoplasms. Therefore, it is essential for contemporary medicine to understand metastatic processes in order to find appropriate, targeted therapeutic options. Thanks to continuous reserearch, there appears more and more detailed knowledge about cancer and metastasis, but these transformations are extremely complicated, e.g., due to the complexity of reactions, the variety of places where they occur, or the participation of both tumor cells and host cells in these transitions. The right target points in tumor metastasis mechanisms are still being researched; that will help us in the proper diagnosis as well as in finding the right treatment.
- spine
- metastasis
- tumor
- cancer
1. Introduction
2. Molecular Basis of Spine Metastases
2.1. Escape from the Primary Site
Tumor is a type of malignant cell growth where abnormal cells multiply uncontrollably with an ability for close or distant tissue invasion. Cells within a tumor may differ in many ways, e.g., in their proliferative potential or the ability to undergo apoptosis or metastasize [13][14][15][16][13,14,15,16]. The route that cancer cells must surmount to reach other organs is burdensome, with escape from the primary site being the first obstacle encountered [17][18][19][17,18,19]. Another essential step in the process of metastasis is the entrance of neoplastic cells into blood or lymphatic vessels through which they reach even remotely located organs [20]. Increased activity of the master regulator of angiogenesis—oxygen-sensitive transcriptional activator HIF (hypoxia-inducible factor-1)—contributes to both provision of the path utilized for metastatic spread as well as tumor cells’ nutrition via modulation of proangiogenic factors that include, e.g., VEGF (vascular endothelial growth factor), Ang-1, Ang-2 (angiopoietins), or PlGF (placental growth factor) [21][22][23][21,22,23]. A hallmark of metastasis, epithelial–mesenchymal transition (EMT) is a biological process where epithelial cells forfeit their adhesive properties and apical–basal polarity and acquire migratory as well as invasive features in order to transform into mesenchymal stem cells [24][25][26][24,25,26]. EMT is involved in both pathological (e.g., cancer formation) and physiological (embryogenesis, organ development, wound healing) processes [27][28][27,28] conditioned by biologically different EMT subtypes [29][30][31][32][29,30,31,32]. Reversibility of this process (MET—mesenchymal–epithelial transition) enables cancer cells to return to their original form in another organ [33][34][33,34]. Both EMT and MET comprise series of biochemical reactions regulated by a plethora of transcriptional factors including Snail/Slug, Twist, Six1, Cripto, TGF-β, and Wnt/β-catenin that, when activated, reprogram gene expression [35][36][37][35,36,37] and, in consequence, alter tumor cell properties. While the cytoskeleton undergoes remodeling, modified protein expression into proteins distinctive for mesenchymal cells, e.g., N-cadherin, FSP-1, α-SMA, α5β1 integrin, αvβ6 integrin, vimentin, type I collagen, laminin 5, and fibrotin lead to the termination of connection with the basal membrane [38][39][40][41][38,39,40,41]. As a result, cancer cells acquire enhanced capability for relocation. Additionally, enzymes produced by tumor cells that break down the extracellular matrix, MMP (matrix metalloproteinase) and ADAM (A disintegrin and metalloproteinase), enable vascular wall penetration [42][43][42,43]. EMT also provides resistance to apoptosis due to diminished cell adhesion. The same mechanism facilitates migration and, in turn, evasion of various factors that lead to cell destruction.2.2. Cancer Cells Dissemination
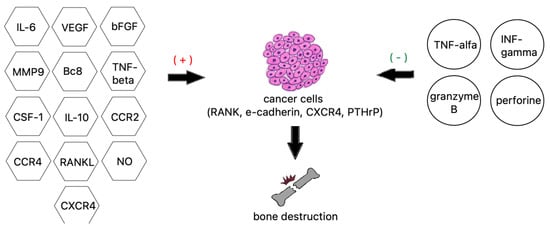
Cancers can spread to bones through various pathways, both venous and arterial, as well as through the lymphatic route or by direct contact [44]. Although the lymphatic system is mentioned as a potential route for spread, the main routes to enter the spinal column are comprised of venous and arterial vessels [45]. The Batson plexus, a network of veins devoid of valves that connect pelvic and thoracic with intraspinal veins contributes to spinal metastasis. Due to the absence of valves, any increase in vena cava pressure is followed by increased blood flow within the plexus, leading to cancer cells dissemination. In turn, neoplastic metastases reach the vertebral body directly through nutritional arteries [46][47][46,47]. Less frequently, neoplastic lesions metastasize through direct contact, e.g., prostate cancer that metastasizes to the lumbosacral spine [48]. Additionally, tumor cells have the ability to adhere to blood cells, including platelets, which serves as protection from the detrimental effects of hemodynamic forces during flow [49][50][51][49,50,51], as well as to bone marrow cells produced by tumor mimic precursors of immune cells, which aids to avoid innate immune response [52].2.3. Bone Invasion
There are two main theories of metastasis. One of them is the so called “seed and soil” theory proposed by Paget over 100 years ago. It says that cancers anchor where they find convenient conditions, similarly to seeds in fertile soil, a process not dependent on anatomical relations [52][53][52,53]. Ewing, in turn, said that metastases are only the result of the structure of the circulatory system and are strictly related to anatomical conditions, such as the diameter of the vessels or connections between organs. In the 1980s, the work by Hart and Fiedler on melanoma metastases confirmed that theory [54][55][54,55]. However, looking at the development of tumors, it seems that both theories are relevant. Bone marrow, due to abundant vascularization, constitutes a part of the bone tissue of relatively high affinity for tumor metastases. The bones of the axial skeleton (skull, spine, sternum, ribs, hips, shoulders) contain a substantial amount of red bone marrow and therefore are a frequent target of tumor spread [56]. Low velocity of the blood flow enables easier adhesion to endothelial cells and, in consequence, quicker integration with endosteum. Bone marrow and bone cells also produce cytokines, hormones, enzymes, as well as growth factors that regulate the immune system and affect the colonization of bone tissue by cancer cells [57][58][57,58]. Tumor cells release a plethora of factors [59][60][59,60], e.g., VEGFR1+—bone marrow-derived progenitor cells that activate VLA-4 that via binding to fibronectin [61][62][63][64][61,62,63,64] enables entrance to the potential metastatic site and create an appropriate environment, a premetastatic niche that facilitates tumor cells implantation [65][66][67][68][69][65,66,67,68,69]. The abovementioned mechanisms are best understood in an example of metastatic breast cancer. It has been found that CXCR4, also known as fusin, is necessary for breast cancer cells migration towards tissues that present a high quantity of its specific ligand—cytokine SDF1 (CXCL12). Among the organs that express high levels of SDF1 are lungs, liver, bone marrow, and brain, which explains the high affinity of breast cancer cells to these tissues [70][71][72][73][70,71,72,73]. Tyrosine kinase Src is activated through the binding of CXCL12 to CXCR4, and downstream effector AKT improves the survival of cancer cells that occupy bone tissues [74]. Primary tumor cells also produce substances that modify the extracellular matrix at sites of metastasis, e.g., lysyl oxidates. Conversely, exosomes and miRNAs are able to influence bone remodeling. Metalloproteinases (MMPs) and bone sialoproteins (BSPs) destroy basal membranes at the site of metastasis, stimulate angiogenesis, and activate various elements involved in the destruction of bone tissue and the spread of tumor cells [70][75][76][77][70,75,76,77]. Once cancer cells reach the bone marrow, their growth depends on multiple factors, including in situ vascularization, available space, type of bone remodeling, or proliferating potential of neoplastic cells [78]. Bone tissue is made up of three main types of cells: osteoblasts, osteoclasts, and osteocytes. Osteoclasts are cells that have the ability to dissolve and resorb bone tissue, while osteoblasts are responsible for the growth and remodeling of bone tissue. Processes that lead to the formation of bone metastases may have different mechanisms: osteolytic and osteoblastic. Sometimes both of these mechanisms work simultaneously. In a healthy organism, the activity of osteoclasts and osteoblasts corresponds to the RANK-RANKL/OPG system [79][80][81][79,80,81]. RANKL (receptor activator for nuclear factor κB ligand) is produced by the osteoblastic line and activated T lymphocytes [80][82][80,82]. It is responsible for activating the process of creating mature osteoclasts. While RANK (receptor activator for nuclear factor κB) is located on osteoclasts and serves as the main regulator during the formation of osteoclasts, RANK combines with RANKL, ligand of the receptor activator of nuclear factor kappa B (NF-κB), which at the same time causes upregulation of nuclear factor of activated T cells 1 (NFATc1) [83][84][83,84]. NFATc1 is the major regulator of cytokine expression in the process of osteoclastogenesis [85][86][85,86]. As a result of these changes, mature osteoblasts are formed. Their main task is old bone reabsorption, which causes the release of nutrients and creates space for osteoblasts. Osteoprotegrin (OPG) binds to RANK and blocks the formation of the RANK–RANKL complex, thereby inhibiting the maturation process of osteoclasts [87][88][89][87,88,89].2.4. Osteocyte Physiology and Pathology
Osteocytes have an impressive lifespan of up to 25 years, during which they undertake several important physiological functions. They differentiate from osteoblasts with four stages of formation, type I preosteocytes (osteoblastic osteocytes), type II preosteocytes (osteoid osteocytes), and type III preosteocytes (young and old osteocytes) [90]. During the process of bone formation, the osteoblastic cell body reduces in size, and its cytoplasm expands, springing processes from out of the cell’s wall. The Golgi apparatus during the type I and II cycles has to be well-developed to efficiently synthesize type I collagen essential for maintaining the bone matrix. Entering the type III preosteocyte phase, the Golgi apparatus is reduced in size, and the osteocyte matrix proceeds from the incompletely mineralized phase to the formation of old osteocytes with high mineral density [90]. Mature osteocytes express such markers as DMP1, Sost, as well as the cx43 protein, which is believed to be critical in the role of keeping the cell from entering apoptosis [90][91][90,91]. It is theorized that cx43 influences bone cell activity by regulating the osteoprotegerin and sclerostin levels [91].2.5. Osteoblast Physiology and Pathology
The aforementioned osteoblasts are generated from pluripotent mesenchymal stem cells that take on the crucial role of bone matrix synthesis by firstly establishing the collagen, OCN, osteonectin, BSP II, and osteopontin proteins alongside decorin and biglycan to create osteoids, which would be further mineralized [90]. Osteoblasts tend to communicate with osteocytes using the RANK–RANKL pathway as a way to order growth factor release from the bone matrix [92]. The aforementioned processes summarize the bone homeostasis. It has been established that the regions of the bone with the largest amount of turnover (trabecular bone) tend to become sites for metastatic cell growth. One very prominent factor of this cancer cell homing is CXC motif chemokine 12, also known as CXCL12 or SDF-1, produced by bone marrow stromal cells and osteoblasts, which was proven to be crucial to metastases [93][94][93,94]. Cancer cells interact with osteoblasts and osteoclasts, as well as the cytokines released by the bone in an otherwise physiological process [95].2.6. Osteoblastic Metastasis Pathogenesis
Bone tissue is considered to be the third place in the aspect of frequency of metastatic changes. Most of these metastatic changes are the result of oncological diseases, primarily breast and prostate cancer [8][96][8,96]. The statistics analyzed in previous years showed bone metastasis to be the effect of up to 70–75% of breast and prostate cancers [17]. A factor that one has to take into consideration is the bone’s extreme metabolic activity derived from the three main cell types: osteocytes, osteoblasts, and osteoclasts. Osteoblasts account for 4–6% of total cells, osteocytes—90–95%, osteoclasts—about 1–4% [90]. Osteoblastic metastasis is characterized by the deposition of new bone rather than lysis of the already existing structures. It is most notably present in prostate cancers, carcinoids, small-cell lung cancers, Hodgkin lymphomas, and medulloblastomas [17]. Tumor cells invading the bone tend to produce growth factors such as bone morphogenic proteins, epidermal growth factors, and platelet-derived growth factors. There have been consistent data proving that the physical contact of osteoblasts and prostate cancer cells promote tumor growth in vitro via protein ECM components, proteoglycans (PGs), and junction-related molecules [97]. Some of the more prominent factors of this process are BMPSs, TGF beta, and endothelin-1. BMP4 has been proven to stimulate osteoblast differentiation after being secreted from PCa-118b prostate cancer cells via the pSmad1–Notch–Hey1 and GSK3 β–β-catenin–Slug pathways [98]. The aforementioned TGF beta, specifically, TGF beta 2, is secreted from the prostate cancer metastasized cells [98] to foster the progression of tumor growth [99]. Another mechanism of growth is the secretion of endothelin-1 which downregulates DKK-1 and stimulates the secretion of the Wnt signaling pathways proven to be associated with lytic lesions and suppressing the growth of bone tissue in myelomas [100]. This occurs because DDK1 inhibits the production of osteoblasts by preventing the binding of low-density lipoprotein receptor-related proteins 5 and 6 (LRP5/6) in osteoblast precursors [101]. The role of PTHrP fragments in the process must not be underestimated. The parathyroid hormone-related protein increases calcium absorption and bone resorption [102], but it greatly increases the metastatic growth of cancer cells [103]. It has been theorized that NH2-terminal fragments of this protein stimulate bone formation via the ETA receptor because of the shared sequence homology to ET-1, which was proven to increase metastatic growth [104]. Other research has proven that PTHrP acts as a mediator in osteoblastogenesis, increasing early osteoblast differentiation and proliferation of bone marrow cells [105]. One other crucial aspect of bone metastasis that has to be taken into consideration is the so-called vicious cycle. Prostate cancer cells that have been freshly metastasized tend to produce PDGF, ET1, and BMPs that activate osteoblastic differentiation and bone matrix formation. As mentioned before, bone turnover marks the sites where tumors tend to grow, as the freshly synthesized structures are rich with growth factors such as IGF, FGF, and TGF-β that attract prostate cancer cells [95]. The physical contact of tumor cells and osteoblasts further promotes the secretion of growth factors—the vicious cycle continues to propel itself until it reaches the physical limits of the metastatic site.2.7. Osteolytic Bone Metastasis
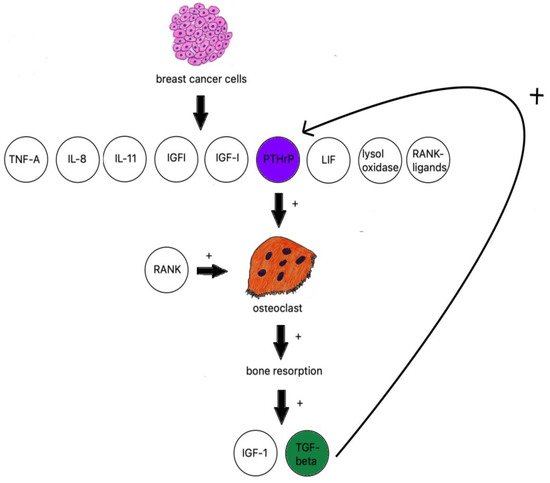
When discussing osteolytic metastases, pweople must look at the research on breast cancer. The majority of breast cancer metastases are lytic; breast cancer cells produce TNF-A, IL-8, IL-11 [106], IGF1, LIF (leukemia inhibitory factor), lysyl oxidase [107], RANK ligands, and PTHrP (Figure 2) [108].